Elucidating Pathway and Anesthetic Mechanism of Action of Clove Oil Nanoformulations in Fish
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Animals and Housing
2.3. Preparation of CO Nanoformulations
2.4. Particle Characterization of CO Nanoformulations
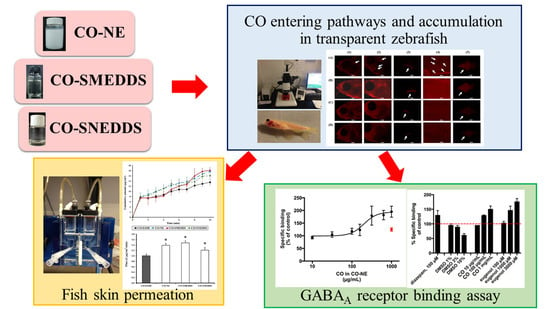
2.5. CO Entering Pathways and Accumulation
2.6. Skin Permeation Study
2.7. Rat Brain Homogenate [3H]Muscimol GABAA Receptor Binding
2.8. Statistical Analysis
3. Results
3.1. Particle Characterization of CO Nanoformulations
3.2. CO Entering Pathways and Accumulation
3.3. Fish Skin Permeation
3.4. GABAA Receptor-Binding Assay
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Summerfelt, R.C.; Smith, L.S. Anaesthesia, surgery and related technigues. In Methods in Fish Biology; Schreck, C.B., Moyle, P., Eds.; American Fisheries Society: Bethesda, MD, USA, 1990; pp. 213–272. [Google Scholar]
- Topic Popovic, N.; Strunjak-Perovic, I.; Coz-Rakovac, R.; Barisic, J.; Jadan, M.; Persin Berakovic, A.; Sauerborn Klobucar, R. Tricaine methane-sulfonate (MS-222) application in fish anaesthesia. J. Appl. Ichthyol. 2012, 28, 553–564. [Google Scholar] [CrossRef] [Green Version]
- Prince, A.; Powell, C. Clove oil as an anesthetic for invasive field procedures on adult rainbow trout. N. Am. J. Fish. Manag. 2000, 20, 1029–1032. [Google Scholar] [CrossRef]
- Cooke, S.J.; Suski, C.D.; Ostrand, K.G.; Tufts, B.L.; Wahl, D.H. Behavioral and physiological assessment of low concentrations of clove oil anaesthetic for handling and transporting largemouth bass (Micropterus salmoides). Aquaculture 2004, 239, 509–529. [Google Scholar] [CrossRef]
- Woody, C.A.; Nelson, J.; Ramstad, K. Clove oil as an anaesthetic for adult sockeye salmon: Field trials. J. Fish Biol. 2002, 60, 340–347. [Google Scholar] [CrossRef]
- Bunyapraphatsara, N. Clove. In Thai Medicinal Plants; Bunyapraphatsara, N., Chokchaijarenporn, O., Eds.; Prachachon: Bangkok, Thailand, 1996; pp. 211–224. [Google Scholar]
- King, V.W.; Hooper, B.; Hillsgrove, S.; Benton, C.; Berlinsky, D.L. The use of clove oil, metomidate, tricaine methanesulphonate and 2-phenoxyethanol for inducing anaesthesia and their effect on the cortisol stress response in black sea bass (Centropristis striata L.). Aquac. Res. 2005, 36, 1442–1449. [Google Scholar] [CrossRef]
- Sanchez-Vazquez, F.J.; Terry, M.I.; Felizardo, V.O.; Vera, L.M. Daily rhythms of toxicity and effectiveness of anesthetics (MS222 and eugenol) in zebrafish (Danio rerio). Chronobiol. Int. 2011, 28, 109–117. [Google Scholar] [CrossRef] [PubMed]
- Songkaew, A.; Chokboonmongkol, C.; Khattiya, R.; Wongsathein, D.; Mengumpun, K.; Pikulkaew, S. Induction time and behavior of anesthesia and recovery in Mekong giant catfish (Pangasianodon gigas) after anesthetized with clove oil and tricaine methanesulfonate (MS-222). J. Thai Vet. Med. Assoc. 2007, 58, 12–21. [Google Scholar]
- Anderson, D.P. 7 Environmental Factors in Fish Health: Immunological Aspects. Fish Physiol. 1997, 15, 289–310. [Google Scholar] [CrossRef]
- Capriotti, K.; Capriotti, J.A. Dimethyl sulfoxide: History, chemistry, and clinical utility in dermatology. J. Clin. Aesthet. Dermatol. 2012, 5, 24–26. [Google Scholar] [PubMed]
- Davaris, P.; Fytiza, R.; Androulakakis, P.; Papacharalampous, N. Carcinogenesis associated with dimethyl sulfoxide. Urol. Int. 1992, 48, 120. [Google Scholar] [CrossRef] [PubMed]
- Kheawfu, K.; Pikulkaew, S.; Rades, T.; Müllertz, A.; Okonogi, S. Development and characterization of clove oil nanoemulsions and self-microemulsifying drug delivery systems. J. Drug Deliv. Sci. Technol. 2018, 46, 330–338. [Google Scholar] [CrossRef]
- Kheawfu, K.; Pikulkaew, S.; Rades, T.; Müllertz, A.; Jørgensen, L.V.G.; Okonogi, S. Design and optimization of self-nanoemulsifying drug delivery systems of clove oil for efficacy enhancement in fish anesthesia. J. Drug Deliv. Sci. Technol. 2021, 61, 102241. [Google Scholar] [CrossRef]
- Solans, C.; Izquierdo, P.; Nolla, J.; Azemar, N.; Garcia-Celma, M.J. Nano-emulsions. Curr. Opin. Colloid Interface Sci. 2005, 10, 102–110. [Google Scholar] [CrossRef]
- Khumpirapang, N.; Pikulkaew, S.; Müllertz, A.; Rades, T.; Okonogi, S. Self-microemulsifying drug delivery system and nanoemulsion for enhancing aqueous miscibility of Alpinia galanga oil. PLoS ONE 2017, 12, e0188848. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Meyer, R.E.; Fish, R.E. Chapter 2: Pharmacology of injectable anesthetics, sedatives, and tranquilizers. In Anesthesia and Analgesia in Laboratory Animals; Fish, R.E., Brown, M.J., Danneman, P.J., Karas, A.Z., Eds.; Academic Press Cambridge: Cambridge, MA, USA, 2008; pp. 27–82. [Google Scholar]
- Delgado, L.; Schmachtenberg, O. Immunohistochemical localization of GABA, GAD65, and the receptor subunits GABAA alpha1 and GABAB1 in the zebrafish cerebellum. Cerebellum 2008, 7, 444–450. [Google Scholar] [CrossRef]
- Garcia, P.S.; Kolesky, S.E.; Jenkins, A. General anesthetic actions on GABAA receptors. Curr. Neuropharmacol. 2010, 8, 2–9. [Google Scholar] [CrossRef] [PubMed]
- Neumcke, B.; Schwarz, W.; Stämpfli, R. Block of Na channels in the membrane of myelinated nerve by benzocaine. Pflügers Arch. Eur. J. Physiol. 1981, 390, 230–236. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Uchida, I. Mechanisms of etomidate potentiation of GABAA receptor-gated currents in cultured postnatal hippocampal neurons. Neuroscience 1996, 73, 69–78. [Google Scholar] [CrossRef]
- Burka, J.F.; Hammell, K.L.; Horsberg, T.E.; Johnson, G.R.; Rainnie, D.J.; Speare, D.J. Drugs in salmonid aquaculture—A review. J. Vet. Pharmacol. Ther. 1997, 20, 333–349. [Google Scholar] [CrossRef]
- Kheawfu, K.; Pikulkaew, S.; Chaisri, W.; Okonogi, S. Nanoemulsion: A suitable nanodelivery system of clove oil for anesthetizing Nile tilapia. Drug Discov. Ther. 2017, 11, 181–185. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Korbut, R.; Mehrdana, F.; Kania, P.W.; Larsen, M.H.; Frees, D.; Dalsgaard, I.; Jørgensen, L.v.G. Antigen uptake during different life stages of zebrafish (Danio rerio) using a GFP-tagged Yersinia ruckeri. PLoS ONE 2016, 11, e0158968. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rana, I.S.; Rana, A.S.; Rajak, R.C. Evaluation of antifungal activity in essential oil of the Syzygium aromaticum (L.) by extraction, purification and analysis of its main component eugenol. Braz. J. Microbiol. 2011, 42, 1269–1277. [Google Scholar] [CrossRef]
- Sintov, A.C.; Botner, S. Transdermal drug delivery using microemulsion and aqueous systems: Influence of skin storage conditions on the in vitro permeability of diclofenac from aqueous vehicle systems. Int. J. Pharm. 2006, 311, 55–62. [Google Scholar] [CrossRef] [PubMed]
- Ransom, R.W.; Stec, N.L. Cooperative modulation of [3H]MK-801 binding to the N-methyl-d-aspartate receptor-ion channel complex by l-glutamate, glycine, and polyamines. J. Neurochem. 1988, 51, 830–836. [Google Scholar] [CrossRef] [PubMed]
- Eghorn, L.F.; Hoestgaard-Jensen, K.; Kongstad, K.T.; Bay, T.; Higgins, D.; Frolund, B.; Wellendorph, P. Positive allosteric modulation of the GHB high-affinity binding site by the GABAA receptor modulator monastrol and the flavonoid catechin. Eur. J. Pharmacol. 2014, 740, 570–577. [Google Scholar] [CrossRef]
- Konrádsdóttir, F.; Loftsson, T.; Sigfússon, S.D. Fish skin as a model membrane: Structure and characteristics. J. Pharm. Pharmacol. 2008, 61, 121–124. [Google Scholar] [CrossRef]
- Campo-Soria, C.; Chang, Y.; Weiss, D.S. Mechanism of action of benzodiazepines on GABAA receptors. Br. J. Pharmacol. 2006, 148, 984–990. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Atucha, E.; Hammerschmidt, F.; Zolle, I.; Sieghart, W.; Berger, M.L. Structure-activity relationship of etomidate derivatives at the GABA(A) receptor: Comparison with binding to 11beta-hydroxylase. Bioorg. Med. Chem. Lett. 2009, 19, 4284–4287. [Google Scholar] [CrossRef] [PubMed]
- Zahl, I.H.; Samuelsen, O.; Kiessling, A. Anaesthesia of farmed fish: Implications for welfare. Fish Physiol. Biochem. 2012, 38, 201–218. [Google Scholar] [CrossRef]
- Javahery, S.; Nekoubin, H.; Moradlu, A.H. Effect of anaesthesia with clove oil in fish (review). Fish Physiol. Biochem. 2012, 38, 1545–1552. [Google Scholar] [CrossRef]
- Li, Q.; Frank, M.; Thisse, C.I.; Thisse, B.V.; Uitto, J. Zebrafish: A model system to study heritable skin diseases. J. Investig. Dermatol. 2011, 131, 565–571. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Másson, M.; Sigfússon, S.D.; Loftsson, T. Fish skin as a model membrane to study transmembrane drug delivery with cyclodextrins. J. Incl. Phenom. Macrocycl. Chem. 2002, 44, 177–182. [Google Scholar] [CrossRef]
- Som, I.; Bhatia, K.; Yasir, M. Status of surfactants as penetration enhancers in transdermal drug delivery. J. Pharm. Bioallied Sci. 2012, 4, 2–9. [Google Scholar] [CrossRef] [PubMed]
- Williams, A.C.; Barry, B.W. Penetration enhancers. Adv. Drug Deliv. Rev. 2004, 56, 603–618. [Google Scholar] [CrossRef]
- Sousa, G.D.; Kishishita, J.; Aquino, K.A.S.; Presgrave, O.A.F.; Leal, L.B.; Santana, D.P. Biopharmaceutical assessment and irritation potential of microemulsions and conventional systems containing oil from Syagrus cearensis for topical delivery of amphotericin b using alternative methods. AAPS PharmSciTech 2017, 18, 1833–1842. [Google Scholar] [CrossRef]
- Intarakumhaeng, R.; Li, S.K. Effects of solvent on percutaneous absorption of nonvolatile lipophilic solute. Int. J. Pharm. 2014, 476, 266–276. [Google Scholar] [CrossRef]
- Pham, Q.D.; Topgaard, D.; Sparr, E. Tracking solvents in the skin through atomically resolved measurements of molecular mobility in intact stratum corneum. Proc. Natl. Acad. Sci. USA 2017, 114, E112–E121. [Google Scholar] [CrossRef] [Green Version]
- Sahin, S.; Eulenburg, V.; Heinlein, A.; Villmann, C.; Pischetsrieder, M. Identification of eugenol as the major determinant of GABAA-receptor activation by aqueous Syzygium aromaticum L. (clove buds) extract. J. Funct. Foods 2017, 37, 641–649. [Google Scholar] [CrossRef]
- Aoshima, H.; Hamamoto, K. Potentiation of GABAA receptors expressed in Xenopus Oocytes by perfume and phytoncid. Biosci. Biotechnol. Biochem. 1999, 63, 743–748. [Google Scholar] [CrossRef]
- Fischer, I.U.; Von Unruh, G.E.; Dengler, H.J. The metabolism of eugenol in man. Xenobiotica 1990, 20, 209–222. [Google Scholar] [CrossRef]
- Galdino, P.M.; Nascimento, M.V.M.; Florentino, I.F.; Lino, R.C.; Fajemiroye, J.O.; Chaibub, B.A.; de Paula, J.R.; de Lima, T.C.M.; Costa, E.A. The anxiolytic-like effect of an essential oil derived from Spiranthera odoratissima A. St. Hil. leaves and its major component, beta-caryophyllene, in male mice. Prog. Neuropsychopharmacol. Biol. Psychiatry 2012, 38, 276–284. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wallner, M.; Hanchar, H.J.; Olsen, R.W. Low dose acute alcohol effects on GABA A receptor subtypes. Pharmacol. Ther. 2006, 112, 513–528. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Du, H.; Yang, X.; Zhai, G. Design of chitosan-based nanoformulations for efficient intracellular release of active compounds. Nanomedicine 2014, 9, 723–740. [Google Scholar] [CrossRef] [PubMed]







| Formulations | Compositions (% w/w) | |||||||
|---|---|---|---|---|---|---|---|---|
| CO | Tween 20 | Water | Ethanol | Isopropanol | Captex 300 | Capmul MCM EP | Kolliphor EL | |
| CO-NE | 20 | 10 | 70 | - | - | - | - | - |
| CO-SMEDDS | 10 | 60 | - | - | 30 | - | - | - |
| CO-SNEDDS | 30 | - | - | 10 | - | 15 | 15 | 30 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kheawfu, K.; Pikulkaew, S.; Wellendorph, P.; Jørgensen, L.v.G.; Rades, T.; Müllertz, A.; Okonogi, S. Elucidating Pathway and Anesthetic Mechanism of Action of Clove Oil Nanoformulations in Fish. Pharmaceutics 2022, 14, 919. https://doi.org/10.3390/pharmaceutics14050919
Kheawfu K, Pikulkaew S, Wellendorph P, Jørgensen LvG, Rades T, Müllertz A, Okonogi S. Elucidating Pathway and Anesthetic Mechanism of Action of Clove Oil Nanoformulations in Fish. Pharmaceutics. 2022; 14(5):919. https://doi.org/10.3390/pharmaceutics14050919
Chicago/Turabian StyleKheawfu, Kantaporn, Surachai Pikulkaew, Petrine Wellendorph, Louise von Gersdorff Jørgensen, Thomas Rades, Anette Müllertz, and Siriporn Okonogi. 2022. "Elucidating Pathway and Anesthetic Mechanism of Action of Clove Oil Nanoformulations in Fish" Pharmaceutics 14, no. 5: 919. https://doi.org/10.3390/pharmaceutics14050919
APA StyleKheawfu, K., Pikulkaew, S., Wellendorph, P., Jørgensen, L. v. G., Rades, T., Müllertz, A., & Okonogi, S. (2022). Elucidating Pathway and Anesthetic Mechanism of Action of Clove Oil Nanoformulations in Fish. Pharmaceutics, 14(5), 919. https://doi.org/10.3390/pharmaceutics14050919