Preparation and Characterization of Pazopanib Hydrochloride-Loaded Four-Component Self-Nanoemulsifying Drug Delivery Systems Preconcentrate for Enhanced Solubility and Dissolution
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. HPLC Conditions
2.3. pH Solubility Test
2.4. Solubility Test in Excipients
2.5. Preparation and Characterization of Two-Component Vehicle (TwCV)
2.6. Preparation and Characterization of Three-Component SNEDDS (TCS)
2.7. Preparation and Characterization of Four-Component SNEDDS (FCS)
2.8. Hydrodynamic Diameter Measurement
2.9. Dissolution Test
2.10. Transmission Electron Microscopy (TEM) Analysis
3. Results and Discussion
3.1. pH Solubility
3.2. Solubility in Excipients
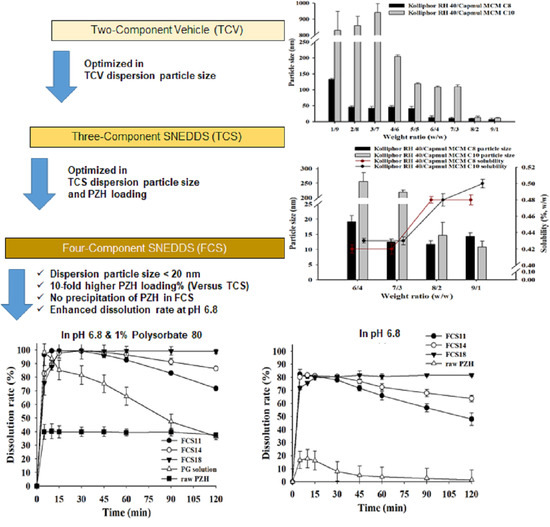
3.3. Characterization of Two-Component Vehicle (TwCV)
3.4. Characterization of Three-Component SNEDDS (TCS)
3.5. Characterization of Four-Component SNEDDS (FCS)
3.6. Dissolution Test
3.7. TEM Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Menezes, T.M.; da Silva Neto, A.M.; Gubert, P.; Neves, J.L. Effects of human serum albumin glycation on the interaction with the tyrosine kinase inhibitor pazopanib unveiled by multi-spectroscopic and bioinformatic tools. J. Mol. Liq. 2021, 340, 116843. [Google Scholar] [CrossRef]
- Méndez-Vidal, M.J.; Molina, Á.; Anido, U.; Chirivella, I.; Etxaniz, O.; Fernández-Parra, E.; Guix, M.; Hernández, C.; Lambea, J.; Montesa, Á.; et al. Pazopanib: Evidence review and clinical practice in the management of advanced renal cell carcinoma. BMC Pharmacol. Toxicol. 2018, 19, 77. [Google Scholar] [CrossRef] [PubMed]
- Herbrink, M.; Groenland, S.L.; Huitema, A.D.; Schellens, J.H.; Beijnen, J.H.; Steeghs, N.; Nuijen, B. Solubility and bioavailability improvement of pazopanib hydrochloride. Int. J. Pharm. 2018, 544, 181–190. [Google Scholar] [CrossRef] [PubMed]
- Heath, E.I.; Infante, J.; Lewis, L.D.; Luu, T.; Stephenson, J.; Tan, A.R.; Kasubhai, S.; LoRusso, P.; Ma, B.; Suttle, A.B.; et al. A randomized, double-blind, placebo-controlled study to evaluate the effect of repeated oral doses of pazopanib on cardiac conduction in patients with solid tumors. Cancer Chemother. Pharmacol. 2013, 71, 565–573. [Google Scholar] [CrossRef] [PubMed]
- Teo, Y.L.; Ho, H.K.; Chan, A. Risk of tyrosine kinase inhibitors-induced hepatotoxicity in cancer patients: A meta-analysis. Cancer Treat. Rev. 2013, 39, 199–206. [Google Scholar] [CrossRef] [PubMed]
- Sugihara, H.; Taylor, L.S. Evaluation of pazopanib phase behavior following pH-induced supersaturation. Mol. Pharm. 2018, 15, 1690–1699. [Google Scholar] [CrossRef]
- Choi, S.U.; Kim, M.J.; Kim, S.T.; Kim, H.C.; Cho, K.H.; Park, S.Y.; Lee, H.M.; Maeng, H.J.; Jang, D.J. Development of Self-Microemulsifying Drug Delivery Systems of Poorly Water-Soluble Pazopanib for Improvement of Oral Absorption. Sci. Adv. Mater. 2020, 12, 152–160. [Google Scholar] [CrossRef]
- Zhang, T.; Wells, E. A review of current methods for food effect prediction during drug development. Curr. Pharmacol. Rep. 2020, 6, 267–279. [Google Scholar] [CrossRef]
- Willemsen, A.E.; Lubberman, F.J.; Tol, J.; Gerritsen, W.R.; Van Herpen, C.M.; Van Erp, N.P. Effect of food and acid-reducing agents on the absorption of oral targeted therapies in solid tumors. Drug Discov. Today 2016, 21, 962–976. [Google Scholar] [CrossRef]
- Wagner, C.; Kesisoglou, F.; Pepin, X.J.; Parrott, N.; Emami Riedmaier, A. Use of physiologically based pharmacokinetic modeling for predicting drug–food interactions: Recommendations for improving predictive performance of low confidence food effect models. AAPS J. 2021, 23, 85. [Google Scholar] [CrossRef]
- Lubberman, F.J.; Gelderblom, H.; Hamberg, P.; Vervenne, W.L.; Mulder, S.F.; Jansman, F.G.; Colbers, A.; Winette, T.A.; van der Graaf, W.T.A.; Burger, D.M.; et al. The effect of using pazopanib with food vs. fasted on pharmacokinetics, patient safety, and preference (DIET study). Clin. Pharmacol. Ther. 2019, 106, 1076–1082. [Google Scholar] [CrossRef] [PubMed]
- Meola, T.R.; Bremmell, K.E.; Williams, D.B.; Schultz, H.B.; Prestidge, C.A. Bio-enabling strategies to mitigate the pharmaceutical food effect: A mini review. Int. J. Pharm. 2022, 619, 121695. [Google Scholar] [CrossRef] [PubMed]
- Poovi, G.; Damodharan, N. Lipid nanoparticles: A challenging approach for oral delivery of BCS Class-II drugs. Future J. Pharm. Sci. 2018, 4, 191–205. [Google Scholar] [CrossRef]
- Kim, W.; Kim, J.S.; Choi, H.G.; Jin, S.G.; Cho, C.W. Novel ezetimibe-loaded fibrous microparticles for enhanced solubility and oral bioavailability by electrospray technique. J. Drug Deliv. Sci. Technol. 2021, 66, 102877. [Google Scholar] [CrossRef]
- Kim, J.S.; Choi, Y.J.; Woo, M.R.; Cheon, S.; Ji, S.H.; Im, D.; Un Din, F.; Kim, J.O.; Youn, Y.S.; Oh, K.T.; et al. New potential application of hydroxypropyl-β-cyclodextrin in solid self-nanoemulsifying drug delivery system and solid dispersion. Carbohydr. Polym. 2021, 271, 118433. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.S.; Choi, H.G.; Jin, S.G. Influence of hydroxypropylmethylcellulose and sodium lauryl sulfate on the solubility and dissolution of sirolimus in solvent-evaporated solid dispersions. Bull. Korean Chem. Soc. 2018, 39, 778–783. [Google Scholar] [CrossRef]
- Bazzo, G.C.; Pezzini, B.R.; Stulzer, H.K. Eutectic mixtures as an approach to enhance solubility, dissolution rate and oral bioavailability of poorly water-soluble drugs. Int. J. Pharm. 2020, 588, 119741. [Google Scholar] [CrossRef]
- AboulFotouh, K.; Allam, A.A.; El-Badry, M.; El-Sayed, A.M. Role of self-emulsifying drug delivery systems in optimizing the oral delivery of hydrophilic macromolecules and reducing interindividual variability. Colloids Surf. B Biointerfaces 2018, 167, 82–92. [Google Scholar] [CrossRef]
- Sun, T.; Morger, A.; Castagner, B.; Leroux, J.C. An oral redox-sensitive self-immolating prodrug strategy. Chem. Commun. 2015, 51, 5721–5724. [Google Scholar] [CrossRef]
- Kim, D.H.; Kim, J.Y.; Kim, R.M.; Maharjan, P.; Ji, Y.G.; Jang, D.J.; Min, K.A.; Koo, T.; Cho, K.H. Orlistat-loaded solid SNEDDS for the enhanced solubility, dissolution, and in vivo performance. Int. J. Nanomed. 2018, 13, 7095–7106. [Google Scholar] [CrossRef] [Green Version]
- Mohammed, A.N.; Ishwarya, S.P.; Nisha, P. Nanoemulsion versus microemulsion systems for the encapsulation of beetroot extract: Comparison of physicochemical characteristics and betalain stability. Food Bioprocess Technol. 2021, 14, 133–150. [Google Scholar] [CrossRef]
- Miao, Y.; Zhao, S.; Zuo, J.; Sun, J.; Wang, J. Reduced the Food Effect and Enhanced the Oral Bioavailability of Ivacaftor by Self-Nanoemulsifying Drug Delivery System (SNEDDS) Using a New Oil Phase. Drug Des. Dev. Ther. 2022, 16, 1531–1546. [Google Scholar] [CrossRef] [PubMed]
- Cao, M.; Xue, X.; Pei, X.; Qian, Y.; Liu, L.; Ren, L.; Chen, G. Formulation optimization and pharmacokinetics evaluation of oral self-microemulsifying drug delivery system for poorly water soluble drug cinacalcet and no food effect. Drug Dev. Ind. Pharm. 2018, 44, 969–981. [Google Scholar] [CrossRef] [PubMed]
- Xue, X.; Cao, M.; Ren, L.; Qian, Y.; Chen, G. Preparation and optimization of rivaroxaban by self-nanoemulsifying drug delivery system (SNEDDS) for enhanced oral bioavailability and no food effect. AAPS PharmSciTech 2018, 19, 1847–1859. [Google Scholar] [CrossRef]
- Miao, Y.; Sun, J.; Chen, G.; Lili, R.; Ouyang, P. Enhanced oral bioavailability of lurasidone by self-nanoemulsifying drug delivery system in fasted state. Drug Dev. Ind. Pharm. 2016, 42, 1234–1240. [Google Scholar] [CrossRef]
- Christiansen, M.L.; Holm, R.; Kristensen, J.; Kreilgaard, M.; Jacobsen, J.; Abrahamsson, B.; Müllertz, A. Cinnarizine food-effects in beagle dogs can be avoided by administration in a Self Nano Emulsifying Drug Delivery System (SNEDDS). Eur. J. Pharm. Sci. 2014, 57, 164–172. [Google Scholar] [CrossRef]
- Buya, A.B.; Beloqui, A.; Memvanga, P.B.; Préat, V. Self-nano-emulsifying drug-delivery systems: From the development to the current applications and challenges in oral drug delivery. Pharmaceutics 2020, 12, 1194. [Google Scholar] [CrossRef]
- Elsayed, N.M.; Abou El Ella, D.A.; Serya, R.A.; Tolba, M.F.; Shalaby, R.; Abouzid, K.A. Design, synthesis and biological evaluation of indazole–pyrimidine based derivatives as anticancer agents with anti-angiogenic and antiproliferative activities. MedChemComm 2016, 7, 881–899. [Google Scholar] [CrossRef]
- Liu, D.Q.; Chen, T.K.; McGuire, M.A.; Kord, A.S. Analytical control of genotoxic impurities in the pazopanib hydrochloride manufacturing process. J. Pharm. Biomed. Anal. 2009, 50, 144–150. [Google Scholar] [CrossRef]
- Kim, Y.H.; Kim, Y.C.; Jang, D.J.; Min, K.A.; Karmacharya, J.; Nguyen, T.T.L.; Maeng, H.J.; Cho, K.H. Development of 20(S)-protopanaxadiol-loaded SNEDDS preconcentrate using comprehensive phase diagram for the enhanced dissolution and oral bioavailability. Pharmaceutics 2020, 12, 362. [Google Scholar] [CrossRef] [Green Version]
- Kim, R.M.; Jang, D.J.; Kim, Y.C.; Yoon, J.H.; Min, K.A.; Maeng, H.J.; Cho, K.H. Flurbiprofen-loaded solid SNEDDS preconcentrate for the enhanced solubility, in-vitro dissolution and bioavailability in rats. Pharmaceutics 2018, 10, 247. [Google Scholar] [CrossRef]
- Zhang, N.; Zhang, F.; Xu, S.; Yun, K.; Wu, W.; Pan, W. Formulation and evaluation of luteolin supersaturatable self-nanoemulsifying drug delivery system (S-SNEDDS) for enhanced oral bioavailability. J. Drug Deliv. Sci. Technol. 2020, 58, 101783. [Google Scholar] [CrossRef]
- Krstić, M.; Medarević, Đ.; Đuriš, J.; Ibrić, S. Self-nanoemulsifying drug delivery systems (SNEDDS) and self-microemulsifying drug delivery systems (SMEDDS) as lipid nanocarriers for improving dissolution rate and bioavailability of poorly soluble drugs. In Lipid Nanocarriers for Drug Targeting; William Andrew Publishing: Norwich, NY, USA, 2018; pp. 473–508. [Google Scholar]
- Choi, J.E.; Kim, J.S.; Choi, M.J.; Baek, K.; Woo, M.R.; Kim, J.O.; Choi, H.G.; Jin, S.G. Effects of different physicochemical characteristics and supersaturation principle of solidified SNEDDS and surface-modified microspheres on the bioavailability of carvedilol. Int. J. Pharm. 2021, 597, 120377. [Google Scholar] [CrossRef] [PubMed]
- Patil, S.C.; Tagalpallewar, A.A.; Kokare, C.R. Natural anti-proliferative agent loaded self-microemulsifying nanoparticles for potential therapy in oral squamous carcinoma. J. Pharm. Investig. 2019, 49, 527–541. [Google Scholar] [CrossRef]







| Test Solution | Solubility (μg/mL) |
|---|---|
| pH 1.2 buffer | 682.64 ± 7.58 |
| pH 4.0 buffer | 3.00 ± 0.25 |
| pH 6.8 buffer | 2.64 ± 1.02 |
| water | 144.08 ± 2.56 |
| FCS | ThCV Composition (%, w/w) | PZH Solubility (%, w/w) | Precipitation Occurrence | Particle Size of Water Dispersion (nm) | |||
|---|---|---|---|---|---|---|---|
| Kolliphor RH40 | Capmul MCM C8 | Capmul MCM C10 | Kollisolv PG | ||||
| FCS1 | 9 | 1 | 0 | 90 | 4.94 ± 0.05 | P | 84.07 ± 30.80 |
| FCS2 | 18 | 2 | 0 | 80 | 4.44 ± 0.10 | P | 15.07 ± 2.81 |
| FCS3 | 27 | 3 | 0 | 70 | 3.97 ± 0.06 | P | 9.70 ± 0.44 |
| FCS4 | 36 | 4 | 0 | 60 | 3.02 ± 0.07 | P | 13.03 ± 3.28 |
| FCS5 | 45 | 5 | 0 | 50 | 2.10 ± 0.06 | N | 11.33 ± 2.35 |
| FCS6 | 54 | 6 | 0 | 40 | 1.75 ± 0.12 | N | 10.27 ± 1.10 |
| FCS7 | 63 | 7 | 0 | 30 | 0.86 ± 0.10 | N | 13.60 ± 3.11 |
| FCS8 | 72 | 8 | 0 | 20 | 0.66 ± 0.05 | N | 12.55 ± 0.07 |
| FCS9 | 81 | 9 | 0 | 10 | 0.50 ± 0.08 | N | 13.60 ± 0.89 |
| FCS10 | 9 | 0 | 1 | 90 | 5.69 ± 0.06 | P | 16.33 ± 1.83 |
| FCS11 | 18 | 0 | 2 | 80 | 5.30 ± 0.13 | N | 14.47 ± 0.31 |
| FCS12 | 27 | 0 | 3 | 70 | 3.34 ± 0.11 | N | 13.57 ± 1.59 |
| FCS13 | 36 | 0 | 4 | 60 | 2.66 ± 0.08 | N | 15.28 ± 1.74 |
| FCS14 | 45 | 0 | 5 | 50 | 2.19 ± 0.04 | N | 16.30 ± 2.90 |
| FCS15 | 54 | 0 | 6 | 40 | 1.44 ± 0.09 | N | 16.73 ± 4.60 |
| FCS16 | 63 | 0 | 7 | 30 | 0.75 ± 0.15 | N | 12.57 ± 2.10 |
| FCS17 | 72 | 0 | 8 | 20 | 0.57 ± 0.06 | N | 10.70 ± 3.87 |
| FCS18 | 81 | 0 | 9 | 10 | 0.53 ± 0.07 | N | 10.43 ± 3.99 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Choi, S.A.; Park, E.J.; Lee, J.H.; Min, K.A.; Kim, S.T.; Jang, D.-J.; Maeng, H.-J.; Jin, S.G.; Cho, K.H. Preparation and Characterization of Pazopanib Hydrochloride-Loaded Four-Component Self-Nanoemulsifying Drug Delivery Systems Preconcentrate for Enhanced Solubility and Dissolution. Pharmaceutics 2022, 14, 1875. https://doi.org/10.3390/pharmaceutics14091875
Choi SA, Park EJ, Lee JH, Min KA, Kim ST, Jang D-J, Maeng H-J, Jin SG, Cho KH. Preparation and Characterization of Pazopanib Hydrochloride-Loaded Four-Component Self-Nanoemulsifying Drug Delivery Systems Preconcentrate for Enhanced Solubility and Dissolution. Pharmaceutics. 2022; 14(9):1875. https://doi.org/10.3390/pharmaceutics14091875
Chicago/Turabian StyleChoi, Seung Ah, Eun Ji Park, Jun Hak Lee, Kyoung Ah Min, Sung Tae Kim, Dong-Jin Jang, Han-Joo Maeng, Sung Giu Jin, and Kwan Hyung Cho. 2022. "Preparation and Characterization of Pazopanib Hydrochloride-Loaded Four-Component Self-Nanoemulsifying Drug Delivery Systems Preconcentrate for Enhanced Solubility and Dissolution" Pharmaceutics 14, no. 9: 1875. https://doi.org/10.3390/pharmaceutics14091875
APA StyleChoi, S. A., Park, E. J., Lee, J. H., Min, K. A., Kim, S. T., Jang, D. -J., Maeng, H. -J., Jin, S. G., & Cho, K. H. (2022). Preparation and Characterization of Pazopanib Hydrochloride-Loaded Four-Component Self-Nanoemulsifying Drug Delivery Systems Preconcentrate for Enhanced Solubility and Dissolution. Pharmaceutics, 14(9), 1875. https://doi.org/10.3390/pharmaceutics14091875