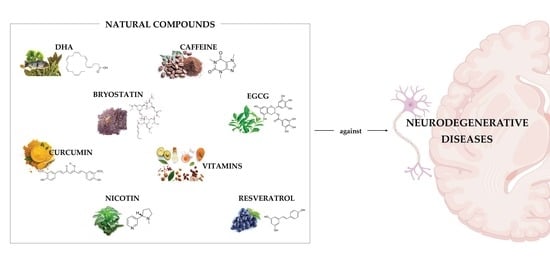
Therapeutic Potential of Natural Compounds in Neurodegenerative Diseases: Insights from Clinical Trials
Abstract
:1. Introduction
2. Most Prevalent Neurodegenerative Diseases
3. Role of Natural Compounds in the Treatment of Neurodegenerative Diseases
4. Natural Compounds in Clinical Trials for Neurodegenerative Diseases
4.1. Natural Compounds in Clinical Trials for Alzheimer’s Disease
4.2. Natural Compounds in Clinical Trials for Parkinson’s Disease
4.3. Natural Compounds in Clinical Trials for Multiple Sclerosis
4.4. Natural Compounds in Clinical Trials for Amyotrophic Lateral Sclerosis
4.5. Natural Compounds in Clinical Trials for Huntington’s Disease
5. Discussion and Concluding Remarks
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ramalho, M.J.; Andrade, S.; Dabur, M.; Loureiro, J.A.; Pereira, M.C. Current vs. Emerging Natural Com-Pounds-Based Treatments for Alzheimer’s Disease. Alzheimer’s Disease and Treatment; MedDocs Publishers: Reno, NV, USA, 2021. [Google Scholar]
- Andrade, S.; Ramalho, M.J.; Loureiro, J.A.; Pereira, M.C. Natural compounds for Alzheimer’s disease therapy: A systematic review of preclinical and clinical studies. Int. J. Mol. Sci. 2019, 20, 2313. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Khan, R.; Mahmood, A.; Ahmed, M. Biomarker Detection of Neurological Disorders through Spectroscopy Analysis. Int. Dent. Med. J. Adv. Res. 2018, 4, 1–9. [Google Scholar] [CrossRef]
- Farooqui, A.A. Neurochemical Aspects of Neurological Disorders. In Trace Amines and Neurological Disorders; Elsevier: Amsterdam, The Netherlands, 2016; pp. 237–256. [Google Scholar] [CrossRef]
- World Health Organization. The Global Dementia Observatory Reference Guide; World Health Organization: Geneva, Switzerland, 2018. [Google Scholar]
- Leong, Y.Q.; Ng, K.Y.; Chye, S.M.; Ling, A.P.K.; Koh, R.Y. Mechanisms of action of amyloid-beta and its precursor protein in neuronal cell death. Metab. Brain Dis. 2020, 35, 11–30. [Google Scholar] [CrossRef] [PubMed]
- International, A.s.D. World Alzheimer Report 2022 Life after Diagnosis: Navigating Treatment, Care and Support; Alzheimer’s Disease International: London, UK, 2022. [Google Scholar]
- Joe, E.; Ringman, J.M. Cognitive symptoms of Alzheimer’s disease: Clinical management and prevention. BMJ 2019, 367, 6217. [Google Scholar] [CrossRef] [Green Version]
- Ricciarelli, R.; Fedele, E. The Amyloid Cascade Hypothesis in Alzheimer’s Disease: It’s Time to Change Our Mind. Curr. Neuropharmacol. 2017, 15, 926–935. [Google Scholar] [CrossRef] [Green Version]
- Tolar, M.; Abushakra, S.; Sabbagh, M. The path forward in Alzheimer’s disease therapeutics: Reevaluating the amyloid cascade hypothesis. Alzheimer’s Dement. 2019, 16, 1553–1560. [Google Scholar] [CrossRef]
- Cabreira, V.; Massano, J. [Parkinson’s Disease: Clinical Review and Update]. Acta Med. Port. 2019, 32, 661–670. [Google Scholar] [CrossRef] [Green Version]
- Kouli, A.; Torsney, K.M.; Kuan, W.L. Parkinson’s Disease: Etiology, Neuropathology, and Pathogenesis. In Parkinson’s Disease: Pathogenesis and Clinical Aspects; Stoker, T.B., Greenland, J.C., Eds.; Codon Publications: Brisbane, Queensland, 2018. [Google Scholar] [CrossRef]
- World Health Organization. Parkinson Disease: A Public Health Approach: Technical Brief; World Health Organization: Geneva, Switzerland, 2022. [Google Scholar]
- Loureiro, J.A.; Gomes, B.; Coelho, M.A.N.; Carmo Pereira, M.D.; Rocha, S. Targeting nanoparticles across the blood–brain barrier with monoclonal antibodies. Nanomedicine 2014, 9, 709–722. [Google Scholar] [CrossRef]
- Poewe, W.; Seppi, K.; Tanner, C.M.; Halliday, G.M.; Brundin, P.; Volkmann, J.; Schrag, A.E.; Lang, A.E. Parkinson disease. Nat. Rev. Dis. Primers 2017, 3, 17013. [Google Scholar] [CrossRef]
- Beitz, J.M. Parkinson’s disease: A review. Front. Biosci. 2014, 6, 65–74. [Google Scholar] [CrossRef]
- Coetzee, T.; Thompson, A.J. Atlas of MS 2020: Informing global policy change. Mult. Scler. J. 2020, 26, 1807–1808. [Google Scholar] [CrossRef] [PubMed]
- Walton, C.; King, R.; Rechtman, L.; Kaye, W.; Leray, E.; Marrie, R.A.; Robertson, N.; La Rocca, N.; Uitdehaag, B.; van der Mei, I. Rising prevalence of multiple sclerosis worldwide: Insights from the Atlas of MS. Mult. Scler. J. 2020, 26, 1816–1821. [Google Scholar] [CrossRef]
- Kennedy, P.G.; George, W.; Yu, X. The Possible Role of Neural Cell Apoptosis in Multiple Sclerosis. Int. J. Mol. Sci. 2022, 23, 7584. [Google Scholar] [CrossRef] [PubMed]
- Silveira, C.; Guedes, R.; Maia, D.; Curral, R.; Coelho, R. Neuropsychiatric Symptoms of Multiple Sclerosis: State of the Art. Psychiatry Investig. 2019, 16, 877–888. [Google Scholar] [CrossRef]
- Alphonsus, K.B.; Su, Y.; D’Arcy, C. The effect of exercise, yoga and physiotherapy on the quality of life of people with multiple sclerosis: Systematic review and meta-analysis. Complement. Ther. Med. 2019, 43, 188–195. [Google Scholar] [CrossRef] [PubMed]
- Chen, P.C.; Wu, D.; Hu, C.J.; Chen, H.Y.; Hsieh, Y.C.; Huang, C.C. Exosomal TAR DNA-binding protein-43 and neurofilaments in plasma of amyotrophic lateral sclerosis patients: A longitudinal follow-up study. J. Neurol. Sci. 2020, 418, 117070. [Google Scholar] [CrossRef]
- Longinetti, E.; Fang, F. Epidemiology of amyotrophic lateral sclerosis: An update of recent literature. Curr. Opin. Neurol. 2019, 32, 771–776. [Google Scholar] [CrossRef]
- Oskarsson, B.; Horton, D.K.; Mitsumoto, H. Potential Environmental Factors in Amyotrophic Lateral Sclerosis. Neurol. Clin. 2015, 33, 877–888. [Google Scholar] [CrossRef]
- Hardiman, O.; Al-Chalabi, A.; Chio, A.; Corr, E.M.; Logroscino, G.; Robberecht, W.; Shaw, P.J.; Simmons, Z.; van den Berg, L.H. Amyotrophic lateral sclerosis. Nat. Rev. Dis. Primers 2017, 3, 17085. [Google Scholar] [CrossRef] [Green Version]
- Pondofe, K.; Marcelino, A.A.; Ribeiro, T.S.; Torres-Castro, R.; Vera-Uribe, R.; Fregonezi, G.A.; Resqueti, V.R. Effects of respiratory physiotherapy in patients with amyotrophic lateral sclerosis: Protocol for a systematic review of randomised controlled trials. BMJ Open 2022, 12, e061624. [Google Scholar] [CrossRef]
- Finkbeiner, S. Huntington’s Disease. Cold Spring Harb. Perspect. Biol. 2011, 3, a007476. [Google Scholar] [CrossRef] [Green Version]
- Medina, A.; Mahjoub, Y.; Shaver, L.; Pringsheim, T. Prevalence and Incidence of Huntington’s Disease: An Updated Systematic Review and Meta-Analysis. Mov. Disord. Off. J. Mov. Disord. Soc. 2022, 37, 2327–2335. [Google Scholar] [CrossRef] [PubMed]
- United Nations Department of Economic and Social Affairs. World Population Prospects 2022: Summary of Results; United Nations Department of Economic and Social Affairs: New York, NY, USA, 2022. [Google Scholar]
- Ross, C.A.; Tabrizi, S.J. Huntington’s disease: From molecular pathogenesis to clinical treatment. Lancet. Neurol. 2011, 10, 83–98. [Google Scholar] [CrossRef] [PubMed]
- Nucifora, F.C., Jr.; Sasaki, M.; Peters, M.F.; Huang, H.; Cooper, J.K.; Yamada, M.; Takahashi, H.; Tsuji, S.; Troncoso, J.; Dawson, V.L.; et al. Interference by huntingtin and atrophin-1 with cbp-mediated transcription leading to cellular toxicity. Science 2001, 291, 2423–2428. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Atanasov, A.G.; Zotchev, S.B.; Dirsch, V.M.; Orhan, I.E.; Banach, M.; Rollinger, J.M.; Barreca, D.; Weckwerth, W.; Bauer, R.; Bayer, E.A.; et al. Natural products in drug discovery: Advances and opportunities. Nat. Rev. Drug Discov. 2021, 20, 200–216. [Google Scholar] [CrossRef]
- Leuci, R.; Brunetti, L.; Poliseno, V.; Laghezza, A.; Loiodice, F.; Tortorella, P.; Piemontese, L. Natural compounds for the prevention and treatment of cardiovascular and neurodegenerative diseases. Foods 2020, 10, 29. [Google Scholar] [CrossRef]
- Springob, K.; Kutchan, T.M. Introduction to the different classes of natural products. In Plant-derived natural products; Springer: Berlin/Heidelberg, Germany, 2009; pp. 3–50. [Google Scholar]
- Neelam; Khatkar, A.; Sharma, K.K. Phenylpropanoids and its derivatives: Biological activities and its role in food, pharmaceutical and cosmetic industries. Crit. Rev. Food Sci. Nutr. 2020, 60, 2655–2675. [Google Scholar] [CrossRef]
- Korkina, L. Phenylpropanoids as naturally occurring antioxidants: From plant defense to human health. Cell. Mol. Biol. 2007, 53, 15–25. [Google Scholar] [PubMed]
- Wang, J.; Zhang, R.; Chen, X.; Sun, X.; Yan, Y.; Shen, X.; Yuan, Q. Biosynthesis of aromatic polyketides in microorganisms using type II polyketide synthases. Microb. Cell Factories 2020, 19, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Cox-Georgian, D.; Ramadoss, N.; Dona, C.; Basu, C. Therapeutic and Medicinal Uses of Terpenes. Med. Plants 2019, 333–359. [Google Scholar] [CrossRef]
- Pal, A.; Das, S. Terpenoids in Treatment of Neurodegenerative Disease. In Terpenoids Against Human Diseases; CRC Press: Boca Raton, FL, USA, 2019; pp. 95–117. [Google Scholar]
- Galasko, D.R.; Peskind, E.; Clark, C.M.; Quinn, J.F.; Ringman, J.M.; Jicha, G.A.; Cotman, C.; Cottrell, B.; Montine, T.J.; Thomas, R.G.; et al. Antioxidants for Alzheimer Disease: A Randomized Clinical Trial With Cerebrospinal Fluid Biomarker Measures. Arch. Neurol. 2012, 69, 836–841. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sano, M.; Ernesto, C.; Thomas, R.G.; Klauber, M.R.; Schafer, K.; Grundman, M.; Woodbury, P.; Growdon, J.; Cotman, C.W.; Pfeiffer, E.; et al. A Controlled Trial of Selegiline, Alpha-Tocopherol, or Both as Treatment for Alzheimer’s Disease. N. Engl. J. Med. 1997, 336, 1216–1222. [Google Scholar] [CrossRef] [Green Version]
- Dysken, M.W.; Sano, M.; Asthana, S.; Vertrees, J.E.; Pallaki, M.; Llorente, M.; Love, S.; Schellenberg, G.D.; McCarten, J.R.; Malphurs, J. Effect of vitamin E and memantine on functional decline in Alzheimer disease: The TEAM-AD VA cooperative randomized trial. JAMA 2014, 311, 33–44. [Google Scholar] [CrossRef]
- Annweiler, C.; Herrmann, F.R.; Fantino, B.; Brugg, B.; Beauchet, O. Effectiveness of the combination of memantine plus vitamin D on cognition in patients with Alzheimer disease: A pre-post pilot study. Cogn. Behav. Neurol. 2012, 25, 121–127. [Google Scholar] [CrossRef] [PubMed]
- Aisen, P.S.; Schneider, L.S.; Sano, M.; Diaz-Arrastia, R.; Van Dyck, C.H.; Weiner, M.F.; Bottiglieri, T.; Jin, S.; Stokes, K.T.; Thomas, R.G. High-dose B vitamin supplementation and cognitive decline in Alzheimer disease: A randomized controlled trial. JAMA 2008, 300, 1774–1783. [Google Scholar] [CrossRef]
- ClinicalTrials.gov. Identifier: NCT00580931. Available online: https://clinicaltrials.gov/ct2/show/NCT00580931 (accessed on 23 October 2022).
- Clinicaltrials.gov. Identifier: NCT01594346. Available online: https://clinicaltrials.gov/ct2/show/NCT01594346 (accessed on 23 October 2022).
- Quinn, J.F.; Raman, R.; Thomas, R.G.; Yurko-Mauro, K.; Nelson, E.B.; Van Dyck, C.; Galvin, J.E.; Emond, J.; Jack, C.R.; Weiner, M.; et al. Docosahexaenoic Acid Supplementation and Cognitive Decline in Alzheimer Disease: A Randomized Trial. JAMA 2010, 304, 1903–1911. [Google Scholar] [CrossRef] [Green Version]
- Farlow, M.R.; Thompson, R.E.; Wei, L.-J.; Tuchman, A.J.; Grenier, E.; Crockford, D.; Wilke, S.; Benison, J.; Alkon, D.L. A Randomized, Double-Blind, Placebo-Controlled, Phase II Study Assessing Safety, Tolerability, and Efficacy of Bryostatin in the Treatment of Moderately Severe to Severe Alzheimer’s Disease. J. Alzheimer’s Dis. 2019, 67, 555–570. [Google Scholar] [CrossRef] [Green Version]
- Salloway, S.; Sperling, R.; Keren, R.; Porsteinsson, A.P.; van Dyck, C.H.; Tariot, P.N.; Gilman, S.; Arnold, D.; Abushakra, S.; Hernandez, C.; et al. A phase 2 randomized trial of ELND005, scyllo-inositol, in mild to moderate Alzheimer disease. Neurology 2011, 77, 1253–1262. [Google Scholar] [CrossRef]
- Turner, R.S.; Thomas, R.G.; Craft, S.; van Dyck, C.H.; Mintzer, J.; Reynolds, B.A.; Brewer, J.B.; Rissman, R.A.; Raman, R.; Aisen, P.S. A randomized, double-blind, placebo-controlled trial of resveratrol for Alzheimer disease. Neurology 2015, 85, 1383–1391. [Google Scholar] [CrossRef] [PubMed]
- Zhu, C.W.; Grossman, H.; Neugroschl, J.; Parker, S.; Burden, A.; Luo, X.; Sano, M. A randomized, double-blind, placebo-controlled trial of resveratrol with glucose and malate (RGM) to slow the progression of Alzheimer’s disease: A pilot study. Alzheimers Dement 2018, 4, 609–616. [Google Scholar] [CrossRef]
- Ringman, J.M.; Frautschy, S.A.; Teng, E.; Begum, A.N.; Bardens, J.; Beigi, M.; Gylys, K.H.; Badmaev, V.; Heath, D.D.; Apostolova, L.G.; et al. Oral curcumin for Alzheimer’s disease: Tolerability and efficacy in a 24-week randomized, double blind, placebo-controlled study. Alzheimer’s Res. Ther. 2012, 4, 43. [Google Scholar] [CrossRef] [Green Version]
- Baum, L.; Lam, C.W.; Cheung, S.K.; Kwok, T.; Lui, V.; Tsoh, J.; Lam, L.; Leung, V.; Hui, E.; Ng, C.; et al. Six-month randomized, placebo-controlled, double-blind, pilot clinical trial of curcumin in patients with Alzheimer disease. J. Clin. Psychopharmacol. 2008, 28, 110–113. [Google Scholar] [CrossRef] [Green Version]
- Rafii, M.S.; Walsh, S.; Little, J.T.; Behan, K.; Reynolds, B.; Ward, C.; Jin, S.; Thomas, R.; Aisen, P.S. A phase II trial of huperzine A in mild to moderate Alzheimer disease. Neurology 2011, 76, 1389–1394. [Google Scholar] [CrossRef] [Green Version]
- Aisen, P.S.; Gauthier, S.; Ferris, S.H.; Saumier, D.; Haine, D.; Garceau, D.; Duong, A.; Suhy, J.; Oh, J.; Lau, W.C.; et al. Tramiprosate in mild-to-moderate Alzheimer’s disease–A randomized, double-blind, placebo-controlled, multi-centre study (the Alphase Study). Arch. Med. Sci. 2011, 7, 102–111. [Google Scholar] [CrossRef] [Green Version]
- Wade, A.G.; Farmer, M.; Harari, G.; Fund, N.; Laudon, M.; Nir, T.; Frydman-Marom, A.; Zisapel, N. Add-on prolonged-release melatonin for cognitive function and sleep in mild to moderate Alzheimer’s disease: A 6-month, randomized, placebo-controlled, multicenter trial. Clin. Interv. Aging 2014, 9, 947. [Google Scholar] [PubMed]
- ClinicalTrials.gov. Identifier: NCT00951834. Available online: https://clinicaltrials.gov/ct2/show/NCT00951834 (accessed on 23 October 2022).
- Sung, S.; Yao, Y.; Uryu, K.; Yang, H.; Lee, V.M.Y.; Trojanowski, J.Q.; Praticò, D. Early vitamin E supplementation in young but not aged mice reduces Aβ levels and amyloid deposition in a transgenic model of Alzheimer’s disease. FASEB J. 2004, 18, 323–325. [Google Scholar] [CrossRef] [PubMed]
- Matsunaga, S.; Kishi, T.; Nomura, I.; Sakuma, K.; Okuya, M.; Ikuta, T.; Iwata, N. The efficacy and safety of memantine for the treatment of Alzheimer’s disease. Expert Opin. Drug Saf. 2018, 17, 1053–1061. [Google Scholar] [CrossRef]
- Grimm, M.O.; Thiel, A.; Lauer, A.A.; Winkler, J.; Lehmann, J.; Regner, L.; Nelke, C.; Janitschke, D.; Benoist, C.; Streidenberger, O. Vitamin D and its analogues decrease amyloid-β (Aβ) formation and increase Aβ-degradation. Int. J. Mol. Sci. 2017, 18, 2764. [Google Scholar] [CrossRef] [PubMed]
- Morello, M.; Landel, V.; Lacassagne, E.; Baranger, K.; Annweiler, C.; Féron, F.; Millet, P. Vitamin D improves neurogenesis and cognition in a mouse model of Alzheimer’s disease. Mol. Neurobiol. 2018, 55, 6463–6479. [Google Scholar] [CrossRef] [Green Version]
- ClinicalTrials.gov. Identifier: NCT01409694. Available online: https://clinicaltrials.gov/ct2/show/NCT01409694 (accessed on 24 October 2022).
- Sinclair, A.J.; Begg, D.; Mathai, M.; Weisinger, R.S. Omega 3 fatty acids and the brain: Review of studies in depression. Asia Pac. J. Clin. Nutr. 2007, 16 (Suppl. 1), 391–397. [Google Scholar]
- Hossain, S.; Hashimoto, M.; Katakura, M.; Miwa, K.; Shimada, T.; Shido, O. Mechanism of docosahexaenoic acid-induced inhibition of in vitro Aβ1–42 fibrillation and Aβ1–42-induced toxicity in SH-S5Y5 cells. J. Neurochem. 2009, 111, 568–579. [Google Scholar] [CrossRef] [PubMed]
- Lim, G.P.; Calon, F.; Morihara, T.; Yang, F.; Teter, B.; Ubeda, O.; Salem, N.; Frautschy, S.A.; Cole, G.M. A Diet Enriched with the Omega-3 Fatty Acid Docosahexaenoic Acid Reduces Amyloid Burden in an Aged Alzheimer Mouse Model. J. Neurosci. 2005, 25, 3032. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kollár, P.; Rajchard, J.; Balounová, Z.; Pazourek, J. Marine natural products: Bryostatins in preclinical and clinical studies. Pharm. Biol. 2014, 52, 237–242. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schrott, L.M.; Jackson, K.; Yi, P.; Dietz, F.; Johnson, G.S.; Basting, T.F.; Purdum, G.; Tyler, T.; Rios, J.D.; Castor, T.P.; et al. Acute Oral Bryostatin-1 Administration Improves Learning Deficits in the APP/PS1 Transgenic Mouse Model of Alzheimer’s Disease. Curr. Alzheimer Res. 2015, 12, 22–31. [Google Scholar] [CrossRef] [PubMed]
- Etcheberrigaray, R.; Tan, M.; Dewachter, I.; Kuipéri, C.; Van der Auwera, I.; Wera, S.; Qiao, L.; Bank, B.; Nelson, T.J.; Kozikowski, A.P.; et al. Therapeutic effects of PKC activators in Alzheimer’s disease transgenic mice. Proc. Natl. Acad. Sci. USA 2004, 101, 11141–11146. [Google Scholar] [CrossRef] [Green Version]
- McLaurin, J.; Kierstead, M.E.; Brown, M.E.; Hawkes, C.A.; Lambermon, M.H.; Phinney, A.L.; Darabie, A.A.; Cousins, J.E.; French, J.E.; Lan, M.F. Cyclohexanehexol inhibitors of Aβ aggregation prevent and reverse Alzheimer phenotype in a mouse model. Nat. Med. 2006, 12, 801–808. [Google Scholar] [CrossRef]
- He, X.; Li, Z.; Rizak, J.D.; Wu, S.; Wang, Z.; He, R.; Su, M.; Qin, D.; Wang, J.; Hu, X. Resveratrol attenuates formaldehyde induced hyperphosphorylation of tau protein and cytotoxicity in N2a cells. Front. Neurosci. 2017, 10, 598. [Google Scholar] [CrossRef] [Green Version]
- Andrade, S.; Loureiro, J.A.; Coelho, M.A.; do Carmo Pereira, M. Interaction studies of amyloid beta-peptide with the natural compound resveratrol. In Proceedings of 2015 IEEE 4th Portuguese Meeting on Bioengineering (ENBENG), Porto, Portugal, 26–28 February 2015; pp. 1–3. [Google Scholar]
- Marambaud, P.; Zhao, H.; Davies, P. Resveratrol promotes clearance of Alzheimer’s disease amyloid-β peptides. J. Biol. Chem. 2005, 280, 37377–37382. [Google Scholar] [CrossRef] [Green Version]
- Yang, F.; Lim, G.P.; Begum, A.N.; Ubeda, O.J.; Simmons, M.R.; Ambegaokar, S.S.; Chen, P.P.; Kayed, R.; Glabe, C.G.; Frautschy, S.A. Curcumin inhibits formation of amyloid β oligomers and fibrils, binds plaques, and reduces amyloid in vivo. J. Biol. Chem. 2005, 280, 5892–5901. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.-J.; Li, Z.-H.; Liu, L.; Tang, W.-X.; Wang, Y.; Dong, M.-R.; Xiao, C. Curcumin Attenuates Beta-Amyloid-Induced Neuroinflammation via Activation of Peroxisome Proliferator-Activated Receptor-Gamma Function in a Rat Model of Alzheimer’s Disease. Front. Pharm. 2016, 7, 261. [Google Scholar] [CrossRef] [Green Version]
- Damar, U.; Gersner, R.; Johnstone, J.T.; Schachter, S.; Rotenberg, A. Huperzine A as a neuroprotective and antiepileptic drug: A review of preclinical research. Expert Rev. Neurother. 2016, 16, 671–680. [Google Scholar] [CrossRef] [PubMed]
- Wright, T.M. Tramiprosate. Drugs Today 2006, 42, 291–298. [Google Scholar] [CrossRef]
- Krzywkowski, P.; Sebastiani, G.; Williams, S.; Delorme, D.; Greenberg, B. Tramiprosate prevents amyloid beta-induced inhibition of long-term potentiation in rat hippocampal slices. In Proceedings of 8th International Conference AD/PD, Salzburg, Austria, 14–18 March 2007. [Google Scholar]
- Gervais, F.; Paquette, J.; Morissette, C.; Krzywkowski, P.; Yu, M.; Azzi, M.; Lacombe, D.; Kong, X.; Aman, A.; Laurin, J.; et al. Targeting soluble Abeta peptide with Tramiprosate for the treatment of brain amyloidosis. Neurobiol. Aging 2007, 28, 537–547. [Google Scholar] [CrossRef]
- Gauthier, S.; Aisen, P.S.; Ferris, S.H.; Saumier, D.; Duong, A.; Haine, D.; Garceau, D.; Suhy, J.; Oh, J.; Lau, W.; et al. Effect of tramiprosate in patients with mild-to-moderate alzheimer’s disease: Exploratory analyses of the MRI sub-group of the alphase study. JNHA—J. Nutr. Health Aging 2009, 13, 550–557. [Google Scholar] [CrossRef] [PubMed]
- Panmanee, J.; Nopparat, C.; Chavanich, N.; Shukla, M.; Mukda, S.; Song, W.; Vincent, B.; Govitrapong, P. Melatonin regulates the transcription of βAPP-cleaving secretases mediated through melatonin receptors in human neuroblastoma SH-SY5Y cells. J. Pineal Res. 2015, 59, 308–320. [Google Scholar] [CrossRef]
- Guo, Y.; Zhao, Y.; Nan, Y.; Wang, X.; Chen, Y.; Wang, S. (-)-Epigallocatechin-3-gallate ameliorates memory impairment and rescues the abnormal synaptic protein levels in the frontal cortex and hippocampus in a mouse model of Alzheimer’s disease. Neuroreport 2017, 28, 590–597. [Google Scholar] [CrossRef]
- Walker, J.M.; Klakotskaia, D.; Ajit, D.; Weisman, G.A.; Wood, W.G.; Sun, G.Y.; Serfozo, P.; Simonyi, A.; Schachtman, T.R. Beneficial effects of dietary EGCG and voluntary exercise on behavior in an Alzheimer’s disease mouse model. J. Alzheimer’s Dis. 2015, 44, 561–572. [Google Scholar] [CrossRef]
- Wobst, H.J.; Sharma, A.; Diamond, M.I.; Wanker, E.E.; Bieschke, J. The green tea polyphenol (−)-epigallocatechin gallate prevents the aggregation of tau protein into toxic oligomers at substoichiometric ratios. FEBS Lett. 2015, 589, 77–83. [Google Scholar] [CrossRef] [Green Version]
- Chesser, A.S.; Ganeshan, V.; Yang, J.; Johnson, G.V. Epigallocatechin-3-gallate enhances clearance of phosphorylated tau in primary neurons. Nutr. Neurosci. 2016, 19, 21–31. [Google Scholar] [CrossRef]
- Lieberman, A.; Lockhart, T.E.; Olson, M.C.; Smith Hussain, V.A.; Frames, C.W.; Sadreddin, A.; McCauley, M.; Ludington, E. Nicotine Bitartrate Reduces Falls and Freezing of Gait in Parkinson Disease: A Reanalysis. Front. Neurol. 2019, 10, 424. [Google Scholar] [CrossRef] [PubMed]
- Postuma, R.; Anang, J.; Pelletier, A.; Joseph, L.; Moscovich, M.; Grimes, D.; Furtado, S.; Munhoz, R.; Appel-Cresswell, S.; Moro, A.; et al. Caffeine as symptomatic treatment for Parkinson disease (Café-PD): A randomized trial. Neurology 2017, 89, 1795–1803. [Google Scholar] [CrossRef] [PubMed]
- Leehey, M.A.; Liu, Y.; Hart, F.; Epstein, C.; Cook, M.; Sillau, S.; Klawitter, J.; Newman, H.; Sempio, C.; Forman, L.; et al. Safety and Tolerability of Cannabidiol in Parkinson Disease: An Open Label, Dose-Escalation Study. Cannabis Cannabinoid Res. 2020, 5, 326–336. [Google Scholar] [CrossRef] [PubMed]
- DiFrancisco-Donoghue, J.; Lamberg, E.M.; Rabin, E.; Elokda, A.; Fazzini, E.; Werner, W.G. Effects of exercise and B vitamins on homocysteine and glutathione in Parkinson’s disease: A randomized trial. Neurodegener Dis 2012, 10, 127–134. [Google Scholar] [CrossRef] [PubMed]
- Wakade, C.; Chong, R.; Seamon, M.; Purohit, S.; Giri, B.; Morgan, J.C. Low-Dose Niacin Supplementation Improves Motor Function in US Veterans with Parkinson’s Disease: A Single-Center, Randomized, Placebo-Controlled Trial. Biomedicines 2021, 9, 1881. [Google Scholar] [CrossRef]
- Hiller, A.L.; Murchison, C.F.; Lobb, B.M.; O’Connor, S.; O’Connor, M.; Quinn, J.F. A randomized, controlled pilot study of the effects of vitamin D supplementation on balance in Parkinson’s disease: Does age matter? PLoS ONE 2018, 13, e0203637. [Google Scholar] [CrossRef] [Green Version]
- Beal, M.F.; Oakes, D.; Shoulson, I.; Henchcliffe, C.; Galpern, W.R.; Haas, R.; Juncos, J.L.; Nutt, J.G.; Voss, T.S.; Ravina, B.; et al. A randomized clinical trial of high-dosage coenzyme Q10 in early Parkinson disease: No evidence of benefit. JAMA Neurol. 2014, 71, 543–552. [Google Scholar] [CrossRef]
- ClinicalTrials.gov. Identifier: NCT01563913. Available online: https://clinicaltrials.gov/ct2/show/NCT01563913 (accessed on 25 October 2022).
- Chan, P.; Qin, Z.; Zheng, Z.; Zhang, L.; Fang, X.; Sun, F.; Gu, Z.; Chen, S.; Ma, J.; Meng, C.; et al. P2.204 A randomized, double-blind, placebo-controlled, delayed start study to assess safty, tolerability and efflcacy of green tea polyphenols in Parkinson’s disease. Park. Relat. Disord.-Park. Relat Disord 2009, 15. [Google Scholar] [CrossRef]
- Quik, M.; Huang, L.Z.; Parameswaran, N.; Bordia, T.; Campos, C.; Perez, X.A. Multiple roles for nicotine in Parkinson’s disease. Biochem. Pharmacol. 2009, 78, 677–685. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- ClinicalTrials.gov. Identifier: NCT03865121. Available online: https://clinicaltrials.gov/ct2/show/NCT03865121 (accessed on 25 October 2022).
- ClinicalTrials.gov. Identifier: NCT02452125. Available online: https://clinicaltrials.gov/ct2/show/NCT02452125 (accessed on 25 October 2022).
- ClinicalTrials.gov. Identifier: NCT00873392. Available online: https://clinicaltrials.gov/ct2/show/NCT00873392 (accessed on 25 October 2022).
- Ren, X.; Chen, J.F. Caffeine and Parkinson’s Disease: Multiple Benefits and Emerging Mechanisms. Front. Neurosci. 2020, 14, 602697. [Google Scholar] [CrossRef]
- Postuma, R.B.; Lang, A.E.; Munhoz, R.P.; Charland, K.; Pelletier, A.; Moscovich, M.; Filla, L.; Zanatta, D.; Rios Romenets, S.; Altman, R.; et al. Caffeine for treatment of Parkinson disease: A randomized controlled trial. Neurology 2012, 79, 651–658. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- ClinicalTrials.gov. Identifier: NCT00459420. Available online: https://clinicaltrials.gov/ct2/show/NCT00459420 (accessed on 26 October 2022).
- ClinicalTrials.gov. Identifier: NCT01190735. Available online: https://clinicaltrials.gov/ct2/show/NCT01190735 (accessed on 26 October 2022).
- Chagas, M.H.N.; Zuardi, A.W.; Tumas, V.; Pena-Pereira, M.A.; Sobreira, E.T.; Bergamaschi, M.M.; dos Santos, A.C.; Teixeira, A.L.; Hallak, J.E.C.; Crippa, J.A.S. Effects of cannabidiol in the treatment of patients with Parkinson’s disease: An exploratory double-blind trial. Journal of Psychopharmacology 2014, 28, 1088–1098. [Google Scholar] [CrossRef] [PubMed]
- ClinicalTrials.gov. Identifier: NCT03582137. Available online: https://clinicaltrials.gov/ct2/show/NCT03582137 (accessed on 26 October 2022).
- Zhao, X.; Zhang, M.; Li, C.; Jiang, X.; Su, Y.; Zhang, Y. Benefits of Vitamins in the Treatment of Parkinson’s Disease. Oxid. Med. Cell. Longev. 2019, 2019, 9426867. [Google Scholar] [CrossRef] [PubMed]
- Chitre, N.M.; Wood, B.J.; Ray, A.; Moniri, N.H.; Murnane, K.S. Docosahexaenoic acid protects motor function and increases dopamine synthesis in a rat model of Parkinson’s disease via mechanisms associated with increased protein kinase activity in the striatum. Neuropharmacology 2020, 167, 107976. [Google Scholar] [CrossRef]
- alar, D.S.; Prasanth, M.I.; Brimson, J.M.; Sharika, R.; Sivamaruthi, B.S.; Chaiyasut, C.; Tencomnao, T. Neuroprotective Properties of Green Tea in Parkinson’s Disease A. Review. Molecules 2020, 25, 3926. [Google Scholar]
- Wang, Y.; Wu, S.; Li, Q.; Lang, W.; Li, W.; Jiang, X.; Wan, Z.; Chen, J.; Wang, H. Epigallocatechin-3-gallate: A phytochemical as a promising drug candidate for the treatment of Parkinson’s disease. Front. Pharmacol. 2022, 13, 977521. [Google Scholar] [CrossRef]
- Camu, W.; Lehert, P.; Pierrot-Deseilligny, C.; Hautecoeur, P.; Besserve, A.; Jean Deleglise, A.-S.; Payet, M.; Thouvenot, E.; Souberbielle, J.C. Cholecalciferol in relapsing-remitting MS: A randomized clinical trial (CHOLINE). Neurol.-Neuroimmunol. Neuroinflamm. 2019, 6, e597. [Google Scholar] [CrossRef] [Green Version]
- Sama, B.; Aliakbar, S.-Y.; Mohammad-Ali, S.; Danesh, S.; Shahriar, N.; Mansoureh, T.; Nahid Beladi, M.; Tina, R.; Niyaz Mohammadzadeh, H.; Mohammad-Hossein, H. Effect of Vitamin A Supplementation on Fatigue and Depression in Multiple Sclerosis Patients: A Double-blind Placebo-controlled Clinical Trial. Iran. J. Allergy Asthma Immunol. 2016, 15, 13–19. [Google Scholar]
- Bitarafan, S.; Saboor-Yaraghi, A.; Sahraian, M.A.; Nafissi, S.; Togha, M.; Beladi Moghadam, N.; Roostaei, T.; Siassi, F.; Eshraghian, M.R.; Ghanaati, H.; et al. Impact of Vitamin A Supplementation on Disease Progression in Patients with Multiple Sclerosis. Arch. Iran. Med. 2015, 18, 435–440. [Google Scholar]
- Spain, R.; Powers, K.; Murchison, C.; Heriza, E.; Winges, K.; Yadav, V.; Cameron, M.; Kim, E.; Horak, F.; Simon, J.; et al. Lipoic acid in secondary progressive MS: A randomized controlled pilot trial. Neurol. Neuroimmunol. Neuroinflamm 2017, 4, e374. [Google Scholar] [CrossRef] [Green Version]
- ClinicalTrials.gov. Identifier: NCT00010842. Available online: https://clinicaltrials.gov/ct2/show/NCT00010842 (accessed on 27 October 2022).
- ClinicalTrials.gov. Identifier: NCT02133664. Available online: https://clinicaltrials.gov/ct2/show/NCT02133664 (accessed on 27 October 2022).
- Ramirez-Ramirez, V.; Macias-Islas, M.A.; Ortiz, G.G.; Pacheco-Moises, F.; Torres-Sanchez, E.D.; Sorto-Gomez, T.E.; Cruz-Ramos, J.A.; Orozco-Aviña, G.; Celis de la Rosa, A.J. Efficacy of Fish Oil on Serum of TNF<i>α</i>, IL-1<i>β</i>, and IL-6 Oxidative Stress Markers in Multiple Sclerosis Treated with Interferon Beta-1b. Oxidative Med. Cell. Longev. 2013, 2013, 709493. [Google Scholar] [CrossRef] [Green Version]
- Tina, R.; Mohammad Ali, S.; Sara, H.; Taha, G.; Mansoureh, T.; Bahaadin, S.; Sepideh, M.; Zahra, M.; Maryam Aghazadeh, A.; Mohammad Hossein, H. Impact of Melatonin on Motor, Cognitive and Neuroimaging Indices in Patients with Multiple Sclerosis. Iran. J. Allergy Asthma Immunol. 2015, 14, 589–595. [Google Scholar]
- ClinicalTrials.gov. Identifier: NCT03740295. Available online: https://clinicaltrials.gov/ct2/show/NCT03740295?term=egcg&cond=Multiple+Sclerosis&draw=2&rank=4 (accessed on 27 October 2022).
- de la Rubia Ortí, J.E.; Platero, J.L.; Yang, I.H.; Ceron, J.J.; Tvarijonaviciute, A.; Sabater, P.S.; Benlloch, M.; Sancho-Cantus, D.; Sancho, S. Possible Role of Butyrylcholinesterase in Fat Loss and Decreases in Inflammatory Levels in Patients with Multiple Sclerosis after Treatment with Epigallocatechin Gallate and Coconut Oil: A Pilot Study. Nutrients 2021, 13, 3230. [Google Scholar] [CrossRef] [PubMed]
- Benlloch, M.; Cuerda Ballester, M.; Drehmer, E.; Platero, J.L.; Carrera-Juliá, S.; López-Rodríguez, M.M.; Ceron, J.J.; Tvarijonaviciute, A.; Navarro, M.Á.; Moreno, M.L.; et al. Possible Reduction of Cardiac Risk after Supplementation with Epigallocatechin Gallate and Increase of Ketone Bodies in the Blood in Patients with Multiple Sclerosis. A Pilot Study. Nutrients 2020, 12, 3792. [Google Scholar] [CrossRef] [PubMed]
- Platero, J.L.; Cuerda-Ballester, M.; Sancho-Cantus, D.; Benlloch, M.; Ceron, J.J.; Peres Rubio, C.; García-Pardo, M.P.; López-Rodríguez, M.M.; de la Rubia Ortí, J.E. The Impact of Epigallocatechin Gallate and Coconut Oil Treatment on Cortisol Activity and Depression in Multiple Sclerosis Patients. Life 2021, 11, 353. [Google Scholar] [CrossRef] [PubMed]
- Petracca, M.; Quarantelli, M.; Moccia, M.; Vacca, G.; Satelliti, B.; D’Ambrosio, G.; Carotenuto, A.; Ragucci, M.; Assogna, F.; Capacchione, A.; et al. ProspeCtive study to evaluate efficacy, safety and tOlerability of dietary supplemeNT of Curcumin (BCM95) in subjects with Active relapsing MultIple Sclerosis treated with subcutaNeous Interferon beta 1a 44 mcg TIW (CONTAIN): A randomized, controlled trial. Mult. Scler. Relat. Disord. 2021, 56, 103274. [Google Scholar] [CrossRef]
- Feige, J.; Moser, T.; Bieler, L.; Schwenker, K.; Hauer, L.; Sellner, J. Vitamin D Supplementation in Multiple Sclerosis: A Critical Analysis of Potentials and Threats. Nutrients 2020, 12, 783. [Google Scholar] [CrossRef] [Green Version]
- ClinicalTrials.gov. Identifier: NCT03610139. Available online: https://clinicaltrials.gov/ct2/show/NCT03610139 (accessed on 28 October 2022).
- ClinicalTrials.gov. Identifier: NCT05340985. Available online: https://clinicaltrials.gov/ct2/show/NCT05340985 (accessed on 28 October 2022).
- ClinicalTrials.gov. Identifier: NCT01817166. Available online: https://clinicaltrials.gov/ct2/show/NCT01817166 (accessed on 28 October 2022).
- ClinicalTrials.gov. Identifier: NCT02096133. Available online: https://clinicaltrials.gov/ct2/show/NCT02096133 (accessed on 28 October 2022).
- ClinicalTrials.gov. Identifier: NCT01005095. Available online: https://clinicaltrials.gov/ct2/show/NCT01005095 (accessed on 28 October 2022).
- ClinicalTrials.gov. Identifier: NCT01440062. Available online: https://clinicaltrials.gov/ct2/show/NCT01440062 (accessed on 28 October 2022).
- ClinicalTrials.gov. Identifier: NCT01490502. Available online: https://clinicaltrials.gov/ct2/show/NCT01490502 (accessed on 28 October 2022).
- ClinicalTrials.gov. Identifier: NCT01432704. Available online: https://clinicaltrials.gov/ct2/show/NCT01432704 (accessed on 28 October 2022).
- ClinicalTrials.gov. Identifier: NCT03385356. Available online: https://clinicaltrials.gov/ct2/show/NCT03385356 (accessed on 28 October 2022).
- Reza Dorosty-Motlagh, A.; Mohammadzadeh Honarvar, N.; Sedighiyan, M.; Abdolahi, M. The Molecular Mechanisms of Vitamin A Deficiency in Multiple Sclerosis. J. Mol. Neurosci. 2016, 60, 82–90. [Google Scholar] [CrossRef]
- Yokote, H.; Kamata, T.; Toru, S.; Sanjo, N.; Yokota, T. Serum retinol levels are associated with brain volume loss in patients with multiple sclerosis. Mult. Scler. J.—Exp. Transl. Clin. 2017, 3, 2055217317729688. [Google Scholar] [CrossRef] [Green Version]
- Waslo, C.; Bourdette, D.; Gray, N.; Wright, K.; Spain, R. Lipoic Acid and Other Antioxidants as Therapies for Multiple Sclerosis. Curr. Treat. Options Neurol. 2019, 21, 26. [Google Scholar] [CrossRef]
- Xie, H.; Yang, X.; Cao, Y.; Long, X.; Shang, H.; Jia, Z. Role of lipoic acid in multiple sclerosis. CNS Neurosci. Ther. 2022, 28, 319–331. [Google Scholar] [CrossRef]
- ClinicalTrials.gov. Identifier: NCT00676156. Available online: https://clinicaltrials.gov/ct2/show/NCT00676156 (accessed on 28 October 2022).
- Fiedler, S.E.; Yadav, V.; Kerns, A.R.; Tsang, C.; Markwardt, S.; Kim, E.; Spain, R.; Bourdette, D.; Salinthone, S. Lipoic Acid Stimulates cAMP Production in Healthy Control and Secondary Progressive MS Subjects. Mol. Neurobiol. 2018, 55, 6037–6049. [Google Scholar] [CrossRef]
- Cameron, M.; Taylor, C.; Lapidus, J.; Ramsey, K.; Koop, D.; Spain, R. Gastrointestinal Tolerability and Absorption of R- Versus R,S-Lipoic Acid in Progressive Multiple Sclerosis: A Randomized Crossover Trial. J. Clin. Pharmacol. 2020, 60, 1099–1106. [Google Scholar] [CrossRef]
- ClinicalTrials.gov. Identifier: NCT03161028. Available online: https://clinicaltrials.gov/ct2/show/NCT03161028 (accessed on 28 October 2022).
- Khalili, M.; Soltani, M.; Moghadam, S.A.; Dehghan, P.; Azimi, A.; Abbaszadeh, O. Effect of alpha-lipoic acid on asymmetric dimethylarginine and disability in multiple sclerosis patients: A randomized clinical trial. Electron. Physician 2017, 9, 4899–4905. [Google Scholar] [CrossRef] [PubMed]
- Khalili, M.; Eghtesadi, S.; Mirshafiey, A.; Eskandari, G.; Sanoobar, M.; Sahraian, M.A.; Motevalian, A.; Norouzi, A.; Moftakhar, S.; Azimi, A. Effect of lipoic acid consumption on oxidative stress among multiple sclerosis patients: A randomized controlled clinical trial. Nutr. Neurosci. 2014, 17, 16–20. [Google Scholar] [CrossRef] [PubMed]
- AlAmmar, W.A.; Albeesh, F.H.; Ibrahim, L.M.; Algindan, Y.Y.; Yamani, L.Z.; Khattab, R.Y. Effect of omega-3 fatty acids and fish oil supplementation on multiple sclerosis: A systematic review. Nutr. Neurosci. 2021, 24, 569–579. [Google Scholar] [CrossRef] [PubMed]
- Skarlis, C.; Anagnostouli, M. The role of melatonin in Multiple Sclerosis. Neurol. Sci. 2020, 41, 769–781. [Google Scholar] [CrossRef] [PubMed]
- ClinicalTrials.gov. Identifier: NCT03498131. Available online: https://clinicaltrials.gov/ct2/show/NCT03498131 (accessed on 28 October 2022).
- ClinicalTrials.gov. Identifier: NCT03540485. Available online: https://clinicaltrials.gov/ct2/show/NCT03540485 (accessed on 28 October 2022).
- Klumbies, K.; Rust, R.; Dörr, J.; Konietschke, F.; Paul, F.; Bellmann-Strobl, J.; Brandt, A.U.; Zimmermann, H.G. Retinal thickness analysis in progressive multiple sclerosis patients treated with epigallocatechin gallate: Optical coherence tomography results from the SUPREMES study. Front. Neurol. 2021, 12, 615790. [Google Scholar] [CrossRef]
- Ghanaatian, N.; Lashgari, N.-A.; Abdolghaffari, A.H.; Rajaee, S.M.; Panahi, Y.; Barreto, G.E.; Butler, A.E.; Sahebkar, A. Curcumin as a therapeutic candidate for multiple sclerosis: Molecular mechanisms and targets. J. Cell. Physiol. 2019, 234, 12237–12248. [Google Scholar] [CrossRef] [PubMed]
- Juntas-Morales, R.; Pageot, N.; Bendarraz, A.; Alphandery, S.; Sedel, F.; Seigle, S.; Camu, W. High-dose pharmaceutical grade biotin (MD1003) in amyotrophic lateral sclerosis: A pilot study. EClinicalMedicine 2020, 19, 100254. [Google Scholar] [CrossRef] [Green Version]
- Kaji, R.; Kuzuhara, S.; Iwasaki, Y.; Okamoto, K.; Nakagawa, M.; Imai, T.; Takase, T.; Shimizu, H.; Tashiro, K. Ultra-high dose methylcobalamin (E0302) prolongs survival of ALS: Report of 7 years’ randomised double-blind, phase 3 clinical trial (ClinicalTrials.gov NCT00444613) (P7.060). Neurology 2015, 84, P7.060. [Google Scholar]
- ClinicalTrials.gov. Identifier: NCT05095571. Available online: https://clinicaltrials.gov/ct2/show/NCT05095571 (accessed on 31 October 2022).
- ClinicalTrials.gov. Identifier: NCT00372879. Available online: https://clinicaltrials.gov/ct2/show/NCT00372879 (accessed on 31 October 2022).
- ClinicalTrials.gov. Identifier: NCT04244630. Available online: https://clinicaltrials.gov/ct2/show/NCT04244630 (accessed on 31 October 2022).
- ClinicalTrials.gov. Identifier: NCT00919555. Available online: https://clinicaltrials.gov/ct2/show/NCT00919555 (accessed on 31 October 2022).
- ClinicalTrials.gov. Identifier: NCT04499963. Available online: https://clinicaltrials.gov/ct2/show/NCT04499963 (accessed on 31 October 2022).
- ClinicalTrials.gov. Identifier: NCT04654689. Available online: https://clinicaltrials.gov/ct2/show/NCT04654689 (accessed on 31 October 2022).
- ClinicalTrials.gov. Identifier: NCT04997954. Available online: https://clinicaltrials.gov/ct2/show/NCT04997954 (accessed on 31 October 2022).
- Ikeda, K.; Iwasaki, Y.; Kaji, R. Neuroprotective effect of ultra-high dose methylcobalamin in wobbler mouse model of amyotrophic lateral sclerosis. J Neurol Sci 2015, 354, 70–74. [Google Scholar] [CrossRef]
- ClinicalTrials.gov. Identifier: NCT03427086. Available online: https://clinicaltrials.gov/ct2/show/NCT03427086 (accessed on 31 October 2022).
- Yang, Z.; Chang, Y.-J.; Yu, I.; Yeh, S.; Wu, C.-C.; Miyamoto, H.; Merry, D.E.; Sobue, G.; Chen, L.-M.; Chang, S.-S. ASC-J9 ameliorates spinal and bulbar muscular atrophy phenotype via degradation of androgen receptor. Nat. Med. 2007, 13, 348–353. [Google Scholar] [CrossRef]
- Bott, L.C.; Badders, N.M.; Chen, K.-l.; Harmison, G.G.; Bautista, E.; Shih, C.C.-Y.; Katsuno, M.; Sobue, G.; Taylor, J.P.; Dantuma, N.P. A small-molecule Nrf1 and Nrf2 activator mitigates polyglutamine toxicity in spinal and bulbar muscular atrophy. Hum. Mol. Genet. 2016, 25, 1979–1989. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- ClinicalTrials.gov. Identifier: NCT03944447. Available online: https://clinicaltrials.gov/ct2/show/NCT03944447 (accessed on 31 October 2022).
- Urbi, B.; Owusu, M.A.; Hughes, I.; Katz, M.; Broadley, S.; Sabet, A. Effects of cannabinoids in Amyotrophic Lateral Sclerosis (ALS) murine models: A systematic review and meta-analysis. J. Neurochem. 2019, 149, 284–297. [Google Scholar] [CrossRef] [PubMed]
- Mancuso, R.; del Valle, J.; Modol, L.; Martinez, A.; Granado-Serrano, A.B.; Ramirez-Nunez, O.; Pallas, M.; Portero-Otin, M.; Osta, R.; Navarro, X. Resveratrol improves motoneuron function and extends survival in SOD1(G93A) ALS mice. Neurotherapeutics 2014, 11, 419–432. [Google Scholar] [CrossRef] [Green Version]
- López-Sendón Moreno, J.L.; García Caldentey, J.; Trigo Cubillo, P.; Ruiz Romero, C.; García Ribas, G.; Alonso Arias, M.A.A.; García de Yébenes, M.J.; Tolón, R.M.; Galve-Roperh, I.; Sagredo, O.; et al. A double-blind, randomized, cross-over, placebo-controlled, pilot trial with Sativex in Huntington’s disease. J. Neurol. 2016, 263, 1390–1400. [Google Scholar] [CrossRef]
- ClinicalTrials.gov. Identifier: NCT01357681. Available online: https://clinicaltrials.gov/ct2/show/study/NCT01357681 (accessed on 31 October 2022).
- ClinicalTrials.gov. Identifier: NCT02336633. Available online: https://clinicaltrials.gov/ct2/show/NCT02336633 (accessed on 31 October 2022).
- ClinicalTrials.gov. Identifier: NCT03034122. Available online: https://clinicaltrials.gov/ct2/show/NCT03034122 (accessed on 31 October 2022).
- ClinicalTrials.gov. Identifier: NCT04478734. Available online: https://clinicaltrials.gov/ct2/show/NCT04478734 (accessed on 31 October 2022).
- Dash, R.; Ali, M.C.; Jahan, I.; Munni, Y.A.; Mitra, S.; Hannan, M.A.; Timalsina, B.; Oktaviani, D.F.; Choi, H.J.; Moon, I.S. Emerging potential of cannabidiol in reversing proteinopathies. Ageing Res. Rev. 2021, 65, 101209. [Google Scholar] [CrossRef]
- Naia, L.; Rosenstock, T.R.; Oliveira, A.M.; Oliveira-Sousa, S.I.; Caldeira, G.L.; Carmo, C.; Laço, M.N.; Hayden, M.R.; Oliveira, C.R.; Rego, A.C. Comparative Mitochondrial-Based Protective Effects of Resveratrol and Nicotinamide in Huntington’s Disease Models. Mol. Neurobiol. 2017, 54, 5385–5399. [Google Scholar] [CrossRef]
- Solayman, M.; Islam, M.A.; Alam, F.; Khalil, M.I.; Kamal, M.A.; Gan, S.H. Natural Products Combating Neurodegeneration: Parkinson’s Disease. Curr Drug Metab 2017, 18, 50–61. [Google Scholar] [CrossRef] [PubMed]
- Yu, S.; Liu, M.; Hu, K. Natural products: Potential therapeutic agents in multiple sclerosis. Int. Immunopharmacol. 2019, 67, 87–97. [Google Scholar] [CrossRef] [PubMed]
- Nabavi, S.F.; Daglia, M.; D’Antona, G.; Sobarzo-Sánchez, E.; Talas, Z.S.; Nabavi, S.M. Natural compounds used as therapies targeting to amyotrophic lateral sclerosis. Curr. Pharm. Biotechnol. 2015, 16, 211–218. [Google Scholar] [CrossRef] [PubMed]
- Lum, P.T.; Sekar, M.; Gan, S.H.; Bonam, S.R.; Shaikh, M.F. Protective Effect of Natural Products against Huntington’s Disease: An Overview of Scientific Evidence and Understanding Their Mechanism of Action. ACS Chem. Neurosci. 2021, 12, 391–418. [Google Scholar] [CrossRef] [PubMed]
- Cummings, J.; Feldman, H.H.; Scheltens, P. The “rights” of precision drug development for Alzheimer’s disease. Alzheimer’s Res. Ther. 2019, 11, 76. [Google Scholar] [CrossRef] [Green Version]
- Sharma, P.; Srivastava, P.; Seth, A.; Tripathi, P.N.; Banerjee, A.G.; Shrivastava, S.K. Comprehensive review of mechanisms of pathogenesis involved in Alzheimer’s disease and potential therapeutic strategies. Prog. Neurobiol. 2019, 174, 53–89. [Google Scholar] [CrossRef]
- Jiang, Y.; Krishnan, N.; Heo, J.; Fang, R.H.; Zhang, L. Nanoparticle-hydrogel superstructures for biomedical applications. J. Control Release 2020, 324, 505–521. [Google Scholar] [CrossRef]
- Pinheiro, R.G.R.; Granja, A.; Loureiro, J.A.; Pereira, M.C.; Pinheiro, M.; Neves, A.R.; Reis, S. RVG29-Functionalized Lipid Nanoparticles for Quercetin Brain Delivery and Alzheimer’s Disease. Pharm. Res. 2020, 37, 139. [Google Scholar] [CrossRef]

| Natural Compound | Condition | Nº of Subjects | Duration | Phase | Main Outcomes | Status | Starting Year | Ref. |
|---|---|---|---|---|---|---|---|---|
| Vitamin E, vitamin C, alpha-lipoic acid or coenzyme Q | AD | 75 | 16 weeks | I | Vitamin E, vitamin C and alpha-lipoic acid group had shown less oxidative stress in the brain | Completed | 2006 | [40] |
| Vitamin E and selegiline | Moderate AD | 341 | 2 years | n.d. | Delay of symptoms and patients’ death | Completed | n.d. | [41] |
| Vitamin E and memantine | Mild to moderate AD | 613 | 5 years | III | Vitamin E slows functional decline. Memantine alone and vitamin E with memantine without effect | Completed | 2007 | [42] |
| Vitamin D3 and memantine | Moderate AD | 43 | 6 months | Pilot study | Improvement of patients’ cognition | Completed | 2011 | [43] |
| Vitamin B9, B6, and B12 | Mild to moderate AD | 340 | 18 weeks | III | No effect | Completed | 2003 | [44] |
| Vitamin B3 | Mild to moderate AD | 50 | 24 weeks | II | No results posted | Completed | 2008 | [45] |
| Vitamin E | AD with Down syndrome | 349 | 36 months | III | No results posted | Completed | 2000 | [46] |
| DHA | Mild to moderate AD | 295 | 18 months | III | No effect | Completed | 2007 | [47] |
| Bryostatin | Severe AD | 150 | 12 weeks | II | Improvement in cognitive function with lower dose; Safe and well-tolerated | Completed | 2015 | [48] |
| Scyllo-inositol | Mild to moderate AD | 353 | 78 weeks | II | Acceptable safety; No therapeutic effects | Completed | 2007 | [49] |
| Resveratrol | Mild to moderate AD | 119 | 52 weeks | II | Side effects; No therapeutic effects | Completed | 2012 | [50] |
| Resveratrol, glucose, and malate | Mild to moderate AD | 39 | 1 year | III | Safe and well tolerated; No therapeutic effects | Completed | 2007 | [51] |
| Curcumin | Mild to moderate AD | 36 | 24 weeks | II | Mostly well tolerated; No therapeutic effects | Completed | 2003 | [52] |
| Curcumin and Ginkgo extract | AD | 34 | 24 weeks | II | Lower oxidative stress in the brain | Completed | 2004 | [53] |
| Huperzine A | Mild to moderate AD | 177 | 16 weeks | II | Cognitive enhancement with higher dose; Safe and well tolerated at both doses | Completed | 2004 | [54] |
| Homotaurine | Mild to moderate AD | 1052 | 78 weeks | III | Slowing hippocampal atrophy | n.d | 2004 | [55] |
| Melatonin | Mild to moderate AD | 80 | 24 weeks | II | Improvement in cognitive performance | Completed | 2009 | [56] |
| EGCG | Early stage of AD | 21 | 18 months | III | No results posted | Completed | 2009 | [57] |
| Natural Compound | Condition | Nº of Subjects | Duration | Phase | Main Outcomes | Status | Starting Year | Ref. |
|---|---|---|---|---|---|---|---|---|
| Nicotine | Idiopathic PD | 65 | 14 weeks | I and II | Improvement of motor symptoms | Completed | 2009 | [85] |
| Caffeine | Idiopathic PD | 119 | 18 months | III | Decline in cognitive functions and improvement of alertness | Completed | 2012 | [86] |
| Cannabidiol | Idiopathic PD | 13 | 5 weeks | II | Improvement in several motor and non-motor symptoms | Completed | 2016 | [87] |
| Vitamins B6, B9, and B12 | PD | 40 | 6 weeks | n.d. | Improvement of homocysteine and glutathione levels | Completed | 2008 | [88] |
| Vitamin B3 | Early- and late-stage PD | 47 | 6 months | n.d. | Improvement of motor impairment; reduction of neuroinflammation | Completed | 2016 | [89] |
| Vitamin D | PD | 101 | 16 weeks | II | Improvement of PD patient’s balance | Completed | 2010 | [90] |
| Vitamin E with coenzyme Q10 | Early PD | 600 | 16 months | III | No therapeutic effect | Completed | 2008 | [91] |
| DHA | PD | 33 | 1.5 years | I | Reduced dyskinesia | Completed | 2018 | [92] |
| EGCG | de-novo PD | 480 | 1 year | II | Slight clinical benefit; Well tolerated | Completed | 2006 | [93] |
| Natural Compound | Condition | Nº of Subjects | Duration | Phase | Main Outcomes | Status | Starting Year | Ref. |
|---|---|---|---|---|---|---|---|---|
| Vitamin D3 | Relapsing-remitting MS | 129 | 96 weeks | II | No changes in the relapse rate; Less axonal loss and matrix destruction | Completed | 2009 | [108] |
| Vitamin A | Relapsing-remitting MS | 100 | 1 year | IV | No changes in the relapse rate and brain lesions but ameliorated symptoms | Completed | 2011 | [109,110] |
| Lipoic acid | MS | 54 | 2 years | III | Prevention of brain volume loss and amelioration of symptoms; Safe and well tolerated | Completed | 2010 | [111] |
| Ginkgo biloba, alpha-lipoic acid, essential fatty acids, vitamin E, and selenium | MS | n.d. | 4 years | II | No results posted | Completed | 1999 | [112] |
| Lipoic acid and omega-3 fatty acids | MS with cognitive impairment | 54 | 12 weeks | II | No significant improvement in cognitive function | Completed | 2014 | [113] |
| DHA and EPA | Relapsing-remitting MS | 50 | 1 year | IV | Decreased inflammatory markers with no other clinical benefits | Completed | 2010 | [114] |
| Melatonin | Relapsing-remitting MS | 25 | 1 year | II | Improved the relapse rate or brain injury; Safe and well tolerated | Completed | 2010 | [115] |
| EGCG and coconut oil | MS | 60 | 4 months | II | Decreased inflammatory markers and biomarkers for cardiovascular risk; Decrease in levels of depression | Completed | 2018 | [116,117,118,119] |
| Curcumin | Active relapsing MS | 80 | 2 years | II | No neuroprotective effects | Completed | 2012 | [120] |
| Natural Compound | Condition | Nº of Subjects | Duration | Phase | Main Outcomes | Status | Starting Year | Ref. |
|---|---|---|---|---|---|---|---|---|
| Vitamin B7 | Probable or confirmed ALS | 30 | 1 year | II | Safe and well tolerated | Completed | 2016 | [147] |
| Methylcobalamin (the active form of vitamin B12) | Probable or confirmed ALS | 373 | 3.5 years | II and III | Prolongs survival and retards the progression | Completed | 2007 | [148] |
| Vitamin B3 | Newly diagnosed or early ALS | 300 | 1 year | n.a. | n.a. | Recruiting | 2021 | [149] |
| Vitamin E | Probable or confirmed ALS | 32 | 10 weeks | III | No results posted | Completed | 2006 | [150] |
| Vitamin E, NAc cysteine, L-cystine, nicotinamide, and taurursodiol | Probable or confirmed ALS | 60 | 1 year | II | n.a. | Recruiting | 2022 | [151] |
| Tretinoin and pioglitazone | Probable or confirmed ALS | 28 | 3 years | I and II | No results posted | Completed | 2008 | [152] |
| Theracurcumin | Sporadic or familial ALS | 68 | 6 months | II | n.a. | Completed | 2020 | [153] |
| Resveratrol and curcumin in liposomes | Patients with clear diagnosis and symptomatology of ALS since at least 6 months | 60 | 4 months | II | n.a. | Recruiting | 2021 | [154] |
| Cannabidiol | Probable or confirmed ALS | 25 | 6 months | IV | n.a. | Not yet recruiting | n.a. | [155] |
| Natural Compound | Condition | Nº of Subjects | Duration | Phase | Main Outcomes | Status | Starting Year | Ref. |
|---|---|---|---|---|---|---|---|---|
| Cannabidiol and THC | HD | 24 | 12 weeks | II | No improvement in symptoms | Completed | 2011 | [163] |
| EGCG | HD | 54 | 1 year | II | No results posted | Completed | 2011 | [164] |
| Resveratrol | HD | 102 | 1 year | n.d. | No results posted | Completed | 2015 | [165] |
| Caffeine | HD | 100 | 2 years | n.d. | n.a. | Recruiting | n.a. | [166] |
| Vitamins B1 and B7 | HD with motor symptoms and/or neuropsychiatric | 24 | 52 weeks | II | n.a. | Not yet recruiting | n.a. | [167] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Andrade, S.; Nunes, D.; Dabur, M.; Ramalho, M.J.; Pereira, M.C.; Loureiro, J.A. Therapeutic Potential of Natural Compounds in Neurodegenerative Diseases: Insights from Clinical Trials. Pharmaceutics 2023, 15, 212. https://doi.org/10.3390/pharmaceutics15010212
Andrade S, Nunes D, Dabur M, Ramalho MJ, Pereira MC, Loureiro JA. Therapeutic Potential of Natural Compounds in Neurodegenerative Diseases: Insights from Clinical Trials. Pharmaceutics. 2023; 15(1):212. https://doi.org/10.3390/pharmaceutics15010212
Chicago/Turabian StyleAndrade, Stéphanie, Débora Nunes, Meghna Dabur, Maria J. Ramalho, Maria C. Pereira, and Joana A. Loureiro. 2023. "Therapeutic Potential of Natural Compounds in Neurodegenerative Diseases: Insights from Clinical Trials" Pharmaceutics 15, no. 1: 212. https://doi.org/10.3390/pharmaceutics15010212
APA StyleAndrade, S., Nunes, D., Dabur, M., Ramalho, M. J., Pereira, M. C., & Loureiro, J. A. (2023). Therapeutic Potential of Natural Compounds in Neurodegenerative Diseases: Insights from Clinical Trials. Pharmaceutics, 15(1), 212. https://doi.org/10.3390/pharmaceutics15010212