RETRACTED: Amitriptyline-Based Biodegradable PEG-PLGA Self-Assembled Nanoparticles Accelerate Cutaneous Wound Healing in Diabetic Rats
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Preparation of Amitrip Self-Assembled PEG-PLGA Biodegradable NPs
2.3. Characterization of Amitrip-NPs’ Size
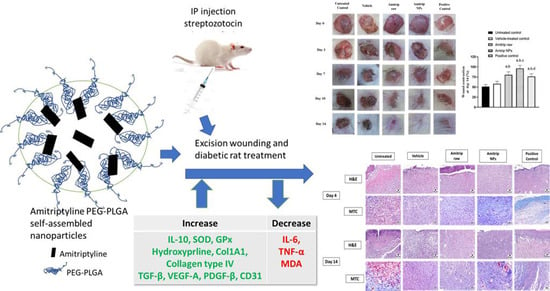
2.4. In Vivo Study
2.5. Excision Wounding and Animal Treatment
2.6. Wound Contraction Calculation
2.7. Preparation of Tissue Homogenate
2.8. Biochemical Analysis
2.9. Quantitative Real-Time PCR (qRT-PCR)
2.10. Histopathology
2.11. Determination of IL-6, TNF-α, IL-10, TGF-β1, VEGF-A and PDGF-B, Collagen IV and CD31 Immunohistochemically
2.12. Statistical Analysis
3. Results
3.1. Preparation of Amitrip Self-Assembled PEG-PLGA Biodegradable NPs
3.2. Wound Healing Assessment
3.3. Histopathological Analysis
3.4. Effect of Amitrip-NPs on Expression of Inflammatory Biomarkers
3.5. Effect of Amitrip-NPs on Biomarkers on Oxidative Status
3.6. Effect of Amitrip-NPs on Collagen Deposition Markers
3.7. Effect of Amitrip-NPs on Expression of TGF-β1, VEGF-A and PDGF-B
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wagner, R.; Heni, M.; Tabák, A.G.; Machann, J.; Schick, F.; Randrianarisoa, E.; Hrabě de Angelis, M.; Birkenfeld, A.L.; Stefan, N.; Peter, A.; et al. Pathophysiology-based subphenotyping of individuals at elevated risk for type 2 diabetes. Nat. Med. 2021, 27, 49–57. [Google Scholar] [CrossRef] [PubMed]
- Patel, S.; Srivastava, S.; Singh, M.R.; Singh, D. Mechanistic insight into diabetic wounds: Pathogenesis, molecular targets and treatment strategies to pace wound healing. Biomed Pharm. 2019, 112, 108615. [Google Scholar] [CrossRef] [PubMed]
- Wan, R.; Weissman, J.P.; Grundman, K.; Lang, L.; Grybowski, D.J.; Galiano, R.D. Diabetic wound healing: The impact of diabetes on myofibroblast activity and its potential therapeutic treatments. Wound Repair Regen. 2021, 29, 573–581. [Google Scholar] [CrossRef] [PubMed]
- Baltzis, D.; Eleftheriadou, I.; Veves, A. Pathogenesis and Treatment of Impaired Wound Healing in Diabetes Mellitus: New Insights. Adv. Ther. 2014, 31, 817–836. [Google Scholar] [CrossRef]
- Sen, C.K. Human Wound and Its Burden: Updated 2020 Compendium of Estimates. Adv. Wound Care 2021, 10, 281–292. [Google Scholar] [CrossRef]
- Nussbaum, S.R.; Carter, M.J.; Fife, C.E.; DaVanzo, J.; Haught, R.; Nusgart, M.; Cartwright, D. An Economic Evaluation of the Impact, Cost, and Medicare Policy Implications of Chronic Nonhealing Wounds. Value Health 2018, 21, 27–32. [Google Scholar] [CrossRef]
- Bhattacharya, D.; Ghosh, B.; Mukhopadhyay, M. Development of nanotechnology for advancement and application in wound healing: A review. IET Nanobiotechnol. 2019, 13, 778–785. [Google Scholar] [CrossRef]
- Lawson, K. A brief review of the pharmacology of amitriptyline and clinical outcomes in treating fibromyalgia. Biomedicines 2017, 5, 24. [Google Scholar] [CrossRef]
- Mercadante, S. Topical amitriptyline and ketamine for the treatment of neuropathic pain. Expert Rev. Neurother. 2015, 15, 1249–1253. [Google Scholar] [CrossRef]
- Alhakamy, N.A.; Caruso, G.; Privitera, A.; Ahmed, O.A.A.; Fahmy, U.A.; Md, S.; Mohamed, G.A.; Ibrahim, S.R.M.; Eid, B.G.; Abdel-Naim, A.B.; et al. Fluoxetine Ecofriendly Nanoemulsion Enhances Wound Healing in Diabetic Rats: In Vivo Efficacy Assessment. Pharmaceutics 2022, 14, 1133. [Google Scholar] [CrossRef]
- Penn, J.W.; Grobbelaar, A.O.; Rolfe, K.J. The role of the TGF-β family in wound healing, burns and scarring: A review. Int. J. Burns Trauma 2012, 2, 18–28. [Google Scholar] [PubMed]
- Olianas, M.C.; Dedoni, S.; Onali, P. Antidepressants induce profibrotic responses via the lysophosphatidic acid receptor LPA1. Eur. J. Pharmacol. 2020, 873, 172963. [Google Scholar] [CrossRef] [PubMed]
- Bai, Q.; Han, K.; Dong, K.; Zheng, C.; Zhang, Y.; Long, Q.; Lu, T. Potential applications of nanomaterials and technology for diabetic wound healing. Int. J. Nanomed. 2020, 15, 9717–9743. [Google Scholar] [CrossRef] [PubMed]
- Ali, A.; Ahmed, S. A review on chitosan and its nanocomposites in drug delivery. Int. J. Biol. Macromol. 2018, 109, 273–286. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.P.; Pei, Y.Y.; Zhang, X.Y.; Gu, Z.H.; Zhou, Z.H.; Yuan, W.F.; Zhou, J.J.; Zhu, J.H.; Gao, X.J. PEGylated PLGA nanoparticles as protein carriers: Synthesis, preparation and biodistribution in rats. J. Control. Release 2001, 71, 203–211. [Google Scholar] [CrossRef]
- Chen, S.; Cheng, S.X.; Zhuo, R.X. Self-Assembly Strategy for the Preparation of Polymer-Based Nanoparticles for Drug and Gene Delivery. Macromol. Biosci. 2011, 11, 576–589. [Google Scholar] [CrossRef]
- Ashjari, M.; Khoee, S.; Mahdavian, A.R.; Rahmatolahzadeh, R. Self-assembled nanomicelles using PLGA-PEG amphiphilic block copolymer for insulin delivery: A physicochemical investigation and determination of CMC values. J. Mater. Sci. Mater. Med. 2012, 23, 943–953. [Google Scholar] [CrossRef]
- Yueying, H.; Yan, Z.; Chunhua, G.; Weifeng, D.; Meidong, L. Micellar carrier based on methoxy poly(ethylene glycol)-block-poly(ε- caprolactone) block copolymers bearing ketone groups on the polyester block for doxorubicin delivery. J. Mater. Sci. Mater. Med. 2010, 21, 567–574. [Google Scholar] [CrossRef]
- Rafiei, P.; Haddadi, A. Docetaxel-loaded PLGA and PLGA-PEG nanoparticles for intravenous application: Pharmacokinetics and biodistribution profile. Int. J. Nanomed. 2017, 12, 935–947. [Google Scholar] [CrossRef]
- Khalil, N.M.; do Nascimento, T.C.F.; Casa, D.M.; Dalmolin, L.F.; de Mattos, A.C.; Hoss, I.; Romano, M.A.; Mainardes, R.M. Pharmacokinetics of curcumin-loaded PLGA and PLGA-PEG blend nanoparticles after oral administration in rats. Colloids Surf. B Biointerfaces 2013, 101, 353–360. [Google Scholar] [CrossRef]
- Anari, E.; Akbarzadeh, A.; Zarghami, N. Chrysin-loaded PLGA-PEG nanoparticles designed for enhanced effect on the breast cancer cell line. Artif. Cells Nanomed. Biotechnol. 2016, 44, 1410–1416. [Google Scholar] [CrossRef] [PubMed]
- Zhang, K.; Tang, X.; Zhang, J.; Lu, W.; Lin, X.; Zhang, Y.; Tian, B.; Yang, H.; He, H. PEG-PLGA copolymers: Their structure and structure-influenced drug delivery applications. J. Control. Release 2014, 183, 77–86. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, O.A.A.; Badr-Eldin, S.M. Biodegradable self-assembled nanoparticles of PEG-PLGA amphiphilic diblock copolymer as a promising stealth system for augmented vinpocetine brain delivery. Int. J. Pharm. 2020, 588, 119778. [Google Scholar] [CrossRef] [PubMed]
- Eid, B.G.; Alhakamy, N.A.; Fahmy, U.A.; Ahmed, O.A.A.; Md, S.; Abdel-Naim, A.B.; Caruso, G.; Caraci, F. Melittin and diclofenac synergistically promote wound healing in a pathway involving TGF-β1. Pharmacol. Res. 2022, 175, 105993. [Google Scholar] [CrossRef]
- Ahmed, O.A.A.; Afouna, M.I.; El-Say, K.M.; Abdel-Naim, A.B.; Khedr, A.; Banjar, Z.M. Optimization of self-nanoemulsifying systems for the enhancement of in vivo hypoglycemic efficacy of glimepiride transdermal patches. Expert Opin. Drug Deliv. 2014, 11, 1005–1013. [Google Scholar] [CrossRef]
- Abdel-Lateff, A.; Abdel-Naim, A.B.; Alarif, W.M.; Algandaby, M.M.; Alburae, N.A.; Alghamdi, A.M.; Nasrullah, M.Z.; Fahmy, U.A. Euryops arabicus Promotes Healing of Excised Wounds in Rat Skin: Emphasis on Its Collagen-Enhancing, Antioxidant, and Anti-Inflammatory Activities. Oxid. Med. Cell. Longev. 2021, 2021, 8891445. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Houreld, N.N. Shedding light on a new treatment for diabetic wound healing: A review on phototherapy. Sci. World J. 2014, 2014, 398412. [Google Scholar] [CrossRef]
- Rosique, R.G.; Rosique, M.J.; Farina Junior, J.A. Curbing inflammation in skin wound healing: A review. Int. J. Inflam. 2015, 2015, 316235. [Google Scholar] [CrossRef]
- McDonald, T.O.; Siccardi, M.; Moss, D.; Liptrott, N.; Giardiello, M.; Rannard, S.; Owen, A. The Application of Nanotechnology to Drug Delivery in Medicine. In Nanoengineering: Global Approaches to Health and Safety Issues; Elsevier: Amsterdam, The Netherlands, 2015; pp. 173–223. ISBN 9780444627452. [Google Scholar]
- De Jong, W.H.; Borm, P.J.A. Drug delivery and nanoparticles: Applications and hazards. Int. J. Nanomed. 2008, 3, 133–149. [Google Scholar] [CrossRef]
- Blanco, E.; Shen, H.; Ferrari, M. Principles of nanoparticle design for overcoming biological barriers to drug delivery. Nat. Biotechnol. 2015, 33, 941–951. [Google Scholar] [CrossRef] [PubMed]
- De Almeida Prado, P.S.; Soares, M.F.; Lima, F.O.; Schor, N.; Teixeira, V.P.C. Amitriptyline aggravates the fibrosis process in a rat model of infravesical obstruction. Int. J. Exp. Pathol. 2012, 93, 218–224. [Google Scholar] [CrossRef] [PubMed]
- Lichtman, M.K.; Otero-Vinas, M.; Falanga, V. Transforming growth factor beta (TGF-β) isoforms in wound healing and fibrosis. Wound Repair Regen. 2016, 24, 215–222. [Google Scholar] [CrossRef] [PubMed]
- Kany, S.; Vollrath, J.T.; Relja, B. Cytokines in inflammatory disease. Int. J. Mol. Sci. 2019, 20, 6008. [Google Scholar] [CrossRef]
- Sen, C.K. Wound healing essentials: Let there be oxygen. Wound Repair Regen. 2009, 17, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Dejban, P.; Sahraei, M.; Chamanara, M.; Dehpour, A.; Rashidian, A. Anti-inflammatory effect of amitriptyline in a rat model of acetic acid-induced colitis: The involvement of the TLR4/NF-kB signaling pathway. Fundam. Clin. Pharmacol. 2021, 35, 843–851. [Google Scholar] [CrossRef]
- Hajhashemi, V.; Sadeghi, H.; Minaiyan, M.; Movahedian, A.; Talebi, A. The role of central mechanisms in the anti-inflammatory effect of amitriptyline on carrageenan-induced paw edema in rats. Clinics 2010, 65, 1183–1187. [Google Scholar] [CrossRef] [PubMed]
- Vismari, L.; Alves, G.J.; Palermo-Neto, J. Amitriptyline and acute inflammation: A study using intravital microscopy and the carrageenan-induced paw edema model. Pharmacology 2010, 86, 231–239. [Google Scholar] [CrossRef] [PubMed]
- Scheuermann, K.; Orellano, L.A.A.; Viana, C.T.R.; Machado, C.T.; Lazari, M.G.T.; Capettini, L.S.A.; Andrade, S.P.; Campos, P.P. Amitriptyline Downregulates Chronic Inflammatory Response to Biomaterial in Mice. Inflammation 2021, 44, 580–591. [Google Scholar] [CrossRef]
- Yaron, I.; Shirazi, I.; Judovich, R.; Levartovsky, D.; Caspi, D.; Yaron, M. Fluoxetine and amitriptyline inhibit nitric oxide, prostaglandin E2, and hyaluronic acid production in human synovial cells and synovial tissue cultures. Arthritis Rheum. 1999, 42, 2561–2568. [Google Scholar] [CrossRef]
- Saha, S.; Mishra, A. Rutin-loaded polymeric nanorods alleviate nephrolithiasis by inhibiting inflammation and oxidative stress in vivo and in vitro. Food Funct. 2022, 13, 3632–3648. [Google Scholar] [CrossRef] [PubMed]
- Bilgen, F.; Ural, A.; Kurutas, E.B.; Bekerecioglu, M. The effect of oxidative stress and Raftlin levels on wound healing. Int. Wound J. 2019, 16, 1178–1184. [Google Scholar] [CrossRef] [PubMed]
- Deng, L.; Du, C.; Song, P.; Chen, T.; Rui, S.; Armstrong, D.G.; Deng, W. The Role of Oxidative Stress and Antioxidants in Diabetic Wound Healing. Oxid. Med. Cell. Longev. 2021, 2021, 8852759. [Google Scholar] [CrossRef]
- Kandil, E.A.; Abdelkader, N.F.; El-Sayeh, B.M.; Saleh, S. Imipramine and amitriptyline ameliorate the rotenone model of Parkinson’s disease in rats. Neuroscience 2016, 332, 26–37. [Google Scholar] [CrossRef]
- Zhang, L.; Peng, X.; Ai, Y.; Li, L.; Zhao, S.; Liu, Z.; Peng, Q.; Deng, S.; Huang, Y.; Mo, Y.; et al. Amitriptyline Reduces Sepsis-Induced Brain Damage Through TrkA Signaling Pathway. J. Mol. Neurosci. 2020, 70, 2049–2057. [Google Scholar] [CrossRef] [PubMed]
- Kolla, N.; Wei, Z.; Richardson, J.S.; Li, X.M. Amitriptyline and fluoxetine protect PC12 cells from cell death induced by hydrogen peroxide. J. Psychiatry Neurosci. 2005, 30, 196–201. [Google Scholar]
- Wang, L.; Xu, L.; Du, J.; Zhao, X.; Liu, M.; Feng, J.; Hu, K. Nose-to-brain delivery of borneol modified tanshinone IIA nanoparticles in prevention of cerebral ischemia/reperfusion injury. Drug Deliv. 2021, 28, 1363–1375. [Google Scholar] [CrossRef]
- Bainbridge, P. Wound healing and the role of fibroblasts. J. Wound Care 2013, 22, 407–412. [Google Scholar]
- Ibrahim, N.‘I.; Wong, S.K.; Mohamed, I.N.; Mohamed, N.; Chin, K.Y.; Ima-Nirwana, S.; Shuid, A.N. Wound healing properties of selected natural products. Int. J. Environ. Res. Public Health 2018, 15, 2360. [Google Scholar] [CrossRef]
- Morikawa, M.; Derynck, R.; Miyazono, K. TGF-β and the TGF-β family: Context-dependent roles in cell and tissue physiology. Cold Spring Harb. Perspect. Biol. 2016, 8, a021873. [Google Scholar] [CrossRef]
- den Dekker, A.; Davis, F.M.; Kunkel, S.L.; Gallagher, K.A. Targeting epigenetic mechanisms in diabetic wound healing. Transl. Res. 2019, 204, 39–50. [Google Scholar] [CrossRef] [PubMed]
- Tonnesen, M.G.; Feng, X.; Clark, R.A.F. Angiogenesis in wound healing. J. Investig. Dermatol. Symp. Proc. 2000, 5, 40–46. [Google Scholar] [CrossRef] [PubMed]
- Veith, A.P.; Henderson, K.; Spencer, A.; Sligar, A.D.; Baker, A.B. Therapeutic strategies for enhancing angiogenesis in wound healing. Adv. Drug Deliv. Rev. 2019, 146, 97–125. [Google Scholar] [CrossRef] [PubMed]
- Barrientos, S.; Stojadinovic, O.; Golinko, M.S.; Brem, H.; Tomic-Canic, M. Growth factors and cytokines in wound healing. Wound Repair Regen. 2008, 16, 585–601. [Google Scholar] [CrossRef]
- Boku, S.; Hisaoka-Nakashima, K.; Nakagawa, S.; Kato, A.; Kajitani, N.; Inoue, T.; Kusumi, I.; Takebayashi, M. Tricyclic antidepressant amitriptyline indirectly increases the proliferation of adult dentate gyrus-derived neural precursors: An involvement of astrocytes. PLoS ONE 2013, 8, e79371. [Google Scholar]
- Olianas, M.C.; Dedoni, S.; Onali, P. LPA1 mediates antidepressant-induced ERK1/2 signaling and protection from oxidative stress in glial cells. J. Pharmacol. Exp. Ther. 2016, 359, 340–353. [Google Scholar] [CrossRef]
- Volmer-Thole, M.; Lobmann, R. Neuropathy and diabetic foot syndrome. Int. J. Mol. Sci. 2016, 17, 917. [Google Scholar] [CrossRef]
- Barker, A.R.; Rosson, G.D.; Dellon, A.L. Wound healing in denervated tissue. Ann. Plast. Surg. 2006, 57, 339–342. [Google Scholar] [CrossRef]
- Generini, S.; Tuveri, M.A.; Matucci Cerinic, M.; Mastinu, F.; Manni, L.; Aloe, L. Topical application of nerve growth factor in human diabetic foot ulcers. A study of three cases. Exp. Clin. Endocrinol. Diabetes 2004, 112, 542–544. [Google Scholar] [CrossRef]
- Rafehi, H.; El-Osta, A.; Karagiannis, T.C. Epigenetic mechanisms in the pathogenesis of diabetic foot ulcers. J. Diabetes Complicat. 2012, 26, 554–561. [Google Scholar] [CrossRef]
- Moore, R.A.; Derry, S.; Aldington, D.; Cole, P.; Wiffen, P.J. Amitriptyline for neuropathic pain and fibromyalgia in adults. Cochrane Database Syst. Rev. 2012, 12, CD008242. [Google Scholar] [PubMed]
- Tran, N.Q.V.; Nguyen, A.N.; Takabe, K.; Yamagata, Z.; Miyake, K. Pre-treatment with amitriptyline causes epigenetic up-regulation of neuroprotection-associated genes and has anti-apoptotic effects in mouse neuronal cells. Neurotoxicol. Teratol. 2017, 62, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.R.; Liu, Z.W.; Peng, S.; Duan, M.Y.; Feng, J.W.; Wang, W.F.; Xu, Y.H.; Tang, X.; Zhang, X.Z.; Ren, B.X.; et al. The Neuroprotective Effect of Amitriptyline on Radiation-Induced Impairment of Hippocampal Neurogenesis. Dose-Response 2019, 17, 1559325819895912. [Google Scholar] [CrossRef] [PubMed]






| Gene | NCBI Reference Sequence | ||
|---|---|---|---|
| Col1A1 | Forward | ATCAGCCCAAACCCCAAGGAGA | NM_053304.1 |
| Reverse | CGCAGGAAGGTCAGCTGGATAG | ||
| GAPDH | Forward | CCATTCTTCCACCTTTGATGCT | NM_017008.4 |
| Reverse | TGTTGCTGTAGCCATATTCATTGT |
| Group | Re-Epithelization | Fibroblast Proliferation | Collagen Deposition | Inflammatory Cell Infiltration | Phase I | Phase II | Phase III |
|---|---|---|---|---|---|---|---|
| Untreated control | - | +++ | ++ | ++ | +++ | ++ | - |
| Vehicle-treated | - | +++ | ++ | ++ | ++ | ++ | - |
| Raw Amitrip | + | + | ++ | + | + | +++ | + |
| Amitrip-NPs | ++ | + | +++ | +/- | + | +++ | ++ |
| Positive control | ++ | + | ++ | + | + | ++ | + |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Asfour, H.Z.; Alhakamy, N.A.; Ahmed, O.A.A.; Fahmy, U.A.; El-moselhy, M.A.; Rizg, W.Y.; Alghaith, A.F.; Eid, B.G.; Abdel-Naim, A.B. RETRACTED: Amitriptyline-Based Biodegradable PEG-PLGA Self-Assembled Nanoparticles Accelerate Cutaneous Wound Healing in Diabetic Rats. Pharmaceutics 2022, 14, 1792. https://doi.org/10.3390/pharmaceutics14091792
Asfour HZ, Alhakamy NA, Ahmed OAA, Fahmy UA, El-moselhy MA, Rizg WY, Alghaith AF, Eid BG, Abdel-Naim AB. RETRACTED: Amitriptyline-Based Biodegradable PEG-PLGA Self-Assembled Nanoparticles Accelerate Cutaneous Wound Healing in Diabetic Rats. Pharmaceutics. 2022; 14(9):1792. https://doi.org/10.3390/pharmaceutics14091792
Chicago/Turabian StyleAsfour, Hani Z., Nabil A. Alhakamy, Osama A. A. Ahmed, Usama A. Fahmy, Mohamed A. El-moselhy, Waleed Y. Rizg, Adel F. Alghaith, Basma G. Eid, and Ashraf B. Abdel-Naim. 2022. "RETRACTED: Amitriptyline-Based Biodegradable PEG-PLGA Self-Assembled Nanoparticles Accelerate Cutaneous Wound Healing in Diabetic Rats" Pharmaceutics 14, no. 9: 1792. https://doi.org/10.3390/pharmaceutics14091792
APA StyleAsfour, H. Z., Alhakamy, N. A., Ahmed, O. A. A., Fahmy, U. A., El-moselhy, M. A., Rizg, W. Y., Alghaith, A. F., Eid, B. G., & Abdel-Naim, A. B. (2022). RETRACTED: Amitriptyline-Based Biodegradable PEG-PLGA Self-Assembled Nanoparticles Accelerate Cutaneous Wound Healing in Diabetic Rats. Pharmaceutics, 14(9), 1792. https://doi.org/10.3390/pharmaceutics14091792