Fluoroquinolones Hybrid Molecules as Promising Antibacterial Agents in the Fight against Antibacterial Resistance
Abstract
:1. Introduction
2. The Research Methodology and Literature Review
3. Antibacterial Quinolones (QNs)
3.1. Structural Characterization of Antibacterial QNs
- Naphthyridine derivatives (nalidixic acid, enoxacin, trovafloxacin, zabofloxacin);
- Quinoline derivatives (cinoxacin);
- Pyrido-pyrimidine derivatives (pyromidic acid, pipemidic acid);
- Quinoline derivatives (norfloxacin, ciprofloxacin, enrofloxacin, moxifloxacin, besifloxacin, delafloxacin, finafloxacin, lascufloxacin, nemonoxacin);
- Compounds with different structures (flumequine, ofloxacin, marbofloxacin, nadifloxacin, and levonadifloxacin).
- -
- Non-fluorinated quinolones (nemonoxacin);
- -
- Monofluoroquinolones (ciprofloxacin, enoxacin, marbofloxacin, moxifloxacin, finafloxacin, pradofloxacin, nadifloxacin and levonadifloxacin, zabofloxacin);
- -
- Difluoroquinolones (lomefloxacin, sarafloxacin, sparfloxacin, garenoxacin);
- -
- Trifluoroquinolones (fleroxacin, temafloxacin, trovafloxacin, lascufloxacin);
- -
- Monochloro- and monofluoroquinolones (besifloxacin);
- -
- Monochloro- and difluoroquinolones (sitafloxacin);
- -
3.2. Physicochemical Properties of FQNs
3.3. Mechanism of Action of Antibacterial FQNs
- The slow death is caused by the unprocessed complexes that block replication and transcription;
- The immediate death occurs when the complexes are processed (by dissociation of the gyrase subunits or by removal of the gyrase from the DNA). In this case, the cell is killed due to the fragmentation of the chromosome, which results when the broken DNA is not repaired.
3.4. Indications, Spectrum of Activity, and Pharmacokinetics Data
3.5. Aspects to Be Considered Regarding the Inclusion of FQNs in Hybrid Compounds
4. Antimicrobial Resistance
4.1. Highlights of the Most Resistant Bacteria Worldwide
4.2. The Development of Antibacterial Resistance over Time
4.3. The Emergence of Resistance to Antibiotics Relatively Recently Introduced in Therapy
4.4. New Mechanisms for Bacterial Resistance
4.5. Resistance to FQNs
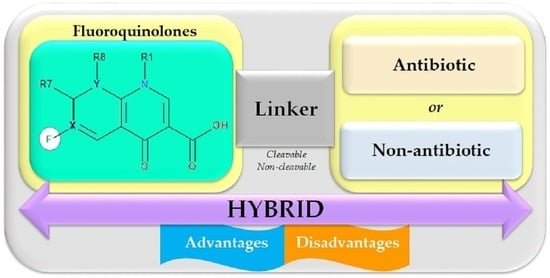
5. Antibiotic Hybrids
5.1. Antibiotic Hybrids as Tools against Antimicrobial Resistance
Prodrug versus Hybrid Comparison
5.2. Structural Considerations regarding Antibiotic Hybrids
5.3. Obtained Hybrids with Antibiotics
5.4. Hybrids with FQNs
5.4.1. Antibiotic–Antibiotic Hybrids
5.4.2. Antibiotic–Non-Antibiotic Hybrids
5.5. FQN Hybrids with Other Biological Effects
6. Future Research Direction of FQN Hybrids
7. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Hospital-Acquired Complication-3. Healthcare-Associated Infection Fact Sheet|Australian Commission on Safety and Quality in Health Care. Available online: https://www.safetyandquality.gov.au/publications-and-resources/resource-library/hospital-acquired-complication-3-healthcare-associated-infection-fact-sheet (accessed on 26 July 2022).
- Global Guidelines for the Prevention of Surgical Site Infection, 2nd ed.; WHO: Geneva, Switzerland, 2018; Available online: https://www.who.int/publications-detail-redirect/global-guidelines-for-the-prevention-of-surgical-site-infection-2nd-ed (accessed on 16 March 2022).
- Millanao, A.R.; Mora, A.Y.; Villagra, N.A.; Bucarey, S.A.; Hidalgo, A.A. Biological Effects of Quinolones: A Family of Broad-Spectrum Antimicrobial Agents. Molecules 2021, 26, 7153. [Google Scholar] [CrossRef]
- Fair, R.J.; Tor, Y. Antibiotics and Bacterial Resistance in the 21st Century. Perspect. Med. Chem. 2014, 6, 25–64. [Google Scholar] [CrossRef] [Green Version]
- Bush, N.G.; Diez-Santos, I.; Abbott, L.R.; Maxwell, A. Quinolones: Mechanism, Lethality and Their Contributions to Antibiotic Resistance. Molecules 2020, 25, 5662. [Google Scholar] [CrossRef]
- Horta, P.; Secrieru, A.; Coninckx, A.; Cristiano, M. Quinolones for Applications in Medicinal Chemistry: Synthesis and Structure in Targets in Heterocyclic Systems; Società Chimica Italiana: Rome, Italy, 2018; Chapter 11; pp. 260–297. [Google Scholar]
- Gupta, V.; Datta, P. Next-Generation Strategy for Treating Drug Resistant Bacteria: Antibiotic Hybrids. Indian J. Med. Res. 2019, 149, 97–106. [Google Scholar] [CrossRef]
- Lesher, G.Y.; Froelich, E.J.; Gruett, M.D.; Bailey, J.H.; Brundage, R.P. 1,8-Naphthyridine Derivatives. A New Class of Chemotherapeutic Agents. J. Med. Pharm. Chem. 1962, 5, 1063–1065. [Google Scholar] [CrossRef]
- Tillotson, G.S. Quinolones: Structure-Activity Relationships and Future Predictions. J. Med. Microbiol. 1996, 44, 320–324. [Google Scholar] [CrossRef] [Green Version]
- Wetzel, C.; Lonneman, M.; Wu, C. Polypharmacological Drug Actions of Recently FDA Approved Antibiotics. Eur. J. Med. Chem. 2021, 209, 112931. [Google Scholar] [CrossRef]
- Delafloxacin. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/Delafloxacin (accessed on 29 June 2021).
- Finafloxacin. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/Finafloxacin (accessed on 29 June 2021).
- Andersson, M.I.; MacGowan, A.P. Development of the Quinolones. J. Antimicrob. Chemother. 2003, 51 (Suppl. 1), 1–11. [Google Scholar] [CrossRef]
- Emmerson, A.M.; Jones, A.M. The Quinolones: Decades of Development and Use. J. Antimicrob. Chemother. 2003, 51 (Suppl. 1), 13–20. [Google Scholar] [CrossRef] [Green Version]
- Ezelarab, H.A.A.; Abbas, S.H.; Hassan, H.A.; Abuo-Rahma, G.E.-D.A. Recent Updates of Fluoroquinolones as Antibacterial Agents. Arch. Pharm. (Weinheim) 2018, 351, e1800141. [Google Scholar] [CrossRef]
- Bisacchi, G.S. Origins of the Quinolone Class of Antibacterials: An Expanded “Discovery Story”. J. Med. Chem. 2015, 58, 4874–4882. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, J.J. Laughing Gas, Viagra, and Lipitor: The Human Stories Behind the Drugs We Use; Oxford University Press: New York, NY, USA, 2006; ISBN 978-0-19-530099-4. [Google Scholar]
- Wilson, C.O.; Beale, J.M.; Block, J.H. (Eds.) Wilson and Gisvold’s Textbook of Organic Medicinal and Pharmaceutical Chemistry, 12th ed.; Lippincott Williams & Wilkins: Baltimore, MD, USA, 2011; ISBN 978-0-7817-7929-6. [Google Scholar]
- Zhang, G.-F.; Zhang, S.; Pan, B.; Liu, X.; Feng, L.-S. 4-Quinolone Derivatives and Their Activities against Gram Positive Pathogens. Eur. J. Med. Chem. 2018, 143, 710–723. [Google Scholar] [CrossRef]
- Ball, P. Quinolone Generations: Natural History or Natural Selection? J. Antimicrob. Chemother. 2000, 46 (Suppl. T1), 17–24. [Google Scholar] [CrossRef] [Green Version]
- Andriole, V. Chapter 1-The Quinolones: History and Overview. In The Quinolones, 3rd ed.; Academic Press: San Diego, CA, USA, 2000; pp. 1–31. ISBN 978-0-12-059517-4. [Google Scholar]
- Tótoli, E.G.; Salgado, H.R.N. Besifloxacin: A Critical Review of Its Characteristics, Properties, and Analytical Methods. Crit. Rev. Anal. Chem. 2018, 48, 132–142. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Markham, A. Delafloxacin: First Global Approval. Drugs 2017, 77, 1481–1486. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Blondeau, J.M. Fluoroquinolones: Mechanism of Action, Classification, and Development of Resistance. Surv. Ophthalmol. 2004, 49 (Suppl. 2), S73–S78. [Google Scholar] [CrossRef]
- Rusu, A.; Lungu, I.-A.; Moldovan, O.-L.; Tanase, C.; Hancu, G. Structural Characterization of the Millennial Antibacterial (Fluoro)Quinolones—Shaping the Fifth Generation. Pharmaceutics 2021, 13, 1289. [Google Scholar] [CrossRef]
- Emami, S.; Shafiee, A.; Foroumadi, A. Quinolones: Recent Structural and Clinical Developments. Iran. J. Pharm. Res. 2010, 4, 123–136. [Google Scholar] [CrossRef]
- Zhanel, G.G.; Walkty, A.; Vercaigne, L.; Karlowsky, J.A.; Embil, J.; Gin, A.S.; Hoban, D.J. The New Fluoroquinolones: A Critical Review. Can. J. Infect. Dis. 1999, 10, 207–238. [Google Scholar] [CrossRef]
- Andriole, V. Chapter 2-Chemistry and Mechanism of Action of the Quinolone Antibacterials. In The Quinolones, 3rd ed.; Academic Press: San Diego, CA, USA, 2000; pp. 33–97. ISBN 978-0-12-059517-4. [Google Scholar]
- Suaifan, G.A.R.Y.; Mohammed, A.A.M. Fluoroquinolones Structural and Medicinal Developments (2013–2018): Where Are We Now? Bioorg. Med. Chem. 2019, 27, 3005–3060. [Google Scholar] [CrossRef]
- Uivarosi, V. Metal Complexes of Quinolone Antibiotics and Their Applications: An Update. Mol. Basel Switz. 2013, 18, 11153–11197. [Google Scholar] [CrossRef]
- Hooper, D.C. Mechanisms of Action of Antimicrobials: Focus on Fluoroquinolones. Clin. Infect. Dis. 2001, 32, S9–S15. [Google Scholar] [CrossRef] [PubMed]
- Aldred, K.J.; Kerns, R.J.; Osheroff, N. Mechanism of Quinolone Action and Resistance. Biochemistry 2014, 53, 1565–1574. [Google Scholar] [CrossRef] [PubMed]
- Hooper, D.C.; Jacoby, G.A. Topoisomerase Inhibitors: Fluoroquinolone Mechanisms of Action and Resistance. Cold Spring Harb. Perspect. Med. 2016, 6, a025320. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Domagala, J.M. Structure-Activity and Structure-Side-Effect Relationships for the Quinolone Antibacterials. J. Antimicrob. Chemother. 1994, 33, 685–706. [Google Scholar] [CrossRef] [PubMed]
- Blum, M.D.; Graham, D.J.; McCloskey, C.A. Temafloxacin Syndrome: Review of 95 Cases. Clin. Infect. Dis. Off. Publ. Infect. Dis. Soc. Am. 1994, 18, 946–950. [Google Scholar] [CrossRef]
- European Pharmacopoeia Online. Available online: https://pheur.edqm.eu/home (accessed on 5 August 2022).
- Merck. Merck Index, 13th ed.; O’Neil, M.J., Smith, A., Heckelman, P.E., Budavari, S., Eds.; Merck: Whitehouse Station, NJ, USA, 2001; ISBN 978-0-911910-13-1. [Google Scholar]
- Ross, D.L.; Riley, C.M. Physicochemical Properties of the Fluoroquinolone Antimicrobials V. Effect of Fluoroquinolone Structure and PH on the Complexation of Various Fluoroquinolones with Magnesium and Calcium Ions. Int. J. Pharm. 1993, 93, 121–129. [Google Scholar] [CrossRef]
- Wishart, D.S.; Feunang, Y.D.; Guo, A.C.; Lo, E.J.; Marcu, A.; Grant, J.R.; Sajed, T.; Johnson, D.; Li, C.; Sayeeda, Z.; et al. DrugBank 5.0: A Major Update to the DrugBank Database for 2018. Nucleic Acids Res. 2018, 46, D1074–D1082. [Google Scholar] [CrossRef]
- NCATS Inxight Drugs—Nemonoxacin Malate Hemihydrate. Available online: https://drugs.ncats.io/drug/Y97F3051FH (accessed on 5 August 2022).
- Baker, W.R.; Cai, S.; Dimitroff, M.; Fang, L.; Huh, K.K.; Ryckman, D.R.; Shang, X.; Shawar, R.M.; Therrien, J.H. A Prodrug Approach toward the Development of Water Soluble Fluoroquinolones and Structure—Activity Relationships of Quinoline-3-Carboxylic Acids. J. Med. Chem. 2004, 47, 4693–4709. [Google Scholar] [CrossRef]
- Sharma, P.C.; Piplani, M.; Mittal, M.; Pahwa, R. Insight into Prodrugs of Quinolones and Fluoroquinolones. Infect. Disord. Drug Targets 2016, 16, 140–161. [Google Scholar] [CrossRef]
- Bhawsar, S.; Kale, R.; Deshpande, P.; Yeole, R.; Bhagwat, S.; Patel, M. Design and Synthesis of an Oral Prodrug Alalevonadifloxacin for the Treatment of MRSA Infection. Bioorg. Med. Chem. Lett. 2021, 54, 128432. [Google Scholar] [CrossRef] [PubMed]
- Lambert, A.; Regnouf-de-Vains, J.-B.; Ruiz-López, M.F. Structure of Levofloxacin in Hydrophilic and Hydrophobic Media: Relationship to Its Antibacterial Properties. Chem. Phys. Lett. 2007, 442, 281–284. [Google Scholar] [CrossRef]
- Jacobs, M.R.; Appelbaum, P.C. Nadifloxacin: A Quinolone for Topical Treatment of Skin Infections and Potential for Systemic Use of Its Active Isomer, WCK 771. Expert Opin. Pharmacother. 2006, 7, 1957–1966. [Google Scholar] [CrossRef] [PubMed]
- Sharma, P.C.; Jain, A.; Jain, S. Fluoroquinolone Antibacterials: A Review on Chemistry, Microbiology and Therapeutic Prospects. Acta Pol. Pharm. 2009, 66, 587–604. [Google Scholar] [PubMed]
- Blokhina, S.V.; Sharapova, A.V.; Ol’khovich, M.V.; Volkova, T.V.; Perlovich, G.L. Solubility, Lipophilicity and Membrane Permeability of Some Fluoroquinolone Antimicrobials. Eur. J. Pharm. Sci. 2016, 93, 29–37. [Google Scholar] [CrossRef]
- Rusu, A.; Tóth, G.; Szőcs, L.; Kökösi, J.; Kraszni, M.; Gyéresi, Á.; Noszál, B. Triprotic Site-Specific Acid–Base Equilibria and Related Properties of Fluoroquinolone Antibacterials. J. Pharm. Biomed. Anal. 2012, 66, 50–57. [Google Scholar] [CrossRef]
- Park, H.-R.; Kim, T.H.; Bark, K.-M. Physicochemical Properties of Quinolone Antibiotics in Various Environments. Eur. J. Med. Chem. 2002, 37, 443–460. [Google Scholar] [CrossRef]
- Perletti, G.; Wagenlehner, F.M.E.; Naber, K.G.; Magri, V. Enhanced Distribution of Fourth-Generation Fluoroquinolones in Prostatic Tissue. Int. J. Antimicrob. Agents 2009, 33, 206–210. [Google Scholar] [CrossRef]
- Shah, P.; Westwell, A.D. The Role of Fluorine in Medicinal Chemistry. J. Enzyme Inhib. Med. Chem. 2007, 22, 527–540. [Google Scholar] [CrossRef] [Green Version]
- O’Hagan, D. Fluorine in Health Care: Organofluorine Containing Blockbuster Drugs. J. Fluor. Chem. 2010, 131, 1071–1081. [Google Scholar] [CrossRef]
- Gillis, E.P.; Eastman, K.J.; Hill, M.D.; Donnelly, D.J.; Meanwell, N.A. Applications of Fluorine in Medicinal Chemistry. J. Med. Chem. 2015, 58, 8315–8359. [Google Scholar] [CrossRef] [PubMed]
- Wermuth, C.G.; Aldous, D.; Raboisson, P.; Rognan, D. Chapter 13-Substituent Groups. In The Practice of Medicinal Chemistry; Elsevier: San Diego, CA, USA, 2015; pp. 319–357. ISBN 978-0-12-417205-0. [Google Scholar]
- Peterson, L.R. Quinolone Molecular Structure-Activity Relationships: What We Have Learned about Improving Antimicrobial Activity. Clin. Infect. Dis. Off. Publ. Infect. Dis. Soc. Am. 2001, 33 (Suppl. 3), S180–S186. [Google Scholar] [CrossRef] [PubMed]
- Takács-Novák, K.; Józan, M.; Hermecz, I.; Szász, G. Lipophilicity of Antibacterial Fluoroquinolones. Int. J. Pharm. 1992, 79, 89–96. [Google Scholar] [CrossRef]
- Kłosińska-Szmurło, E.; Grudzień, M.; Betlejewska-Kielak, K.; Pluciński, F.; Biernacka, J.; Mazurek, A.P. Physicochemical Properties of Lomefloxacin, Levofloxacin, and Moxifloxacin Relevant to the Biopharmaceutics Classification System. Acta Chim. Slov. 2014, 61, 827–834. [Google Scholar] [PubMed]
- Fedorowicz, J.; Sączewski, J. Modifications of Quinolones and Fluoroquinolones: Hybrid Compounds and Dual-Action Molecules. Mon. Chem. 2018, 149, 1199–1245. [Google Scholar] [CrossRef] [Green Version]
- Serafin, A.; Stańczak, A. The Complexes of Metal Ions with Fluoroquinolones. Russ. J. Coord. Chem. 2009, 35, 81–95. [Google Scholar] [CrossRef]
- Correia, S.; Poeta, P.; Hébraud, M.; Capelo, J.L.; Igrejas, G. Mechanisms of Quinolone Action and Resistance: Where Do We Stand? J. Med. Microbiol. 2017, 66, 551–559. [Google Scholar] [CrossRef]
- Fàbrega, A.; Madurga, S.; Giralt, E.; Vila, J. Mechanism of Action of and Resistance to Quinolones. Microb. Biotechnol. 2009, 2, 40–61. [Google Scholar] [CrossRef] [Green Version]
- Mustaev, A.; Malik, M.; Zhao, X.; Kurepina, N.; Luan, G.; Oppegard, L.M.; Hiasa, H.; Marks, K.R.; Kerns, R.J.; Berger, J.M.; et al. Fluoroquinolone-Gyrase-DNA Complexes. J. Biol. Chem. 2014, 289, 12300–12312. [Google Scholar] [CrossRef] [Green Version]
- Levine, C.; Hiasa, H.; Marians, K.J. DNA Gyrase and Topoisomerase IV: Biochemical Activities, Physiological Roles during Chromosome Replication, and Drug Sensitivities. Biochim. Biophys. Acta 1998, 1400, 29–43. [Google Scholar] [CrossRef]
- Champoux, J.J. DNA Topoisomerases: Structure, Function, and Mechanism. Annu. Rev. Biochem. 2001, 70, 369–413. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pommier, Y.; Leo, E.; Zhang, H.; Marchand, C. DNA Topoisomerases and Their Poisoning by Anticancer and Antibacterial Drugs. Chem. Biol. 2010, 17, 421–433. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Blower, T.R.; Williamson, B.H.; Kerns, R.J.; Berger, J.M. Crystal Structure and Stability of Gyrase–Fluoroquinolone Cleaved Complexes from Mycobacterium Tuberculosis. Proc. Natl. Acad. Sci. USA 2016, 113, 1706–1713. [Google Scholar] [CrossRef] [Green Version]
- Hooper, D.C.; Strahilevitz, J. Quinolones. In Mandell, Douglas, and Bennett’s Principles and Practice of Infectious Diseases; Elsevier: Amsterdam, The Netherlands, 2020; pp. 426–448. [Google Scholar]
- Laponogov, I.; Pan, X.-S.; Veselkov, D.A.; Cirz, R.T.; Wagman, A.; Moser, H.E.; Fisher, L.M.; Sanderson, M.R. Exploring the Active Site of the Streptococcus Pneumoniae Topoisomerase IV–DNA Cleavage Complex with Novel 7,8-Bridged Fluoroquinolones. Open Biol. 2016, 6, 160157. [Google Scholar] [CrossRef] [Green Version]
- Gonzalez, J.P.; Henwood, J.M. Pefloxacin. A Review of Its Antibacterial Activity, Pharmacokinetic Properties and Therapeutic Use. Drugs 1989, 37, 628–668. [Google Scholar] [CrossRef] [PubMed]
- Martindale, W.; Sweetman, S.C. (Eds.) Martindale: The Complete Drug Reference, 36th ed.; Pharmaceuticale Press, PhP: London, UK; Chicago, IL, USA, 2009; ISBN 978-0-85369-840-1. [Google Scholar]
- Nenoff, P. Acne Vulgaris and Bacterial Skin Infections: Review of the Topical Quinolone Nadifloxacin. Expert Rev. Dermatol. 2006, 1, 643–654. [Google Scholar] [CrossRef]
- Sukul, P.; Spiteller, M. Fluoroquinolone Antibiotics in the Environment. Rev. Environ. Contam. Toxicol. 2007, 191, 131–162. [Google Scholar] [CrossRef] [PubMed]
- Ware, G. (Ed.) Reviews of Environmental Contamination and Toxicology 191; Reviews of Environmental Contamination and Toxicology; Springer: New York, NY, USA, 2007; ISBN 978-0-387-69162-6. [Google Scholar]
- Limberakis, C. Quinolone Antibiotics: Levofloxacin (Levaquin®), Moxifloxacin (Avelox®), Gemifloxacin (Factive®), and Garenoxacin (T-3811). In The Art of Drug Synthesis; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2007; pp. 39–69. ISBN 978-0-470-13497-9. [Google Scholar]
- Saravolatz, L.D.; Leggett, J. Gatifloxacin, Gemifloxacin, and Moxifloxacin: The Role of 3 Newer Fluoroquinolones. Clin. Infect. Dis. Off. Publ. Infect. Dis. Soc. Am. 2003, 37, 1210–1215. [Google Scholar] [CrossRef]
- Caeiro, J.-P.; Iannini, P.B. Moxifloxacin (Avelox®): A Novel Fluoroquinolone with a Broad Spectrum of Activity. Expert Rev. Anti Infect. Ther. 2003, 1, 363–370. [Google Scholar] [CrossRef]
- Al Omari, M.M.H.; Jaafari, D.S.; Al-Sou’od, K.A.; Badwan, A.A. Chapter Seven-Moxifloxacin Hydrochloride. In Profiles of Drug Substances, Excipients and Related Methodology; Brittain, H.G., Ed.; Academic Press: Cambridge, MA, USA, 2014; Volume 39, pp. 299–431. [Google Scholar]
- Avelox, Moxifloxacin Systemic (Moxifloxacin) Dosing, Indications, Interactions, Adverse Effects, and More. Available online: https://reference.medscape.com/drug/avelox-moxifloxacin-systemic-moxifloxacin-342537 (accessed on 15 July 2021).
- Pham, T.D.M.; Ziora, Z.M.; Blaskovich, M.A.T. Quinolone Antibiotics. MedChemComm 2019, 10, 1719–1739. [Google Scholar] [CrossRef]
- Anonymous Factive: Withdrawn Application. Available online: https://www.ema.europa.eu/en/medicines/human/withdrawn-applications/factive (accessed on 14 July 2021).
- Factive (Gemifloxacin) Dosing, Indications, Interactions, Adverse Effects, and More. Available online: https://reference.medscape.com/drug/factive-gemifloxacin-342529 (accessed on 15 July 2021).
- Besifloxacin Ophthalmic (Rx) (Voreloxin) Dosing, Indications, Interactions, Adverse Effects, and More. Available online: https://reference.medscape.com/drug/besivance-besifloxacin-ophthalmic-999210 (accessed on 15 July 2021).
- XTORO (Finafloxacin Otic Suspension) 0.3% for Topical Otic Administration Highlights of Prescribing Information (206307s000lbl.Pdf). Available online: https://www.accessdata.fda.gov/drugsatfda_docs/label/2014/206307s000lbl.pdf (accessed on 15 July 2021).
- Baxdela (Delafloxacin) Dosing, Indications, Interactions, Adverse Effects, and More. Available online: https://reference.medscape.com/drug/baxdela-delafloxacin-1000153 (accessed on 15 July 2021).
- Stein, G.E. Review of the Bioavailability and Pharmacokinetics of Oral Norfloxacin. Am. J. Med. 1987, 82, 18–21. [Google Scholar] [CrossRef]
- TABLETS NOROXIN® (NORFLOXACIN). Available online: https://www.accessdata.fda.gov/drugsatfda_docs/label/2013/019384s066lbl.pdf (accessed on 21 October 2021).
- Highlights of Prescribing Information CIPRO® (Ciprofloxacin Hydrochloride) Tablet, for Oral Use CIPRO® (Ciprofloxacin Hydrochloride), for Oral Suspension. Available online: https://www.accessdata.fda.gov/drugsatfda_docs/label/2016/019537s086lbl.pdf (accessed on 21 October 2021).
- Pefloxacin. Available online: https://go.drugbank.com/drugs/DB00487 (accessed on 21 October 2021).
- Narayanan, V.; Motlekar, S.; Kadhe, G.; Bhagat, S. Efficacy and Safety of Nadifloxacin for Bacterial Skin Infections: Results from Clinical and Post-Marketing Studies. Dermatol. Ther. 2014, 4, 233–248. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fish, D.N.; Chow, A.T. The Clinical Pharmacokinetics of Levofloxacin. Clin. Pharmacokinet. 1997, 32, 101–119. [Google Scholar] [CrossRef]
- Keating, G.M. Levofloxacin 0.5% Ophthalmic Solution. Drugs 2009, 69, 1267–1286. [Google Scholar] [CrossRef] [PubMed]
- Cervantes, L.J.; Mah, F.S. Clinical Use of Gatifloxacin Ophthalmic Solution for Treatment of Bacterial Conjunctivitis. Clin. Ophthalmol. 2011, 5, 495–502. [Google Scholar] [CrossRef] [Green Version]
- Drug Approval Package: Zymar (Gatifloxacin) NDA #021493. Available online: https://www.accessdata.fda.gov/drugsatfda_docs/nda/2003/021493_Zymar.cfm (accessed on 10 August 2022).
- Drug Approval Package: Zymaxid (Gatifloxacin) NDA #022548. Available online: https://www.accessdata.fda.gov/drugsatfda_docs/nda/2010/022548s000_zymaxid_toc.cfm (accessed on 10 August 2022).
- Rhee, C.K.; Chang, J.H.; Choi, E.G.; Kim, H.K.; Kwon, Y.-S.; Kyung, S.Y.; Lee, J.-H.; Park, M.J.; Yoo, K.H.; Oh, Y.M. Zabofloxacin versus Moxifloxacin in Patients with COPD Exacerbation: A Multicenter, Double-Blind, Double-Dummy, Randomized, Controlled, Phase III, Non-Inferiority Trial. Int. J. Chron. Obstruct. Pulmon. Dis. 2015, 10, 2265–2275. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Drug Approval Package: Avelox (Moxifloxacin Hydrochloride) NDA# 21-085. Available online: https://www.accessdata.fda.gov/drugsatfda_docs/nda/99/21-085_Avelox.cfm (accessed on 10 August 2022).
- Drug Approval Package: Vigamox (Monofloxacin Hydrochloride) NDA #021598. Available online: https://www.accessdata.fda.gov/drugsatfda_docs/nda/2003/21-598_Vigamox.cfm (accessed on 10 August 2022).
- Baxdela (Delafloxacin) Tablets and Injection. Available online: https://www.accessdata.fda.gov/drugsatfda_docs/nda/2017/208610Orig1s000,208611Orig1s000TOC.cfm (accessed on 8 August 2022).
- Van Bambeke, F. Delafloxacin, a Non-Zwitterionic Fluoroquinolone in Phase III of Clinical Development: Evaluation of Its Pharmacology, Pharmacokinetics, Pharmacodynamics and Clinical Efficacy. Future Microbiol. 2015, 10, 1111–1123. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Singh, C.L.; Singh, A.; Kumar, S.; Majumdar, D.K. Besifloxacin the fourth generation fluoroquinolone: A review. J. Drug Deliv. Ther. 2014, 4, 39–44. [Google Scholar] [CrossRef]
- Highlights of Prescribing Information BesivanceTM (Besifloxacin Ophthalmic Suspension) 0.6%. Available online: https://www.accessdata.fda.gov/drugsatfda_docs/label/2009/022308lbl.pdf (accessed on 21 October 2021).
- McKeage, K. Finafloxacin: First Global Approval. Drugs 2015, 75, 687–693. [Google Scholar] [CrossRef]
- Barnes, K.B.; Zumbrun, S.D.; Halasohoris, S.A.; Desai, P.D.; Miller, L.L.; Richards, M.I.; Russell, P.; Bentley, C.; Harding, S.V. Demonstration of the Broad-Spectrum In Vitro Activity of Finafloxacin against Pathogens of Biodefense Interest. Antimicrob. Agents Chemother. 2019, 63, e01470-19. [Google Scholar] [CrossRef] [Green Version]
- Alksne, L. Balofloxacin Choongwae. Curr. Opin. Investig. Drugs Lond. Engl. 2000 2003, 4, 224–229. [Google Scholar]
- Yang, Z.; Wang, X.; Qin, W.; Zhao, H. Capillary Electrophoresis–Chemiluminescence Determination of Norfloxacin and Prulifloxacin. Anal. Chim. Acta 2008, 623, 231–237. [Google Scholar] [CrossRef] [PubMed]
- Ghebremedhin, B. Bacterial Infections in the Elderly Patient: Focus on Sitafloxacin. Clin. Med. Insights Ther. 2012, 4, CMT.S7435. [Google Scholar] [CrossRef] [Green Version]
- Chen, C.-K.; Cheng, I.-L.; Chen, Y.-H.; Lai, C.-C. Efficacy and Safety of Sitafloxacin in the Treatment of Acute Bacterial Infection: A Meta-Analysis of Randomized Controlled Trials. Antibiotics 2020, 9, 106. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Poole, R.M. Nemonoxacin: First Global Approval. Drugs 2014, 74, 1445–1453. [Google Scholar] [CrossRef]
- Kocsis, B.; Szabo, D. Zabofloxacin for Chronic Bronchitis. Drugs Today Barc. Spain 1998 2016, 52, 495–500. [Google Scholar] [CrossRef]
- Davenport, J.M.; Covington, P.; Gotfried, M.; Medlock, M.; Watanalumlerd, P.; McIntyre, G.; Turner, L.; Almenoff, J. Summary of Pharmacokinetics and Tissue Distribution of a Broad-Spectrum Fluoroquinolone, JNJ-Q2. Clin. Pharmacol. Drug Dev. 2012, 1, 121–130. [Google Scholar] [CrossRef]
- Wright, D.H.; Brown, G.H.; Peterson, M.L.; Rotschafer, J.C. Application of Fluoroquinolone Pharmacodynamics. J. Antimicrob. Chemother. 2000, 46, 669–683. [Google Scholar] [CrossRef]
- Anand, N.; Remers, W.A. Synthetic Antibacterial Agents. In Burger’s Medicinal Chemistry and Drug Discovery; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2010; pp. 481–562. ISBN 978-0-471-26694-5. [Google Scholar]
- Kocsis, B.; Gulyás, D.; Szabó, D. Delafloxacin, Finafloxacin, and Zabofloxacin: Novel Fluoroquinolones in the Antibiotic Pipeline. Antibiotics 2021, 10, 1506. [Google Scholar] [CrossRef]
- Stuck, A.E.; Kim, D.K.; Frey, F.J. Fleroxacin Clinical Pharmacokinetics. Clin. Pharmacokinet. 1992, 22, 116–131. [Google Scholar] [CrossRef]
- Rodvold, K.A.; Gotfried, M.H.; Chugh, R.; Gupta, M.; Yeole, R.; Patel, A.; Bhatia, A. Intrapulmonary Pharmacokinetics of Levonadifloxacin Following Oral Administration of Alalevonadifloxacin to Healthy Adult Subjects. Antimicrob. Agents Chemother. 2018, 62, e02297-17. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kocsis, B.; Domokos, J.; Szabo, D. Chemical Structure and Pharmacokinetics of Novel Quinolone Agents Represented by Avarofloxacin, Delafloxacin, Finafloxacin, Zabofloxacin and Nemonoxacin. Ann. Clin. Microbiol. Antimicrob. 2016, 15, 34. [Google Scholar] [CrossRef] [Green Version]
- Bressolle, F.; Gonçalves, F.; Gouby, A.; Galtier, M. Pefloxacin Clinical Pharmacokinetics. Clin. Pharmacokinet. 1994, 27, 418–446. [Google Scholar] [CrossRef]
- Granneman, G.R.; Carpentier, P.; Morrison, P.J.; Pernet, A.G. Pharmacokinetics of Temafloxacin in Humans after Single Oral Doses. Antimicrob. Agents Chemother. 1991, 35, 436–441. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Han, H.; Kim, S.E.; Shin, K.-H.; Lim, C.; Lim, K.S.; Yu, K.-S.; Cho, J.-Y. Comparison of Pharmacokinetics between New Quinolone Antibiotics: The Zabofloxacin Hydrochloride Capsule and the Zabofloxacin Aspartate Tablet. Curr. Med. Res. Opin. 2013, 29, 1349–1355. [Google Scholar] [CrossRef] [PubMed]
- NegGram® Caplets (Nalidixic Acid, USP). Available online: https://www.accessdata.fda.gov/drugsatfda_docs/label/2012/014214s060lbl.pdf (accessed on 21 October 2021).
- FLOXIN® Tablets (Ofloxacin Tablets). Available online: https://www.accessdata.fda.gov/drugsatfda_docs/label/2008/019735s059lbl.pdf (accessed on 21 October 2021).
- Core Safety Profile Active Substance: Nadifloxacin Pharmaceutical Form(s)/Strength: 1% Cream. Available online: https://www.bfarm.de/SharedDocs/Downloads/EN/Drugs/vigilance/PSURs/csp/m-p/nadifloxacin.pdf?__blob=publicationFile&v=3 (accessed on 21 October 2021).
- Highlights of Prescribing Information LEVAQUIN® (Levofloxacin) Tablet, Film Coated for Oral Use LEVAQUIN® (Levofloxacin) Solution for Oral Use LEVAQUIN® (Levofloxacin) Injection, Solution, Concentrate for Intravenous Use LEVAQUIN® (Levofloxacin) Injection, Solution for Intravenous Use. Available online: https://www.accessdata.fda.gov/drugsatfda_docs/label/2013/020634s065,020635s071,021721s032lbl.pdf (accessed on 21 October 2021).
- Highlights of Prescribing Information AVELOX (Moxifloxacin Hydrochloride) Tablets, for Oral Use AVELOX (Moxifloxacin Hydrochloride) Injection, for Intravenous Use. Available online: https://www.accessdata.fda.gov/drugsatfda_docs/label/2016/021085s063lbl.pdf (accessed on 21 October 2021).
- Prescribing Information FACTIVE® (Gemifloxacin Mesylate) Tablets. Available online: https://www.accessdata.fda.gov/drugsatfda_docs/label/2007/021158s007lbl.pdf (accessed on 21 October 2021).
- Cross-Discipline Team Leader Review XTORO (Finafloxacin Otic Suspension) 0.3%. Available online: https://www.accessdata.fda.gov/drugsatfda_docs/nda/2014/206307Orig1s000CrossR.pdf (accessed on 21 October 2021).
- Highlights of Prescribing Information BAXDELA (Delafloxacin) Tablets, for Oral Use BAXDELA (Delafloxacin) for Injection, for Intravenous Use. Available online: https://www.accessdata.fda.gov/drugsatfda_docs/label/2017/208610s000,208611s000lbl.pdf (accessed on 21 October 2021).
- Rubinstein, E. History of Quinolones and Their Side Effects. Chemotherapy 2001, 47, 3–8. [Google Scholar] [CrossRef]
- FDA Drug Safety Communication: FDA Updates Warnings for Oral and Injectable Fluoroquinolone Antibiotics Due to Disabling Side Effects. Available online: https://www.fda.gov/drugs/drug-safety-and-availability/fda-drug-safety-communication-fda-updates-warnings-oral-and-injectable-fluoroquinolone-antibiotics (accessed on 15 July 2021).
- Redgrave, L.S.; Sutton, S.B.; Webber, M.A.; Piddock, L.J.V. Fluoroquinolone Resistance: Mechanisms, Impact on Bacteria, and Role in Evolutionary Success. Trends Microbiol. 2014, 22, 438–445. [Google Scholar] [CrossRef]
- Gao, F.; Wang, P.; Yang, H.; Miao, Q.; Ma, L.; Lu, G. Recent Developments of Quinolone-Based Derivatives and Their Activities against Escherichia Coli. Eur. J. Med. Chem. 2018, 157, 1223–1248. [Google Scholar] [CrossRef]
- Abushaheen, M.A.; Muzaheed; Fatani, A.J.; Alosaimi, M.; Mansy, W.; George, M.; Acharya, S.; Rathod, S.; Divakar, D.D.; Jhugroo, C.; et al. Antimicrobial Resistance, Mechanisms and Its Clinical Significance. Dis. Mon. 2020, 66, 100971. [Google Scholar] [CrossRef]
- Munita, J.M.; Arias, C.A. Mechanisms of Antibiotic Resistance. Microbiol. Spectr. 2016, 4, 481–511. [Google Scholar] [CrossRef] [Green Version]
- Frieri, M.; Kumar, K.; Boutin, A. Antibiotic Resistance. J. Infect. Public Health 2017, 10, 369–378. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Antimicrobial Resistance. Available online: https://www.who.int/en/news-room/fact-sheets/detail/antimicrobial-resistance (accessed on 16 June 2021).
- Antimicrobial Resistance Information from FDA. Available online: https://www.fda.gov/emergency-preparedness-and-response/mcm-issues/antimicrobial-resistance-information-fda (accessed on 21 July 2021).
- Marston, H.D.; Dixon, D.M.; Knisely, J.M.; Palmore, T.N.; Fauci, A.S. Antimicrobial Resistance. JAMA 2016, 316, 1193–1204. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mayers, D.L.; Sobel, J.D.; Ouellette, M.; Kaye, K.S.; Marchaim, D. (Eds.) Antimicrobial Drug Resistance: Clinical and Epidemiological Aspects, 2nd ed.; Springer International Publishing: Berlin, Germany, 2017; Volume 2, ISBN 978-3-319-47264-5. [Google Scholar]
- Petchiappan, A.; Chatterji, D. Antibiotic Resistance: Current Perspectives. ACS Omega 2017, 2, 7400–7409. [Google Scholar] [CrossRef] [PubMed]
- Schaenzer, A.J.; Wright, G.D. Antibiotic Resistance by Enzymatic Modification of Antibiotic Targets. Trends Mol. Med. 2020, 26, 768–782. [Google Scholar] [CrossRef]
- McClelland, S.; Lamoureux, B.; Larson, E. Trends in Antimicrobial Resistance Legislation 2011-2019: A Review of the US Policy Response to the Antimicrobial Resistance Threat and Its Public Health Impact. Am. J. Infect. Control 2021, 49, 813–817. [Google Scholar] [CrossRef]
- Bulteel, A.J.B.; Larson, E.L.; Getahun, H. Identifying Global Research Gaps to Mitigate Antimicrobial Resistance: A Scoping Review. Am. J. Infect. Control 2021, 49, 818–824. [Google Scholar] [CrossRef]
- Hershberg, R. Antibiotic-Independent Adaptive Effects of Antibiotic Resistance Mutations. Trends Genet. 2017, 33, 521–528. [Google Scholar] [CrossRef]
- Knöppel, A.; Näsvall, J.; Andersson, D.I. Evolution of Antibiotic Resistance without Antibiotic Exposure. Antimicrob. Agents Chemother. 2017, 61, e01495-17. [Google Scholar] [CrossRef] [Green Version]
- WHO Publishes List of Bacteria for Which New Antibiotics Are Urgently Needed. Available online: https://www.who.int/news/item/27-02-2017-who-publishes-list-of-bacteria-for-which-new-antibiotics-are-urgently-needed (accessed on 3 July 2021).
- Hooper, D.C.; Jacoby, G.A. Mechanisms of Drug Resistance: Quinolone Resistance. Ann. N. Y. Acad. Sci. 2015, 1354, 12–31. [Google Scholar] [CrossRef] [Green Version]
- Owens, R.C.; Ambrose, P.G. Clinical use of the fluoroquinolones. Med. Clin. N. Am. 2000, 84, 1447–1469. [Google Scholar] [CrossRef]
- Kim, E.S.; Hooper, D.C. Clinical Importance and Epidemiology of Quinolone Resistance. Infect. Chemother. 2014, 46, 226–238. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Watanabe, T. Infective heredity of multiple drug resistance in bacteria. Bacteriol. Rev. 1963, 27, 87–115. [Google Scholar] [CrossRef] [PubMed]
- Wax, R.G.; Lewis, K.; Salyers, A.A.; Taber, H. Bacterial Resistance to Antimicrobials; CRC Press: Boca Raton, FL, USA, 2008; ISBN 978-1-4200-0875-3. [Google Scholar]
- Podolsky, S.H. The Evolving Response to Antibiotic Resistance (1945–2018). Palgrave Commun. 2018, 4, 1–8. [Google Scholar] [CrossRef]
- Antibiotic Resistance Threats in the United States (Ar-Threats-2013-508.Pdf) 2013. Available online: https://www.cdc.gov/drugresistance/pdf/ar-threats-2013-508.pdf (accessed on 4 July 2021).
- Centers for Disease Control and Prevention (U.S.). Antibiotic Resistance Threats in the United States, 2019; Centers for Disease Control and Prevention (U.S.): Atlanta, GA, USA, 2019.
- Johnson, A.P. Surveillance of Antibiotic Resistance. Philos. Trans. R. Soc. B Biol. Sci. 2015, 370, 20140080. [Google Scholar] [CrossRef] [Green Version]
- Dalhoff, A. Global Fluoroquinolone Resistance Epidemiology and Implictions for Clinical Use. Interdiscip. Perspect. Infect. Dis. 2012, 2012, 976273. [Google Scholar] [CrossRef] [Green Version]
- Davies, J.; Davies, D. Origins and Evolution of Antibiotic Resistance. Microbiol. Mol. Biol. Rev. MMBR 2010, 74, 417–433. [Google Scholar] [CrossRef] [Green Version]
- Livermore, D.M.; Hope, R.; Reynolds, R.; Blackburn, R.; Johnson, A.P.; Woodford, N. Declining Cephalosporin and Fluoroquinolone Non-Susceptibility among Bloodstream Enterobacteriaceae from the UK: Links to Prescribing Change? J. Antimicrob. Chemother. 2013, 68, 2667–2674. [Google Scholar] [CrossRef] [Green Version]
- Spellberg, B.; Doi, Y. The Rise of Fluoroquinolone-Resistant Escherichia Coli in the Community: Scarier Than We Thought. J. Infect. Dis. 2015, 212, 1853–1855. [Google Scholar] [CrossRef] [Green Version]
- Carlet, J. World alliance against antibiotic resistance: The WAAAR declaration against antibiotic resistance. Med. Intensiva 2015, 39, 34–39. [Google Scholar] [CrossRef]
- Fernandes, P.; Martens, E. Antibiotics in Late Clinical Development. Biochem. Pharmacol. 2017, 133, 152–163. [Google Scholar] [CrossRef] [Green Version]
- TYGACIL® (Tigecycline) for Injection for Intravenous Use-Prescribing Information (021821s021lbl.Pdf). Available online: https://www.accessdata.fda.gov/drugsatfda_docs/label/2010/021821s021lbl.pdf (accessed on 4 July 2021).
- Wang, L.; Liu, D.; Lv, Y.; Cui, L.; Li, Y.; Li, T.; Song, H.; Hao, Y.; Shen, J.; Wang, Y.; et al. Novel Plasmid-Mediated Tet(X5) Gene Conferring Resistance to Tigecycline, Eravacycline, and Omadacycline in a Clinical Acinetobacter Baumannii Isolate. Antimicrob. Agents Chemother. 2019, 64, e01326-19. [Google Scholar] [CrossRef] [PubMed]
- ZERBAXA® (Ceftolozane and Tazobactam) for Injection, for Intravenous Use-Prescribing Information (Zerbaxa_pi.Pdf). Available online: https://www.merck.com/product/usa/pi_circulars/z/zerbaxa/zerbaxa_pi.pdf (accessed on 4 July 2021).
- Wi, Y.M.; Greenwood-Quaintance, K.E.; Schuetz, A.N.; Ko, K.S.; Peck, K.R.; Song, J.-H.; Patel, R. Activity of Ceftolozane-Tazobactam against Carbapenem-Resistant, Non-Carbapenemase-Producing Pseudomonas Aeruginosa and Associated Resistance Mechanisms. Antimicrob. Agents Chemother. 2017, 62, e01970-17. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- AVYCAZ (Ceftazidime and Avibactam) for Injection, for Intravenous Use-Prescribing Information (Avycaz_Final_PI_CBE-0_10_2019.Pdf). Available online: https://media.allergan.com/actavis/actavis/media/allergan-pdf-documents/product-prescribing/Avycaz_Final_PI_CBE-0_10_2019.pdf (accessed on 4 July 2021).
- Wang, Y.; Wang, J.; Wang, R.; Cai, Y. Resistance to Ceftazidime–Avibactam and Underlying Mechanisms. J. Glob. Antimicrob. Resist. 2020, 22, 18–27. [Google Scholar] [CrossRef] [PubMed]
- Wise, M.G.; Estabrook, M.A.; Sahm, D.F.; Stone, G.G.; Kazmierczak, K.M. Prevalence of Mcr-Type Genes among Colistin-Resistant Enterobacteriaceae Collected in 2014-2016 as Part of the INFORM Global Surveillance Program. PLoS ONE 2018, 13, e0195281. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- de Jonge, B.L.M.; Karlowsky, J.A.; Kazmierczak, K.M.; Biedenbach, D.J.; Sahm, D.F.; Nichols, W.W. In Vitro Susceptibility to Ceftazidime-Avibactam of Carbapenem-Nonsusceptible Enterobacteriaceae Isolates Collected during the INFORM Global Surveillance Study (2012 to 2014). Antimicrob. Agents Chemother. 2016, 60, 3163–3169. [Google Scholar] [CrossRef] [Green Version]
- Kazmierczak, K.M.; Bradford, P.A.; Stone, G.G.; de Jonge, B.L.M.; Sahm, D.F. In Vitro Activity of Ceftazidime-Avibactam and Aztreonam-Avibactam against OXA-48-Carrying Enterobacteriaceae Isolated as Part of the International Network for Optimal Resistance Monitoring (INFORM) Global Surveillance Program from 2012 to 2015. Antimicrob. Agents Chemother. 2018, 62, e00592-18. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kazmierczak, K.M.; de Jonge, B.L.M.; Stone, G.G.; Sahm, D.F. In Vitro Activity of Ceftazidime/Avibactam against Isolates of Enterobacteriaceae Collected in European Countries: INFORM Global Surveillance 2012-15. J. Antimicrob. Chemother. 2018, 73, 2782–2788. [Google Scholar] [CrossRef]
- Sader, H.S.; Castanheira, M.; Shortridge, D.; Mendes, R.E.; Flamm, R.K. Antimicrobial Activity of Ceftazidime-Avibactam Tested against Multidrug-Resistant Enterobacteriaceae and Pseudomonas Aeruginosa Isolates from U.S. Medical Centers, 2013 to 2016. Antimicrob. Agents Chemother. 2017, 61, e01045-17. [Google Scholar] [CrossRef] [Green Version]
- Sader, H.S.; Castanheira, M.; Mendes, R.E.; Flamm, R.K. Frequency and Antimicrobial Susceptibility of Gram-Negative Bacteria Isolated from Patients with Pneumonia Hospitalized in ICUs of US Medical Centres (2015–17). J. Antimicrob. Chemother. 2018, 73, 3053–3059. [Google Scholar] [CrossRef]
- Senchyna, F.; Gaur, R.L.; Sandlund, J.; Truong, C.; Tremintin, G.; Kültz, D.; Gomez, C.A.; Tamburini, F.B.; Andermann, T.; Bhatt, A.; et al. Diversity of Resistance Mechanisms in Carbapenem-Resistant Enterobacteriaceae at a Health Care System in Northern California, from 2013 to 2016. Diagn. Microbiol. Infect. Dis. 2019, 93, 250–257. [Google Scholar] [CrossRef]
- Yin, D.; Wu, S.; Yang, Y.; Shi, Q.; Dong, D.; Zhu, D.; Hu, F. China Antimicrobial Surveillance Network (CHINET) Study Group Results from the China Antimicrobial Surveillance Network (CHINET) in 2017 of the In Vitro Activities of Ceftazidime-Avibactam and Ceftolozane-Tazobactam against Clinical Isolates of Enterobacteriaceae and Pseudomonas Aeruginosa. Antimicrob. Agents Chemother. 2019, 63, e02431-18. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Karlowsky, J.A.; Kazmierczak, K.M.; Bouchillon, S.K.; de Jonge, B.L.M.; Stone, G.G.; Sahm, D.F. In Vitro Activity of Ceftazidime-Avibactam against Clinical Isolates of Enterobacteriaceae and Pseudomonas Aeruginosa Collected in Asia-Pacific Countries: Results from the INFORM Global Surveillance Program, 2012 to 2015. Antimicrob. Agents Chemother. 2018, 62, e02569-17. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hackel, M.; Kazmierczak, K.M.; Hoban, D.J.; Biedenbach, D.J.; Bouchillon, S.K.; de Jonge, B.L.M.; Stone, G.G. Assessment of the In Vitro Activity of Ceftazidime-Avibactam against Multidrug-Resistant Klebsiella Spp. Collected in the INFORM Global Surveillance Study, 2012 to 2014. Antimicrob. Agents Chemother. 2016, 60, 4677–4683. [Google Scholar] [CrossRef] [Green Version]
- Flamm, R.K.; Nichols, W.W.; Sader, H.S.; Farrell, D.J.; Jones, R.N. In Vitro Activity of Ceftazidime/Avibactam against Gram-Negative Pathogens Isolated from Pneumonia in Hospitalised Patients, Including Ventilated Patients. Int. J. Antimicrob. Agents 2016, 47, 235–242. [Google Scholar] [CrossRef] [PubMed]
- Wilson, W.R.; Kline, E.G.; Jones, C.E.; Morder, K.T.; Mettus, R.T.; Doi, Y.; Nguyen, M.H.; Clancy, C.J.; Shields, R.K. Effects of KPC Variant and Porin Genotype on the In Vitro Activity of Meropenem-Vaborbactam against Carbapenem-Resistant Enterobacteriaceae. Antimicrob. Agents Chemother. 2019, 63, e02048-18. [Google Scholar] [CrossRef] [Green Version]
- Gonzalez, M.D.; McMullen, A.R.; Wallace, M.A.; Crotty, M.P.; Ritchie, D.J.; Burnham, C.A.D. Susceptibility of Ceftolozane-Tazobactam and Ceftazidime-Avibactam Against a Collection of β-Lactam-Resistant Gram-Negative Bacteria. Ann. Lab. Med. 2017, 37, 174–176. [Google Scholar] [CrossRef]
- Kazmierczak, K.M.; de Jonge, B.L.M.; Stone, G.G.; Sahm, D.F. In Vitro Activity of Ceftazidime/Avibactam against Isolates of Pseudomonas Aeruginosa Collected in European Countries: INFORM Global Surveillance 2012-15. J. Antimicrob. Chemother. 2018, 73, 2777–2781. [Google Scholar] [CrossRef]
- Sader, H.S.; Castanheira, M.; Flamm, R.K.; Mendes, R.E.; Farrell, D.J.; Jones, R.N. Ceftazidime/Avibactam Tested against Gram-Negative Bacteria from Intensive Care Unit (ICU) and Non-ICU Patients, Including Those with Ventilator-Associated Pneumonia. Int. J. Antimicrob. Agents 2015, 46, 53–59. [Google Scholar] [CrossRef]
- Hachem, R.; Reitzel, R.; Rolston, K.; Chaftari, A.-M.; Raad, I. Antimicrobial Activities of Ceftazidime-Avibactam and Comparator Agents against Clinical Bacteria Isolated from Patients with Cancer. Antimicrob. Agents Chemother. 2017, 61, e02106-16. [Google Scholar] [CrossRef] [Green Version]
- FETROJA (Cefiderocol) for Injection, for Intravenous Use-Prescribing Information (Fetroja.Pdf). Available online: https://www.shionogi.com/content/dam/shionogi/si/products/pdf/fetroja.pdf (accessed on 4 July 2021).
- Malik, S.; Kaminski, M.; Landman, D.; Quale, J. Cefiderocol Resistance in Acinetobacter Baumannii: Roles of β-Lactamases, Siderophore Receptors, and Penicillin Binding Protein 3. Antimicrob. Agents Chemother. 2020, 64, e01221-20. [Google Scholar] [CrossRef]
- Ventola, C.L. The Antibiotic Resistance Crisis. Pharm. Ther. 2015, 40, 277–283. [Google Scholar]
- Chokshi, A.; Sifri, Z.; Cennimo, D.; Horng, H. Global Contributors to Antibiotic Resistance. J. Glob. Infect. Dis. 2019, 11, 36–42. [Google Scholar] [CrossRef] [PubMed]
- Livermore, D.M. Microbiology Society. 8 November 2016. Available online: https://microbiologysociety.org/ (accessed on 26 July 2022).
- Nisnevitch, M. Antibiotic Resistance and Antibiotic Alternatives: Looking towards the Future. Sci. Prog. 2016, 99, 92–96. [Google Scholar] [CrossRef]
- Skandalis, N.; Maeusli, M.; Papafotis, D.; Miller, S.; Lee, B.; Theologidis, I.; Luna, B. Environmental Spread of Antibiotic Resistance. Antibiotics 2021, 10, 640. [Google Scholar] [CrossRef]
- Cunha, C.B.; Opal, S.M. Antibiotic Stewardship: Strategies to Minimize Antibiotic Resistance While Maximizing Antibiotic Effectiveness. Med. Clin. N. Am. 2018, 102, 831–843. [Google Scholar] [CrossRef]
- Alós, J.-I. Resistencia bacteriana a los antibióticos: Una crisis global. Enferm. Infecc. Microbiol. Clínica 2015, 33, 692–699. [Google Scholar] [CrossRef] [PubMed]
- Bhardwaj, S.; Mehra, P.; Dhanjal, D.S.; Sharma, P.; Sharma, V.; Singh, R.; Nepovimova, E.; Chopra, C.; Kuča, K. Antibiotics and Antibiotic Resistance-Flipsides of the Same Coin. Curr. Pharm. Des. 2022. [Google Scholar] [CrossRef] [PubMed]
- Ghotaslou, R.; Bannazadeh Baghi, H.; Alizadeh, N.; Yekani, M.; Arbabi, S.; Memar, M.Y. Mechanisms of Bacteroides Fragilis Resistance to Metronidazole. Infect. Genet. Evol. 2018, 64, 156–163. [Google Scholar] [CrossRef]
- Pang, Z.; Raudonis, R.; Glick, B.R.; Lin, T.-J.; Cheng, Z. Antibiotic Resistance in Pseudomonas Aeruginosa: Mechanisms and Alternative Therapeutic Strategies. Biotechnol. Adv. 2019, 37, 177–192. [Google Scholar] [CrossRef]
- Verderosa, A.D.; de la Fuente-Núñez, C.; Mansour, S.C.; Cao, J.; Lu, T.K.; Hancock, R.E.W.; Fairfull-Smith, K.E. Ciprofloxacin-Nitroxide Hybrids with Potential for Biofilm Control. Eur. J. Med. Chem. 2017, 138, 590–601. [Google Scholar] [CrossRef] [Green Version]
- Eisenreich, W.; Rudel, T.; Heesemann, J.; Goebel, W. Link Between Antibiotic Persistence and Antibiotic Resistance in Bacterial Pathogens. Front. Cell. Infect. Microbiol. 2022, 12, 900848. [Google Scholar] [CrossRef] [PubMed]
- Jacoby, G.A. Mechanisms of Resistance to Quinolones. Clin. Infect. Dis. Off. Publ. Infect. Dis. Soc. Am. 2005, 41 (Suppl. 2), S120–S126. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, Y.; Li, X.; Mi, K. Mechanisms of Fluoroquinolone Resistance in Mycobacterium Tuberculosis. Yi Chuan Hered. 2016, 38, 918–927. [Google Scholar] [CrossRef]
- Sandra Georgina Solano-Gálvez Mechanisms of Resistance to Quinolones. In Antimicrobial Resistance; Valencia-Segrove, M.F. (Ed.) IntechOpen: Rijeka, Croatia, 2020; p. Ch. 2. ISBN 978-1-83962-433-9. [Google Scholar]
- Cuypers, W.L.; Jacobs, J.; Wong, V.; Klemm, E.J.; Deborggraeve, S.; Van Puyvelde, S. Fluoroquinolone Resistance in Salmonella: Insights by Whole-Genome Sequencing. Microb. Genom. 2018, 4, e000195. [Google Scholar] [CrossRef]
- Roychoudhury, S.; Makin, K.; Twinem, T.; Leunk, R.; Hsu, M.C. In Vitro Resistance Development to Nemonoxacin in Streptococcus Pneumoniae: A Unique Profile for a Novel Nonfluorinated Quinolone. Microb. Drug Resist. 2016, 22, 578–584. [Google Scholar] [CrossRef] [Green Version]
- Phillips-Jones, M.K.; Harding, S.E. Antimicrobial Resistance (AMR) Nanomachines-Mechanisms for Fluoroquinolone and Glycopeptide Recognition, Efflux and/or Deactivation. Biophys. Rev. 2018, 10, 347–362. [Google Scholar] [CrossRef] [Green Version]
- Wei, M.; Tu, N.; Yang, K. Resistance Mechanism of Carbapenem-Resistant Enterobacteriaceae to Quinolones. Clin. Lab. 2021, 67. [Google Scholar] [CrossRef]
- Spizek, J.; Havlicek, V. Tackling Antibiotic Resistance. In Antibiotics: Current Innovations and Future Trends; Caister Academic Press: Wymondham, UK, 2015; pp. 83–94. ISBN 978-1-908230-54-6. [Google Scholar]
- Chellat, M.F.; Raguž, L.; Riedl, R. Targeting Antibiotic Resistance. Angew. Chem. Int. Ed Engl. 2016, 55, 6600–6626. [Google Scholar] [CrossRef]
- Fisher, J.F.; Mobashery, S. Endless Resistance. Endless Antibiotics? MedChemComm 2016, 7, 37–49. [Google Scholar] [CrossRef]
- Domalaon, R.; Idowu, T.; Zhanel, G.G.; Schweizer, F. Antibiotic Hybrids: The Next Generation of Agents and Adjuvants against Gram-Negative Pathogens? Clin. Microbiol. Rev. 2018, 31, e00077-17. [Google Scholar] [CrossRef] [Green Version]
- Gao, C.; Fan, Y.-L.; Zhao, F.; Ren, Q.-C.; Wu, X.; Chang, L.; Gao, F. Quinolone Derivatives and Their Activities against Methicillin-Resistant Staphylococcus Aureus (MRSA). Eur. J. Med. Chem. 2018, 157, 1081–1095. [Google Scholar] [CrossRef] [PubMed]
- Darehkordi, A.; Javanmiri, M.; Ghazi, S.; Assar, S. Synthesis of N-Aryl-2,2,2-Trifluoroacetimidoyl Piperazinylquinolone Derivatives and Their Antibacterial Evaluations. J. Fluor. Chem. 2011, 132, 263–268. [Google Scholar] [CrossRef]
- Shavit, M.; Pokrovskaya, V.; Belakhov, V.; Baasov, T. Covalently Linked Kanamycin–Ciprofloxacin Hybrid Antibiotics as a Tool to Fight Bacterial Resistance. Small Mol. Enabled Chem. Biol. Drug Discov. 2017, 25, 2917–2925. [Google Scholar] [CrossRef]
- Gorityala, B.K.; Guchhait, G.; Goswami, S.; Fernando, D.M.; Kumar, A.; Zhanel, G.G.; Schweizer, F. Hybrid Antibiotic Overcomes Resistance in P. Aeruginosa by Enhancing Outer Membrane Penetration and Reducing Efflux. J. Med. Chem. 2016, 59, 8441–8455. [Google Scholar] [CrossRef] [PubMed]
- Gordeev, M.F.; Hackbarth, C.; Barbachyn, M.R.; Banitt, L.S.; Gage, J.R.; Luehr, G.W.; Gomez, M.; Trias, J.; Morin, S.E.; Zurenko, G.E.; et al. Novel Oxazolidinone-Quinolone Hybrid Antimicrobials. Bioorg. Med. Chem. 2003, 13, 4213–4216. [Google Scholar] [CrossRef]
- Marc, G.; Araniciu, C.; Oniga, S.D.; Vlase, L.; Pîrnău, A.; Nadăș, G.C.; Novac, C.Ș.; Matei, I.A.; Chifiriuc, M.C.; Măruțescu, L.; et al. Design, Synthesis and Biological Evaluation of New Piperazin-4-Yl-(Acetyl-Thiazolidine-2,4-Dione) Norfloxacin Analogues as Antimicrobial Agents. Molecules 2019, 24, 3959. [Google Scholar] [CrossRef] [Green Version]
- Liu, L.; Shao, L.; Li, J.; Cui, H.; Li, B.; Zhou, X.; Lv, P.; Zhang, J. Synthesis, Antibacterial Activities, Mode of Action and Acute Toxicity Studies of New Oxazolidinone-Fluoroquinolone Hybrids. Mol. Basel Switz. 2019, 24, 1641. [Google Scholar] [CrossRef] [Green Version]
- Akhtar, R.; Yousaf, M.; Naqvi, S.A.R.; Irfan, M.; Zahoor, A.F.; Hussain, A.I.; Chatha, S.A.S. Synthesis of Ciprofloxacin-Based Compounds: A Review. Synth. Commun. 2016, 46, 1849–1879. [Google Scholar] [CrossRef]
- Pokrovskaya, V.; Belakhov, V.; Hainrichson, M.; Yaron, S.; Baasov, T. Design, Synthesis, and Evaluation of Novel Fluoroquinolone−Aminoglycoside Hybrid Antibiotics. J. Med. Chem. 2009, 52, 2243–2254. [Google Scholar] [CrossRef]
- Chugunova, E.; Akylbekov, N.; Bulatova, A.; Gavrilov, N.; Voloshina, A.; Kulik, N.; Zobov, V.; Dobrynin, A.; Syakaev, V.; Burilov, A. Synthesis and Biological Evaluation of Novel Structural Hybrids of Benzofuroxan Derivatives and Fluoroquinolones. Eur. J. Med. Chem. 2016, 116, 165–172. [Google Scholar] [CrossRef]
- Hu, Y.-Q.; Zhang, S.; Xu, Z.; Lv, Z.-S.; Liu, M.-L.; Feng, L.-S. 4-Quinolone Hybrids and Their Antibacterial Activities. Eur. J. Med. Chem. 2017, 141, 335–345. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.-N.; Bheemanaboina, R.R.Y.; Gao, W.-W.; Kang, J.; Cai, G.-X.; Zhou, C.-H. Discovery of Benzimidazole-Quinolone Hybrids as New Cleaving Agents toward Drug-Resistant Pseudomonas Aeruginosa DNA. ChemMedChem 2018, 13, 1004–1017. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.-H.; Fan, Y.-L.; Zhou, J. Quinolone–Triazole Hybrids and Their Biological Activities. J. Heterocycl. Chem. 2018, 55, 1854–1862. [Google Scholar] [CrossRef]
- Ji, C.; Xu, X. Recent Advancements in Macrolide Hybrids against Staphylococcus Aureus. Curr. Top. Med. Chem. 2021, 21, 2455–2473. [Google Scholar] [CrossRef] [PubMed]
- Jubeh, B.; Breijyeh, Z.; Karaman, R. Antibacterial Prodrugs to Overcome Bacterial Resistance. Mol. Basel Switz. 2020, 25, 1543. [Google Scholar] [CrossRef] [Green Version]
- Piplani, M.; Rajak, H.; Sharma, P.C. Synthesis and Characterization of N-Mannich Based Prodrugs of Ciprofloxacin and Norfloxacin: In Vitro Anthelmintic and Cytotoxic Evaluation. J. Adv. Res. 2017, 8, 463–470. [Google Scholar] [CrossRef]
- Sharma, P.C.; Piplani, M.; Rajak, H. Synthesis, Characterization and Antimicrobial Evaluation of Lipid Based Norfloxacin Prodrug. Curr. Drug Deliv. 2018, 15, 219–226. [Google Scholar] [CrossRef]
- Pokrovskaya, V.; Baasov, T. Dual-Acting Hybrid Antibiotics: A Promising Strategy to Combat Bacterial Resistance. Expert Opin. Drug Discov. 2010, 5, 883–902. [Google Scholar] [CrossRef]
- Hall, I.H.; Schwab, U.E.; Ward, E.S.; Ives, T.J. Effects of Alatrofloxacin, the Parental Prodrug of Trovafloxacin, on Phagocytic, Anti-Inflammatory and Immunomodulation Events of Human THP-1 Monocytes. Biomed. Pharmacother. 2003, 57, 359–365. [Google Scholar] [CrossRef]
- Tanaka, K.S.E.; Houghton, T.J.; Kang, T.; Dietrich, E.; Delorme, D.; Ferreira, S.S.; Caron, L.; Viens, F.; Arhin, F.F.; Sarmiento, I.; et al. Bisphosphonated Fluoroquinolone Esters as Osteotropic Prodrugs for the Prevention of Osteomyelitis. Bioorg. Med. Chem. 2008, 16, 9217–9229. [Google Scholar] [CrossRef]
- Sobczak, M.; Witkowska, E.; Oledzka, E.; Kolodziejski, W. Synthesis and Structural Analysis of Polyester Prodrugs of Norfloxacin. Molecules 2008, 13, 96–106. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Amin, M.; Abbas, N.; Hussain, M.; Edgar, K.; Tahir, M.; Tremel, W.; Sher, M. Cellulose Ether Derivatives: A New Platform for Prodrug Formation of Fluoroquinolone Antibiotics. Cellulose 2015, 22, 2011–2022. [Google Scholar] [CrossRef]
- Abbas, N.S.; Amin, M.; Hussain, M.A.; Edgar, K.J.; Tahir, M.N.; Tremel, W. Extended Release and Enhanced Bioavailability of Moxifloxacin Conjugated with Hydrophilic Cellulose Ethers. Carbohydr. Polym. 2016, 136, 1297–1306. [Google Scholar] [CrossRef] [PubMed]
- Bremner, J.B.; Ambrus, J.I.; Samosorn, S. Dual Action-Based Approaches to Antibacterial Agents. Curr. Med. Chem. 2007, 14, 1459–1477. [Google Scholar] [CrossRef] [PubMed]
- Wermuth, C.G.; Ganellin, C.R.; Lindberg, P.; Mitscher, L.A. Glossary of Terms Used in Medicinal Chemistry (IUPAC Recommendations 1998). Pure Appl. Chem. 1998, 70, 1129–1143. [Google Scholar] [CrossRef]
- Surur, A.S.; Sun, D. Macrocycle-Antibiotic Hybrids: A Path to Clinical Candidates. Front. Chem. 2021, 9, 659845. [Google Scholar] [CrossRef]
- El-Lababidi, R.M.; Rizk, J.G. Cefiderocol: A Siderophore Cephalosporin. Ann. Pharmacother. 2020, 54, 1215–1231. [Google Scholar] [CrossRef]
- Antibiotics Currently in Global Clinical Development. Available online: http://pew.org/1YkUFkT (accessed on 11 June 2022).
- Gerding, D.N.; Cornely, O.A.; Grill, S.; Kracker, H.; Marrast, A.C.; Nord, C.E.; Talbot, G.H.; Buitrago, M.; Diaconescu, I.G.; de Oliveira, C.M.; et al. Cadazolid for the Treatment of Clostridium Difficile Infection: Results of Two Double-Blind, Placebo-Controlled, Non-Inferiority, Randomised Phase 3 Trials. Lancet Infect. Dis. 2019, 19, 265–274. [Google Scholar] [CrossRef]
- Actelion. A Phase 1, Open-Label, Single Oral Dose Study to Investigate the Pharmacokinetics, Safety, and Tolerability of Cadazolid in Patients With Severe Clostridium Difficile Infection (CDI); Actelion: Allschwil, Switzerland, 2014. [Google Scholar]
- Ma, Z.; Lynch, A.S. Development of a Dual-Acting Antibacterial Agent (TNP-2092) for the Treatment of Persistent Bacterial Infections. J. Med. Chem. 2016, 59, 6645–6657. [Google Scholar] [CrossRef] [Green Version]
- Yuan, Y.; Wang, X.; Xu, X.; Liu, Y.; Li, C.; Yang, M.; Yang, Y.; Ma, Z. Evaluation of a Dual-Acting Antibacterial Agent, TNP-2092, on Gut Microbiota and Potential Application in the Treatment of Gastrointestinal and Liver Disorders. ACS Infect. Dis. 2020, 6, 820–831. [Google Scholar] [CrossRef]
- TenNor Therapeutics Limited. Phase 2, Double-Blind, Randomized, Multicenter, Parallel, Controlled Study to Evaluate the Safety, Tolerability, Pharmacokinetics, and Efficacy of TNP-2092 to Treat Acute Bacterial Skin and Skin Structure Infection in Adults; TenNor Therapeutics Limited: Westfield, NJ, USA, 2020. [Google Scholar]
- Adams, R.A.; Leon, G.; Miller, N.M.; Reyes, S.P.; Thantrong, C.H.; Thokkadam, A.M.; Lemma, A.S.; Sivaloganathan, D.M.; Wan, X.; Brynildsen, M.P. Rifamycin Antibiotics and the Mechanisms of Their Failure. J. Antibiot. (Tokyo) 2021, 74, 786–798. [Google Scholar] [CrossRef]
- Blaskovich, M.A.T.; Hansford, K.A.; Butler, M.S.; Jia, Z.; Mark, A.E.; Cooper, M.A. Developments in Glycopeptide Antibiotics. ACS Infect. Dis. 2018, 4, 715–735. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Theravance Biopharma. A Phase 2, Randomized, Double Blind, Study of Intravenous TD 1792 Versus Vancomycin for Treatment of Complicated Gram Positive Skin and Skin Structure Infections; Theravance Biopharma: San Francisco, CA, USA, 2021. [Google Scholar]
- Stryjewski, M.E.; Potgieter, P.D.; Li, Y.-P.; Barriere, S.L.; Churukian, A.; Kingsley, J.; Corey, G.R. TD-1792 versus Vancomycin for Treatment of Complicated Skin and Skin Structure Infections. Antimicrob. Agents Chemother. 2012, 56, 5476–5483. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bifunctional Beta-Lactam Antibiotics. Available online: https://encyclopedia.pub/entry/7047 (accessed on 27 July 2022).
- Theravance Biopharma. A Randomized, Double-Blind, Placebo-Controlled, Single Ascending Dose (SAD) Study to Evaluate the Safety, Tolerability, and Pharmacokinetics of TD-1607, a Glycopeptide-Cephalosporin Heterodimer Gram-Positive Antibiotic, in Healthy Subjects; Theravance Biopharma: San Francisco, CA, USA, 2021. [Google Scholar]
- Ma, Z.; He, S.; Yuan, Y.; Zhuang, Z.; Liu, Y.; Wang, H.; Chen, J.; Xu, X.; Ding, C.; Molodtsov, V.; et al. Design, Synthesis, and Characterization of TNP-2198, a Dual-Targeted Rifamycin-Nitroimidazole Conjugate with Potent Activity against Microaerophilic and Anaerobic Bacterial Pathogens. J. Med. Chem. 2022, 65, 4481–4495. [Google Scholar] [CrossRef]
- Dalhoff, A.; Rashid, M.-U.; Kapsner, T.; Panagiotidis, G.; Weintraub, A.; Nord, C.E. Analysis of Effects of MCB3681, the Antibacterially Active Substance of Prodrug MCB3837, on Human Resident Microflora as Proof of Principle. Clin. Microbiol. Infect. 2015, 21, 767.e1–767.e4. [Google Scholar] [CrossRef] [Green Version]
- Commissioner, O. FDA Approves New Antibacterial Drug to Treat Complicated Urinary Tract Infections as Part of Ongoing Efforts to Address Antimicrobial Resistance. Available online: https://www.fda.gov/news-events/press-announcements/fda-approves-new-antibacterial-drug-treat-complicated-urinary-tract-infections-part-ongoing-efforts (accessed on 26 July 2022).
- Wu, J.Y.; Srinivas, P.; Pogue, J.M. Cefiderocol: A Novel Agent for the Management of Multidrug-Resistant Gram-Negative Organisms. Infect. Dis. Ther. 2020, 9, 17–40. [Google Scholar] [CrossRef] [Green Version]
- Yao, J.; Wang, J.; Chen, M.; Cai, Y. Cefiderocol: An Overview of Its in-Vitro and in-Vivo Activity and Underlying Resistant Mechanisms. Front. Med. 2021, 8, 741940. [Google Scholar] [CrossRef] [PubMed]
- Shionogi. A Multicenter, Randomized, Open-Label Clinical Study of S-649266 or Best Available Therapy for the Treatment of Severe Infections Caused by Carbapenem-Resistant Gram-Negative Pathogens; Shionogi: Osaka, Japan, 2020. [Google Scholar]
- Zheng, T.; Nolan, E.M. Enterobactin-Mediated Delivery of β-Lactam Antibiotics Enhances Antibacterial Activity against Pathogenic Escherichia Coli. J. Am. Chem. Soc. 2014, 136, 9677–9691. [Google Scholar] [CrossRef] [Green Version]
- Cherian, P.T.; Deshpande, A.; Cheramie, M.N.; Bruhn, D.F.; Hurdle, J.G.; Lee, R.E. Design, Synthesis and Microbiological Evaluation of Ampicillin-Tetramic Acid Hybrid Antibiotics. J. Antibiot. (Tokyo) 2017, 70, 65–72. [Google Scholar] [CrossRef] [Green Version]
- Peck, M.; Rothenberg, M.E.; Deng, R.; Lewin-Koh, N.; She, G.; Kamath, A.V.; Carrasco-Triguero, M.; Saad, O.; Castro, A.; Teufel, L.; et al. A Phase 1, Randomized, Single-Ascending-Dose Study To Investigate the Safety, Tolerability, and Pharmacokinetics of DSTA4637S, an Anti-Staphylococcus Aureus Thiomab Antibody-Antibiotic Conjugate, in Healthy Volunteers. Antimicrob. Agents Chemother. 2019, 63, e02588-18. [Google Scholar] [CrossRef] [Green Version]
- Vogt, J.; Hazenbos, W.; Pillow, T.; Proctor, W. P49-Uncovering Novel Lysosomal PH Changes Caused by DmDNA31, a Novel Rifalog Payload of an Antibody-Antibiotic Conjugate (AAC) in Development to Treat Staphylococcus Aureus Infections. Drug Metab. Pharmacokinet. 2020, 35, S36. [Google Scholar] [CrossRef]
- Genentech, Inc. A Phase IB, Randomized, Double-Blind, Placebo-Controlled, Multiple-Ascending Dose Study to Investigate the Safety, Tolerability, and Pharmacokinetics of DSTA4637S in Patients With Staphylococcus Aureus Bacteremia Receiving Standard-of-Care Antibiotics; Genentech, Inc.: San Francisco, CA, USA, 2020. [Google Scholar]
- Genentech, Inc. A Phase I, Randomized, Double-Blind, Placebo-Controlled, Single-Ascending Dose Study To Investigate The Safety, Tolerability, And Pharmacokinetics Of Dsta4637s In Healthy Volunteers; Genentech, Inc.: San Francisco, CA, USA, 2018. [Google Scholar]
- Parkes, A.L.; Yule, I.A. Hybrid Antibiotics-Clinical Progress and Novel Designs. Expert Opin. Drug Discov. 2016, 11, 665–680. [Google Scholar] [CrossRef]
- Benet, L.Z.; Hosey, C.M.; Ursu, O.; Oprea, T.I. BDDCS, the Rule of 5 and Drugability. Adv. Drug Deliv. Rev. 2016, 101, 89–98. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Doak, B.C.; Kihlberg, J. Drug Discovery beyond the Rule of 5-Opportunities and Challenges. Expert Opin. Drug Discov. 2017, 12, 115–119. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Protti, Í.F.; Rodrigues, D.R.; Fonseca, S.K.; Alves, R.J.; de Oliveira, R.B.; Maltarollo, V.G. Do Drug-Likeness Rules Apply to Oral Prodrugs? ChemMedChem 2021, 16, 1446–1456. [Google Scholar] [CrossRef] [PubMed]
- Pathania, S.; Singh, P.K. Analyzing FDA-Approved Drugs for Compliance of Pharmacokinetic Principles: Should There Be a Critical Screening Parameter in Drug Designing Protocols? Expert Opin. Drug Metab. Toxicol. 2021, 17, 351–354. [Google Scholar] [CrossRef]
- Jukič, M.; Bren, U. Machine Learning in Antibacterial Drug Design. Front. Pharmacol. 2022, 13, 864412. [Google Scholar] [CrossRef]
- Panda, S.S.; Liaqat, S.; Girgis, A.S.; Samir, A.; Hall, C.D.; Katritzky, A.R. Novel Antibacterial Active Quinolone-Fluoroquinolone Conjugates and 2D-QSAR Studies. Bioorg. Med. Chem. Lett. 2015, 25, 3816–3821. [Google Scholar] [CrossRef]
- Senthilkumar, P.; Dinakaran, M.; Yogeeswari, P.; China, A.; Nagaraja, V.; Sriram, D. Antimycobacterial Activities of Novel Fluoroquinolones. Biomed. Pharmacother. 2009, 63, 27–35. [Google Scholar] [CrossRef]
- Sriram, D.; Yogeeswari, P.; Senchani, G.; Banerjee, D. Newer Tetracycline Derivatives: Synthesis, Anti-HIV, Antimycobacterial Activities and Inhibition of HIV-1 Integrase. Bioorg. Med. Chem. Lett. 2007, 17, 2372–2375. [Google Scholar] [CrossRef]
- Robertson, G.T.; Bonventre, E.J.; Doyle, T.B.; Du, Q.; Duncan, L.; Morris, T.W.; Roche, E.D.; Yan, D.; Lynch, A.S. In Vitro Evaluation of CBR-2092, a Novel Rifamycin-Quinolone Hybrid Antibiotic: Microbiology Profiling Studies with Staphylococci and Streptococci. Antimicrob. Agents Chemother. 2008, 52, 2324–2334. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pavlović, D.; Mutak, S. Discovery of 4′′-Ether Linked Azithromycin-Quinolone Hybrid Series: Influence of the Central Linker on the Antibacterial Activity. ACS Med. Chem. Lett. 2011, 2, 331–336. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, K.K.; Stone, L.K.; Lieberman, T.D.; Shavit, M.; Baasov, T.; Kishony, R. A Hybrid Drug Limits Resistance by Evading the Action of the Multiple Antibiotic Resistance Pathway. Mol. Biol. Evol. 2016, 33, 492–500. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Durcik, M.; Skok, Ž.; Ilaš, J.; Zidar, N.; Zega, A.; Szili, P.É.; Draskovits, G.; Révész, T.; Kikelj, D.; Nyerges, A.; et al. Hybrid Inhibitors of DNA Gyrase A and B: Design, Synthesis and Evaluation. Pharmaceutics 2021, 13, 6. [Google Scholar] [CrossRef]
- Wang, X.-D.; Wei, W.; Wang, P.-F.; Tang, Y.-T.; Deng, R.-C.; Li, B.; Zhou, S.-S.; Zhang, J.-W.; Zhang, L.; Xiao, Z.-P.; et al. Novel 3-Arylfuran-2(5H)-One-Fluoroquinolone Hybrid: Design, Synthesis and Evaluation as Antibacterial Agent. Bioorg. Med. Chem. 2014, 22, 3620–3628. [Google Scholar] [CrossRef]
- Xiao, Z.-P.; Wang, X.-D.; Wang, P.-F.; Zhou, Y.; Zhang, J.-W.; Zhang, L.; Zhou, J.; Zhou, S.-S.; Ouyang, H.; Lin, X.-Y.; et al. Design, Synthesis, and Evaluation of Novel Fluoroquinolone-Flavonoid Hybrids as Potent Antibiotics against Drug-Resistant Microorganisms. Eur. J. Med. Chem. 2014, 80, 92–100. [Google Scholar] [CrossRef]
- Yang, P.; Luo, J.-B.; Zhang, L.-L.; Wang, Y.-S.; Xie, X.-B.; Shi, Q.-S.; Zhang, X.-G. Design, Synthesis and Antibacterial Studies of 1,3,4-Oxadiazole-Fluoroquinolone Hybrids and Their Molecular Docking Studies. ChemistrySelect 2021, 6, 13209–13214. [Google Scholar] [CrossRef]
- Ibrahim, N.M.; Fahim, S.H.; Hassan, M.; Farag, A.E.; Georgey, H.H. Design and Synthesis of Ciprofloxacin-Sulfonamide Hybrids to Manipulate Ciprofloxacin Pharmacological Qualities: Potency and Side Effects. Eur. J. Med. Chem. 2022, 228, 114021. [Google Scholar] [CrossRef]
- Ezelarab, H.A.A.; Hassan, H.A.; Abbas, S.H.; Abd El-Baky, R.M.; Aburahama, G.E.-D.A. Design, Synthesis and Antifungal Activity of 1,2,4-Triazole/ or 1,3,4-Oxadiazole-Ciprofloxacin Hybrids. J. Adv. Biomed. Pharm. Sci. 2018, 1, 78–84. [Google Scholar] [CrossRef] [Green Version]
- Labischinski, H.; Cherian, J.; Calanasan, C.; Boyce, R.S. Hybrid Antimicrobial Compounds and Their. Use. Patent WO2010025906A2, 11 March 2010. [Google Scholar]
- Hagihara, M.; Kashiwase, H.; Katsube, T.; Kimura, T.; Komai, T.; Momota, K.; Ohmine, T.; Nishigaki, T.; Kimura, S.; Shimada, K. Synthesis and Anti-HIV Activity of Arylpiperazinyl Fluoroquinolones: A New Class of Anti-HIV Agents. Bioorg. Med. Chem. Lett. 1999, 9, 3063–3068. [Google Scholar] [CrossRef]
- Endres, B.T.; Bassères, E.; Alam, M.J.; Garey, K.W. Cadazolid for the Treatment of Clostridium Difficile. Expert Opin. Investig. Drugs 2017, 26, 509–514. [Google Scholar] [CrossRef]
- Scaiola, A.; Leibundgut, M.; Boehringer, D.; Caspers, P.; Bur, D.; Locher, H.H.; Rueedi, G.; Ritz, D. Structural Basis of Translation Inhibition by Cadazolid, a Novel Quinoxolidinone Antibiotic. Sci. Rep. 2019, 9, 5634. [Google Scholar] [CrossRef] [PubMed]
- Robertson, G.T.; Bonventre, E.J.; Doyle, T.B.; Du, Q.; Duncan, L.; Morris, T.W.; Roche, E.D.; Yan, D.; Lynch, A.S. In Vitro Evaluation of CBR-2092, a Novel Rifamycin-Quinolone Hybrid Antibiotic: Studies of the Mode of Action in Staphylococcus Aureus. Antimicrob. Agents Chemother. 2008, 52, 2313–2323. [Google Scholar] [CrossRef] [Green Version]
- Peek, J.; Koirala, B.; Brady, S.F. Synthesis and Evaluation of Dual-Action Kanglemycin-Fluoroquinolone Hybrid Antibiotics. Bioorg. Med. Chem. Lett. 2022, 57, 128484. [Google Scholar] [CrossRef] [PubMed]
- Islam, R.; Singh, V.; Ammeter, D.; Schweizer, F.; Kuss, S. Electrochemical Characterization of the Antibiotic Hybrid Ciprofloxacin-Tobramycin. Electrochem. Commun. 2020, 119, 106825. [Google Scholar] [CrossRef]
- Seiler, P.; Enderlin-Paput, M.; Pfaff, P.; Weiss, M.; Ritz, D.; Clozel, M.; Locher, H.H. Cadazolid Does Not Promote Intestinal Colonization of Vancomycin-Resistant Enterococci in Mice. Antimicrob. Agents Chemother. 2015, 60, 628–631. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Muhammad, A.; Simcha, W.; Rawish, F.; Sabih, R.; Albert, E.; Ali, N. Cadazolid vs Vancomycin for the Treatment of Clostridioides Difficile Infection: Systematic Review with Meta-Analysis. Curr. Clin. Pharmacol. 2020, 15, 4–10. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Damu, G.L.V.; Lv, J.-S.; Geng, R.-X.; Yang, D.-C.; Zhou, C.-H. Design, Synthesis and Evaluation of Clinafloxacin Triazole Hybrids as a New Type of Antibacterial and Antifungal Agents. Bioorg. Med. Chem. Lett. 2012, 22, 5363–5366. [Google Scholar] [CrossRef]
- Eissa, S.I.; Farrag, A.M.; Abbas, S.Y.; El Shehry, M.F.; Ragab, A.; Fayed, E.A.; Ammar, Y.A. Novel Structural Hybrids of Quinoline and Thiazole Moieties: Synthesis and Evaluation of Antibacterial and Antifungal Activities with Molecular Modeling Studies. Bioorganic Chem. 2021, 110, 104803. [Google Scholar] [CrossRef]
- Kowalczuk, D.; Gładysz, A.; Pitucha, M.; Kamiński, D.M.; Barańska, A.; Drop, B. Spectroscopic Study of the Molecular Structure of the New Hybrid with a Potential Two-Way Antibacterial Effect. Molecules 2021, 26, 1442. [Google Scholar] [CrossRef]
- Zhang, F.Y.; Du, G.J.; Zhang, L.; Zhang, C.L.; Lu, W.L.; Liang, W. Naringenin Enhances the Anti-Tumor Effect of Doxorubicin through Selectively Inhibiting the Activity of Multidrug Resistance-Associated Proteins but Not P-Glycoprotein. Pharm. Res. 2009, 26, 914–925. [Google Scholar] [CrossRef] [PubMed]
- Hryhoriv, H.; Mariutsa, I.; Kovalenko, S.M.; Georgiyants, V.; Perekhoda, L.; Filimonova, N.; Geyderikh, O.; Sidorenko, L. The Search for New Antibacterial Agents among 1,2,3-Triazole Functionalized Ciprofloxacin and Norfloxacin Hybrids: Synthesis, Docking Studies, and Biological Activity Evaluation. Sci. Pharm. 2022, 90, 2. [Google Scholar] [CrossRef]
- Albayrak, F.; Çiçek, M.; Alkaya, D.; Kulu, I. Design, Synthesis and Biological Evaluation of 8-Aminoquinoline-1,2,3-Triazole Hybrid Derivatives as Potential Antimicrobial Agents. Med. Chem. Res. 2022, 31, 652–665. [Google Scholar] [CrossRef]
- Awolade, P.; Cele, N.; Ebenezer, O.; Kerru, N.; Gummidi, L.; Gu, L.; Palma, G.; Kaur, M.; Singh, P. Synthesis of 1H-1,2,3-Triazole-Linked Quinoline-Isatin Molecular Hybrids as Anti-Breast Cancer and Anti-Methicillin-Resistant Staphylococcus Aureus (MRSA) Agents. Anticancer Agents Med. Chem. 2021, 21, 1228–1239. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.-Q.; Gao, C.; Zhang, S.; Xu, L.; Xu, Z.; Feng, L.-S.; Wu, X.; Zhao, F. Quinoline Hybrids and Their Antiplasmodial and Antimalarial Activities. Eur. J. Med. Chem. 2017, 139, 22–47. [Google Scholar] [CrossRef] [PubMed]
- Fan, Y.-L.; Cheng, X.-W.; Wu, J.-B.; Liu, M.; Zhang, F.-Z.; Xu, Z.; Feng, L.-S. Antiplasmodial and Antimalarial Activities of Quinolone Derivatives: An Overview. Eur. J. Med. Chem. 2018, 146, 1–14. [Google Scholar] [CrossRef]
- Abdel-Aziz, M.; Park, S.-E.; Abuo-Rahma, G.E.-D.A.A.; Sayed, M.A.; Kwon, Y. Novel N-4-Piperazinyl-Ciprofloxacin-Chalcone Hybrids: Synthesis, Physicochemical Properties, Anticancer and Topoisomerase I and II Inhibitory Activity. Eur. J. Med. Chem. 2013, 69, 427–438. [Google Scholar] [CrossRef]
- Fallica, A.N.; Barbaraci, C.; Amata, E.; Pasquinucci, L.; Turnaturi, R.; Dichiara, M.; Intagliata, S.; Gariboldi, M.B.; Marras, E.; Orlandi, V.T.; et al. Nitric Oxide Photo-Donor Hybrids of Ciprofloxacin and Norfloxacin: A Shift in Activity from Antimicrobial to Anticancer Agents. J. Med. Chem. 2021, 64, 11597–11613. [Google Scholar] [CrossRef]
- Serafim, M.S.M.; Kronenberger, T.; Oliveira, P.R.; Poso, A.; Honório, K.M.; Mota, B.E.F.; Maltarollo, V.G. The Application of Machine Learning Techniques to Innovative Antibacterial Discovery and Development. Expert Opin. Drug Discov. 2020, 15, 1165–1180. [Google Scholar] [CrossRef]
- Hou, Y.; Zhao, Y.; Li, Y. Environmentally Friendly Fluoroquinolone Derivatives with Lower Plasma Protein Binding Rate Designed Using 3D-QSAR, Molecular Docking and Molecular Dynamics Simulation. Int. J. Environ. Res. Public. Health 2020, 17, 6626. [Google Scholar] [CrossRef]













| Position on the Chemical Structure | Requirements and Possible Implications | References |
|---|---|---|
| 2 | Optimal is a hydrogen moiety; larger moieties may hinder the C3 and C4 positions. | [9] |
| 3 | A carboxyl group is required (essential for interacting with the DNA bases and DNA gyrase). | [5,9,31,32,33] |
| 4 | Oxo-(keto) moiety is required; essential for interacting with the DNA bases and DNA gyrase. | |
| 6 | Small moiety is required (optimal—fluorine); fluorine increases the potency by between 5- and 100-fold compared to any other potential halogen moiety. | [9] |
| 1 | It is involved in the pharmacokinetic properties and overall potency. A cyclopropyl moiety confers activity against Gram-negative bacteria. A 2,4-difluorophenyl substituent determines less potency but heightens activity against anaerobes (e.g., temafloxacin; it was withdrawn shortly after approval due to severe adverse reactions). | [9,34,35] |
| 5 | Specific radicals substituted at this position (-NH2, -CH3) may increase activity against Gram-positive bacteria. | [9,34] |
| 7 | It is involved in pharmacokinetic properties and the spectrum of activity. A five- or six-membered nitrogen heterocycle at this position improves the activity and pharmacokinetic profile. For example, amino pyrrolidine or an alkyl moiety determines enhanced activity against Gram-positive bacteria. On the other hand, piperazine determines better activity against Gram-negative bacteria. | [9,34] |
| 8 | It is involved in the pharmacokinetic properties and activity against anaerobic bacteria. | [9] |
| Compounds (Generation) | Usual Doses | Indications and Administration | References |
|---|---|---|---|
| Nalidixic acid (1st) | 4 g daily (every 6 h); 7 to 14 days in acute infections, reducing after that to half this dose in chronic infections. | Uncomplicated urinary tract infections; Oral administration. | [18,20,70,79] |
| Norfloxacin (2nd) | 400 mg twice a day (every 12 h); 3–7–21–28 days depending on the severity and nature of the infection. | Uncomplicated and complicated urinary tract infections; Acute or chronic prostatitis; Uncomplicated gonorrhea; Oral administration. | [18,21,70,85,86] |
| Ciprofloxacin (2nd) | 250–500 mg (every 12 h); 7 to 14 days or more, depending on the severity and nature of the infection. | Uncomplicated and complicated urinary tract infections, pyelonephritis, sexually transmitted diseases, prostatitis, skin and tissue infections; Oral (as the hydrochloride or base) and parenteral administration (lactate), eye drops, eye ointment, or ear drops (as the hydrochloride). | [18,70,87] |
| Ofloxacin (2nd) | 200–400 mg twice a day (every 12 h); 3 days to 6 weeks, depending on the severity and nature of the infection. | Similar to ciprofloxacin. In addition, Chlamydia or Chlamydophila infections include nongonococcal urethritis and mycobacterial infections (leprosy and tuberculosis); Oral (as a base) and parenteral administration (as a hydrochloride salt). | [18,70] |
| Pefloxacin (2nd) | 400 mg twice daily (every 12 h); similar to norfloxacin. | Uncomplicated gonococcal urethritis in males, Gram-negative bacterial infections in the gastrointestinal system and the genitourinary tract; Oral and parenteral administration (as a mesylate salt). | [18,21,85,88] |
| Nadifloxacin (topical use) (2nd) | Twice a day as cream or ointment (1%). | Acne vulgaris and other skin infections; Topical use. | [45,70,71,89] |
| Levofloxacin (3rd) | 250–500 mg (once or twice daily); 7 to 14 days, depending on the severity and nature of the infection. | Acute and chronic bronchitis, exacerbated forms, acquired pneumonia (nosocomial), and other susceptible infections, including tuberculosis; Oral and parenteral administration (as a hemihydrate); Ophthalmic use (0.5% ophthalmic solution). | [18,70,74,90,91] |
| Gatifloxacin (ophthalmic use) (3rd) | Day 1:1 drop every 2 h in the affected eye(s) while awake, up to 8 times Day 2 to 7:1 drop twice to 4 times daily in the affected eye(s) while awake. | Bacterial conjunctivitis, ophthalmic use (0.3% or 0.5% ophthalmic solution). | [92,93,94] |
| Moxifloxacin (4th) | Oral: 400 mg once a day; 5–10 days depending on the severity and nature of the infection; Ophthalmic administration: one drop in the affected eye 3 times daily for 7 days. | Sexually transmitted diseases, prostatitis, skin and tissue infections, acute and chronic bronchitis, exacerbated forms, acquired pneumonia (nosocomial), intra-abdominal infections, gynecological infections, bacterial conjunctivitis; Oral, parenteral, and ophthalmic administration (0.5%) as a hydrochloride salt. | [75,77,95,96,97] |
| Delafloxacin (4th) | Intravenous: 300 mg over 60 min, every 12 h; Oral: 450 mg every 12 h; 5 to 14 days. | Bacterial skin and skin structure infections; Oral and intravenous administration. | [23,98,99] |
| Besifloxacin (topical, ophthalmic use) (4th) | Ophthalmic administration: 1 drop in the affected eye 3 times daily, 4 to 12 h apart for 7 days. | Bacterial conjunctivitis; Ophthalmic suspension (0.6%). | [22,100,101] |
| Finafloxacin (topical, ophthalmic use) (4th) | Optic administration: 4 drops in the affected ear(s) twice daily for 7 days. | Acute otitis externa; Optic suspension (0.3%). | [83,102,103] |
| FQNs | Single Dose p.o. 1 (g) | Plasmatic Concentration (μg/mL) | Half-Life (Hours) | Binding to Plasma Proteins (%) | Elimination Route | References |
|---|---|---|---|---|---|---|
| Avarofloxacin | 0.25 | 2 | 14 | 65 | renal | [110] |
| Ciprofloxacin | 0.2 | 0.8 | 4–6 | 20–50 | renal, hepatic, feces | [13,18,111,112] |
| Delafloxacin | 0.45 | 5.80–7.17 | 4.2–14.9 | 84 | renal | [98,99,113] |
| Enoxacin * | 0.20 | 1.0 | 5 | 40–60 | renal, hepatic | [13,18,25,112] |
| Fleroxacin * | 0.4 | 5.0 | 10–12 | 23 | renal, hepatic | [25,114] |
| Gatifloxacin * | 0.20 | 2.0 | 7.8 | 20 | renal | [13,25,75,112] |
| Gemifloxacin * | 0.32 | 1.6 | 6.9 | 60–70 | renal and others | [13,25,75] |
| Grepafloxacin * | 0.40 | 0.93 | 12 | 50 | hepatic, renal | [13,25] |
| Lomefloxacin * | 0.2 | 0.7 | 3–4 | 10 | renal | [18,25,112] |
| Levofloxacin | 0.50 | 6.2–8.7 | 6–7 | 24–40 | renal | [13,18,111] |
| (Ala)Levonadifloxacin | 1 | 16.5 | 4.5 | 85 | - | [115] |
| Moxifloxacin | 0.40 | 4.5 | 12 | 30–50 | hepatic, renal | [13,75,112] |
| Nalidixic acid | 1.00 | 20–40 | 6–7 | 93–97 | renal | [13,18,112] |
| Nemonoxacin | 0.5 | 7.02 | 15 | 16 | renal | [116] |
| Norfloxacin | 0.40 | 1.5–2 | 4–8 | 15 | renal, hepatic, feces | [18,85,112] |
| Ofloxacin | 0.20 | 1.5 | 4.5–9 | 32–40 | renal | [13,18,112] |
| Pefloxacin | 0.40 | 3.9–5.8 | 8–13 | 20–30 | hepatic, renal, feces | [117] |
| Sparfloxacin * | 0.40 | 1.1–1.3 | 20 | 40–50 | renal, hepatic | [13,18,25,111,112] |
| Temafloxacin * | 0.60 | 2.43 | 8 | 25 | hepatic, renal | [13,25,118] |
| Trovafloxacin * | 0.10 | 1.0 | 9.1 | 76–85 | hepatic | [13,25,112] |
| Zabofloxacin | 0.4 | 2.0 | 8.24–8.32 | NA 2 | NA 2 | [109,113,116,119] |
| Type | Hybrid (Commercial Name) | Unit 1 (Class) | Linker | Unit 2 (Class) | Possible Indications and Dosage | References |
|---|---|---|---|---|---|---|
| AB-LK-AB | Cadazolid | Tedizolid (oxazolidinones) | NC | Ciprofloxacin (FQNs) | Clostridium difficile-associated diarrhea—Phase 1 clinical trial—single oral dose of 3000 mg | [207,236,237] |
| TNP-2092 (CBR-2092) | Rifamycin (ansamycins) | NC | Ciprofloxacin derivative (FQNs) | Gastrointestinal and liver disorders—Clostridium difficile infection model—6.67 mg/kg, orally, 7 days, Acute bacterial skin and skin structure infection—Phase 2 clinical trial—300 mg intravenously, every 12 h | [238,239,240,241] | |
| Cefilavancin (TD-1792) | Vancomycin (glycopeptide antibiotics) | NC | THRX-169797 (cephalosporins) | Gram-positive complicated skin and skin structure infections—Phase 2 clinical trial—2 mg/kg/day, intravenously | [233,242,243,244,245] | |
| TD-1607 | Vancomycin (glycopeptide antibiotics) | C | THRX-169797 (cephalosporins) | Infections with Gram-positive bacteria—Phase 1 clinical trials to evaluate the tolerability, safety, and pharmacokinetics—single escalating doses, intravenously | [233,246] | |
| TNP-2198 | Rifamycin (ansamycins) | NC | Metronidazole | Helicobacter pylori infection (mouse model), Clostridium difficile infection (hamster model)—5, 15, and 45 mg/kg/day, orally, 5 days;bacterial vaginosis | [233,247] | |
| MCB-3681 | Linezolid (oxazolidinones) | NC | Ciprofloxacin derivative (FQNs) | Infections with Gram-positive bacteria—multiple-dose phase 1 study—6 mg/kg body weight over 12 h for 5 days, intravenously | [248] | |
| AB-LK-NAB | Cefiderocol (Fetroja) | Ceftazidime (cephalosporins) | NC | 2-chloro-3,4-dihydroxybenzoic acid (catechol derivative; siderophore) | Complicated UTI and severe carbapenem-resistant Gram-negative bacterial infection—Phase 3 clinical trial—2 g intravenously over 3 h every 8 h for a period of 7 to 14 days, or 2 g every 6 h for participants with creatinine clearance >120 mL/min | [207,249,250,251,252] |
| - | Ampicillin/ Amoxycillin | NC | Enterobactin (catecholate siderophore) | Escherichia coli Infections—microbiological assay | [253] | |
| - | Ampicillin | NC | Tetramic acid(s) | Gram-negative bacterial infections—microbiological assay | [254] | |
| DSTA4637S | 4-Dimethylaminopiperidino-hydroxybenzoxazino rifamycin (ansamycins) | C | Thiomab human immunoglobulin G1 (IgG1) monoclonal antibody | Staphylococcus aureus infections—Phase 1 clinical trials—low-, intermediate-, and high-dose intravenous infusion | [233,255,256,257,258] |
| Type of Hybrid | Compound Code | Microorganism | MIC | Reference |
|---|---|---|---|---|
| QN-FQN | 10f | Staphylococcus aureus | 3.3 μM | [265] |
| 10b | Streptococcus pyogenes | 7.8 μM | ||
| 11a | Salmonella typhi | 7.6 μM | ||
| 11b | 7.4 μM | |||
| N-alkylations of the C-7 chain of QN | 7l | Mycobacterium tuberculosis H37Rv and multi-drug-resistant Mycobacterium tuberculosis | 0.09 μM | [266] |
| Oxazolidinone-FQN | 2, 5 and 6 | Staphylococcus aureus Enterococcus faecium | ≤1 μg/mL | [212] |
| Tetracycline-FQN | 10 | Mycobacterium tuberculosis | 0.2 μg/mL | [267] |
| Rifamycin-QN | CBR-2092 | 300 clinical isolates of staphylococci and streptococci | 0.008–0.5 μg/mL | [268] |
| Aminoglycoside-FQN | 1i | Escherichia coli (R477-100, ATCC 25922, AG100B, AG100A) | 0.75–3 μg/mL | [216] |
| 1q | 0.38–12 μg/mL | |||
| Azithromycin-QN | 7f | Streptococcus pyogenes | 0.5 μg/mL | [269] |
| 8f | 1 μg/mL | |||
| 7f | Haemophilus influenzae B 0529 | 0.5 μg/mL | ||
| 8f | 0.5 μg/mL | |||
| Aminoglycoside-FQN | 1 | Staphylococcus aureus and methicillin-resistant Staphylococcus aureus | 1 μg/mL | [211] |
| three Pseudomonas aeruginosa strains (including two gentamicin-resistant Pseudomonas aeruginosa strains) | 4−8 μg/mL | |||
| Aminoglycoside-FQN | 1m | Escherichia coli | 6.2 ± 0.7 μM (day 1) 30.3 ± 3.4 μM (day 17) | [270] |
| Aminoglycoside-FQN | 1b | Escherichia coli (R477-100, 25922, AG100B, AG100A) | 0.37–12 μg/mL | [210] |
| Bacillus subtilis | 1.5 μg/mL | |||
| ATP-competitive inhibitors (for DNA Gyrase A and B)-FQN | 3a | Klebsiella pneumoniae | 0.5 µg/mL | [271] |
| Enterobacter cloacae | 4 µg/mL | |||
| Escherichia coli | 2 µg/mL | |||
| 3-arylfuran-2(5H)-one-FQN | 11 | Multiple drug-resistant Escherichia coli | 0.11 μg/mL | [272] |
| Benzimidazole-QN | 5b | Pseudomonas aeruginosa | 1 μg/mL | [219] |
| Staphylococcus aureus and methicillin-resistant Staphylococcus aureus | 8 μg/mL | |||
| Klebsiella pneumoniae | 16 μg/mL | |||
| Benzofuroxane-FQN | 4d | Bacillus cereus 8035 | 0.97 μg/mL | [217] |
| Flavonoids (naringenin)-FQN | 7 | Escherichia coli | 0.71 μg/mL | [273] |
| Bacillus subtilis | 0.062 μg/mL | |||
| Staphylococcus aureus | 0.29 μg/mL | |||
| Candida albicans | 0.14 μg/mL | |||
| 1,3,4-Oxadiazole-FQN | 4 b–d | Staphylococcus aureus | ≤0.125 μg/mL | [274] |
| Sulfonamide-FQN | 3a | Staphylococcus aureus | 0.324 μM | [275] |
| Escherichia coli ATCC8739 | 0.025 μM | |||
| 3b | Staphylococcus aureus | 0.422 μM | ||
| Escherichia coli ATCC8739 | 0.013 μM | |||
| Triazole-FQN | 11 | Candida albicans | 10.23 µg/mL | [276] |
| Trimethoprim-FQN | BP-4Q-002 | Staphylococcus aureus | 0.5 μg/mL | [277] |
| Escherichia coli | 1 μg/mL | |||
| Staphylococcus aureus NRS19 (resistant to ciprofloxacin) | 1 μg/mL |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lungu, I.-A.; Moldovan, O.-L.; Biriș, V.; Rusu, A. Fluoroquinolones Hybrid Molecules as Promising Antibacterial Agents in the Fight against Antibacterial Resistance. Pharmaceutics 2022, 14, 1749. https://doi.org/10.3390/pharmaceutics14081749
Lungu I-A, Moldovan O-L, Biriș V, Rusu A. Fluoroquinolones Hybrid Molecules as Promising Antibacterial Agents in the Fight against Antibacterial Resistance. Pharmaceutics. 2022; 14(8):1749. https://doi.org/10.3390/pharmaceutics14081749
Chicago/Turabian StyleLungu, Ioana-Andreea, Octavia-Laura Moldovan, Victoria Biriș, and Aura Rusu. 2022. "Fluoroquinolones Hybrid Molecules as Promising Antibacterial Agents in the Fight against Antibacterial Resistance" Pharmaceutics 14, no. 8: 1749. https://doi.org/10.3390/pharmaceutics14081749
APA StyleLungu, I. -A., Moldovan, O. -L., Biriș, V., & Rusu, A. (2022). Fluoroquinolones Hybrid Molecules as Promising Antibacterial Agents in the Fight against Antibacterial Resistance. Pharmaceutics, 14(8), 1749. https://doi.org/10.3390/pharmaceutics14081749