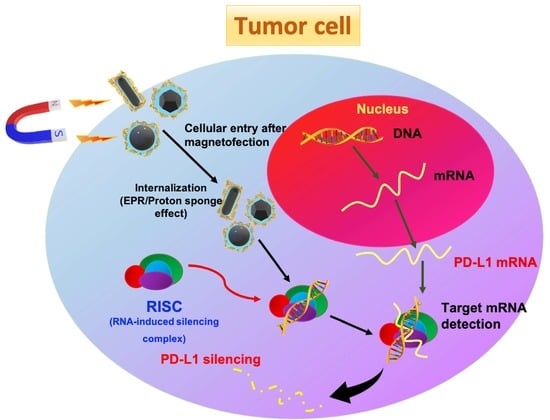
Comparing the Variants of Iron Oxide Nanoparticle-Mediated Delivery of miRNA34a for Efficiency in Silencing of PD-L1 Genes in Cancer Cells
Abstract
:1. Introduction
2. Materials and Methods
2.1. Synthesis of Different Shapes of Iron Oxide (Fe3O4) Nanoparticles
2.1.1. Iron Oxide Nanorods
2.1.2. Iron Oxide Nanospheres
2.1.3. Iron Oxide Truncated Octahedron
2.1.4. Surface Functionalization of Fe3O4 Nanoparticles (Nanorods, Nanospheres, and Truncated Octahedrons) by B-PEI 10K
2.2. Characterizations
2.3. Synthesis of Magnetoplexes and Transfection Efficiency Studies
2.4. Cell Culture and Cytotoxic Studies
2.5. Western Blot Analysis
2.6. Live/Dead Assay to Analyze Apoptosis via Cell Staining
2.7. Flow Cytometry for Cell Apoptosis
2.8. Quantitative Real-Time Polymerase Chain Reaction (qRT-PCR)
2.9. Statistical Analysis
3. Results and Discussion
3.1. Synthesis and Characterizations of Iron Oxide Nanoparticles with Different Shapes and Sizes
3.2. Preparation and Characterization of DNA/PEI@IONP Magnetoplexes for pDNA Delivery
3.3. Cytotoxicity of PEI@IONPs and DNA/PEI@IONPs
3.4. In Vitro Gene Transfection of miRNA34a Using PEI@IONPS
3.5. Apoptosis after Transfection with miRNA34a/PEI@IONPs
3.6. miRNA34a Expression Using Real-Time Quantitative Polymerase Chain Reaction (qRT-PCR)
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Nakamura, Y.; Mochida, A.; Choyke, P.L.; Kobayashi, H. Nanodrug delivery: Is the enhanced permeability and retention effect sufficient for curing cancer? Bioconjugate Chem. 2016, 27, 2225–2238. [Google Scholar] [CrossRef] [PubMed]
- Maggiora, G.; Gokhale, V. Non-specificity of drug-target interactions–consequences for drug discovery. In Proceedings of the Frontiers in Molecular Design and Chemical Information Science-Herman Skolnik Award Symposium 2015, Boston, MA, USA, 5 October 2016; pp. 91–142. [Google Scholar]
- Chenthamara, D.; Subramaniam, S.; Ramakrishnan, S.G.; Krishnaswamy, S.; Essa, M.M.; Lin, F.H.; Qoronfleh, M.W. Therapeutic efficacy of nanoparticles and routes of administration. Biomater. Res. 2019, 23, 1–29. [Google Scholar] [CrossRef] [PubMed]
- Xie, X.; Zhang, Y.; Li, F.; Lv, T.; Li, Z.; Chen, H.; Jia, L.; Gao, Y. Challenges and opportunities from basic cancer biology for nanomedicine for targeted drug delivery. Curr. Cancer Drug Targets 2019, 19, 257–276. [Google Scholar] [CrossRef] [PubMed]
- Soetaert, F.; Korangath, P.; Serantes, D.; Fiering, S.; Ivkov, R. Cancer therapy with iron oxide nanoparticles: Agents of thermal and immune therapies. Adv. Drug Deliv. Rev. 2020, 163, 65–83. [Google Scholar] [CrossRef] [PubMed]
- Niemeyer, C.M. Nanoparticles, proteins, and nucleic acids: Biotechnology meets materials science. Angew. Chem. Int. Ed. 2001, 40, 4128–4158. [Google Scholar] [CrossRef]
- Kamaly, N.; Xiao, Z.; Valencia, P.M.; Radovic-Moreno, A.F.; Farokhzad, O.C. Targeted polymeric therapeutic nanoparticles: Design, development and clinical translation. Chem. Soc. Rev. 2012, 41, 2971–3010. [Google Scholar] [CrossRef]
- Nath, S.; Kaittanis, C.; Ramachandran, V.; Dalal, N.S.; Perez, J.M. Synthesis, magnetic characterization, and sensing applications of novel dextran-coated iron oxide nanorods. Chem. Mater. 2009, 21, 1761–1767. [Google Scholar] [CrossRef] [Green Version]
- Sharma, S.; Javed, M.N.; Pottoo, F.H.; Rabbani, S.A.; Barkat, M.; Sarafroz, M.; Amir, M. Bioresponse inspired nanomaterials for targeted drug and gene delivery. Pharm. Nanotechnol. 2019, 7, 220–233. [Google Scholar] [CrossRef]
- Tiwari, I.; Singh, M.; Pandey, C.M.; Sumana, G. Electrochemical genosensor based on graphene oxide modified iron oxide–chitosan hybrid nanocomposite for pathogen detection. Sens. Actuators B Chem. 2015, 206, 276–283. [Google Scholar] [CrossRef]
- Lugin, M.L.; Lee, R.T.; Kwon, Y.J. Synthetically engineered adeno-associated virus for efficient, safe, and versatile gene therapy applications. ACS Nano 2020, 14, 14262–14283. [Google Scholar] [CrossRef]
- Wang, X.; Zhou, L.; Ma, Y.; Li, X.; Gu, H. Control of aggregate size of polyethyleneimine-coated magnetic nanoparticles for magnetofection. Nano Res. 2009, 2, 365–372. [Google Scholar] [CrossRef] [Green Version]
- Wahajuddin, S.A. Superparamagnetic iron oxide nanoparticles: Magnetic nanoplatforms as drug carriers. Int. J. Nanomed. 2012, 7, 3445. [Google Scholar] [CrossRef] [Green Version]
- Arami, H.; Khandhar, A.; Liggitt, D.; Krishnan, K.M. In vivo delivery, pharmacokinetics, biodistribution and toxicity of iron oxide nanoparticles. Chem. Soc. Rev. 2015, 44, 8576–8607. [Google Scholar] [CrossRef] [Green Version]
- Schladt, T.D.; Schneider, K.; Schild, H.; Tremel, W. Synthesis and bio-functionalization of magnetic nanoparticles for medical diagnosis and treatment. Dalton Trans. 2011, 40, 6315–6343. [Google Scholar] [CrossRef]
- Danbaran, G.R.; Aslani, S.; Sharafkandi, N.; Hemmatzadeh, M.; Hosseinzadeh, R.; Azizi, G.; Jadidi-Niaragh, F.; Babaie, F.; Mohammadi, H. How microRNAs affect the PD-L1 and its synthetic pathway in cancer. Int. Immunopharmacol. 2020, 84, 106594. [Google Scholar] [CrossRef]
- Jiang, X.; Wang, J.; Deng, X.; Xiong, F.; Ge, J.; Xiang, B.; Wu, X.; Ma, J.; Zhou, M.; Li, X. Role of the tumor microenvironment in PD-L1/PD-1-mediated tumor immune escape. Mol. Cancer 2019, 18, 1–17. [Google Scholar] [CrossRef] [Green Version]
- Liu, Y.; Zhou, Z.; Hou, J.; Xiong, W.; Kim, H.; Chen, J.; Zheng, C.; Jiang, X.; Yoon, J.; Shen, J. Tumor Selective Metabolic Reprogramming as a Prospective PD-L1 Depression Strategy to Reactivate Immunotherapy. Adv. Mater. 2022, 34, 2206121. [Google Scholar] [CrossRef]
- Zhou, Z.; Chen, J.; Liu, Y.; Zheng, C.; Luo, W.; Chen, L.; Zhou, S.; Li, Z.; Shen, J. Cascade two-stage tumor re-oxygenation and immune re-sensitization mediated by self-assembled albumin-sorafenib nanoparticles for enhanced photodynamic immunotherapy. Acta Pharm. Sin. B 2022, 12, 4204–4223. [Google Scholar] [CrossRef]
- Cao, W.; Fan, R.; Wang, L.; Cheng, S.; Li, H.; Jiang, J.; Geng, M.; Jin, Y.; Wu, Y. Expression and regulatory function of miRNA-34a in targeting survivin in gastric cancer cells. Tumor Biol. 2013, 34, 963–971. [Google Scholar] [CrossRef]
- Aghdam, S.G.; Ebrazeh, M.; Hemmatzadeh, M.; Seyfizadeh, N.; Shabgah, A.G.; Azizi, G.; Ebrahimi, N.; Babaie, F.; Mohammadi, H. The role of microRNAs in prostate cancer migration, invasion, and metastasis. J. Cell. Physiol. 2019, 234, 9927–9942. [Google Scholar] [CrossRef]
- Wang, X.; Li, J.; Dong, K.; Lin, F.; Long, M.; Ouyang, Y.; Wei, J.; Chen, X.; Weng, Y.; He, T.; et al. Tumor suppressor miR-34a targets PD-L1 and functions as a potential immunotherapeutic target in acute myeloid leukemia. Cell. Signal. 2015, 27, 443–452. [Google Scholar] [CrossRef] [PubMed]
- Deng, S.; Wang, M.; Wang, C.; Zeng, Y.; Qin, X.; Tan, Y.; Liang, B.; Cao, Y. P53 downregulates PD-L1 expression via miR-34a to inhibit the growth of triple-negative breast cancer cells: A potential clinical immunotherapeutic target. Mol. Biol. Rep. 2022, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Shahidi, M.; Abazari, O.; Dayati, P.; Bakhshi, A.; Zavarreza, J.; Modarresi, M.H.; Haghiralsadat, F.; Rahmanian, M.; Naghib, S.M.; Tofighi, D. Multicomponent siRNA/miRNA-loaded modified mesoporous silica nanoparticles targeted bladder cancer for a highly effective combination therapy. Front. Bioeng. Biotechnol. 2022, 10, 9704. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; Cheng, Y.S.L.; Matthen, M.; Yoon, A.; Schwartz, G.K.; Bala, S.; Taylor, A.M.; Momen-Heravi, F. Down-regulation of the tumor suppressor miR-34a contributes to head and neck cancer by up-regulating the MET oncogene and modulating tumor immune evasion. J. Exp. Clin. Cancer Res. 2021, 40, 70. [Google Scholar] [CrossRef] [PubMed]
- Ali, A.; Hira Zafar, M.Z.; ul Haq, I.; Phull, A.R.; Ali, J.S.; Hussain, A. Synthesis, characterization, applications, and challenges of iron oxide nanoparticles. Nanotechnol. Sci. Appl. 2016, 9, 49. [Google Scholar] [CrossRef] [Green Version]
- Mohapatra, J.; Mitra, A.; Tyagi, H.; Bahadur, D.; Aslam, M. Iron oxide nanorods as high-performance magnetic resonance imaging contrast agents. Nanoscale 2015, 7, 9174–9184. [Google Scholar] [CrossRef] [Green Version]
- Park, J.; An, K.; Hwang, Y.; Park, J.G.; Noh, H.J.; Kim, J.Y.; Hyeon, T. Ultra-large-scale syntheses of monodisperse nanocrystals. Nat. Mater. 2004, 3, 891–895. [Google Scholar] [CrossRef]
- Kemp, S.J.; Ferguson, R.M.; Khandhar, A.P.; Krishnan, K.M. Monodisperse magnetite nanoparticles with nearly ideal saturation magnetization. RSC Adv. 2016, 6, 77452–77464. [Google Scholar] [CrossRef]
- Huang, C.C.; Tsai, C.Y.; Sheu, H.S.; Chuang, K.Y.; Su, C.H.; Jeng, U.S.; Cheng, F.Y.; Su, C.H.; Lei, H.Y.; Yeh, C.S. Enhancing transversal relaxation for magnetite nanoparticles in MR imaging using Gd3+–chelated mesoporous silica shells. ACS Nano 2011, 5, 3905–3916. [Google Scholar] [CrossRef]
- Jadhao, M.; Tsai, E.M.; Yang, H.C.; Chen, Y.F.; Liang, S.S.; Wang, T.N.; Teng, Y.N.; Huang, H.W.; Wang, L.F.; Chiu, C.C. The long-term DEHP exposure confers multidrug resistance of triple-negative breast cancer cells through ABC transporters and intracellular ROS. Antioxidants 2021, 10, 949. [Google Scholar] [CrossRef]
- Hasakova, K.; Reis, R.; Vician, M.; Zeman, M.; Herichova, I. Expression of miR-34a-5p is up-regulated in human colorectal cancer and correlates with survival and clock gene PER2 expression. PLoS ONE 2019, 14, e0224396. [Google Scholar] [CrossRef] [Green Version]
- Chen, Y.; He, B.; Huang, T.; Liu, H. Controlled synthesis of palladium icosahedra nanocrystals by reducing H2PdCl4 with tetraethylene glycol. Colloids Surf. A: Physicochem. Eng. Asp. 2009, 348, 145–150. [Google Scholar] [CrossRef]
- Nikolopoulou, S.G.; Boukos, N.; Sakellis, E.; Efthimiadou, E.K. Synthesis of biocompatible silver nanoparticles by a modified polyol method for theranostic applications: Studies on red blood cells, internalization ability and antibacterial activity. J. Inorg. Biochem. 2020, 211, 111177. [Google Scholar] [CrossRef]
- Zhang, L.; Wu, J.; Liao, H.; Hou, Y.; Gao, S. Octahedral Fe3O4 nanoparticles and their assembled structures. Chem. Commun. 2009, 4378–4380. [Google Scholar] [CrossRef]
- Ding, Y.; Jiang, Z.; Saha, K.; Kim, C.S.; Kim, S.T.; Landis, R.F.; Rotello, V.M. Gold nanoparticles for nucleic acid delivery. Mol. Ther. 2014, 22, 1075–1083. [Google Scholar] [CrossRef] [Green Version]
- Gokduman, K.; Gok, A. In Vitro Investigation of Therapeutic Potential of Bare Magnetite (Fe3O4) Nanoparticles (≤ 100 ppm) on Hepatocellular Carcinoma Cells. J. Nanosci. Nanotechnol. 2020, 20, 1391–1400. [Google Scholar] [CrossRef]
- Mohapatra, J.; Mitra, A.; Bahadur, D.; Aslam, M. Surface controlled synthesis of MFe2O4 (M= Mn, Fe, Co, Ni and Zn) nanoparticles and their magnetic characteristics. CrystEngComm 2013, 15, 524–532. [Google Scholar] [CrossRef]
- Jun, Y.W.; Huh, Y.M.; Choi, J.S.; Lee, J.H.; Song, H.T.; Kim, S.; Yoon, S.; Kim, K.S.; Shin, J.S.; Suh, J.S. Nanoscale size effect of magnetic nanocrystals and their utilization for cancer diagnosis via magnetic resonance imaging. J. Am. Chem. Soc. 2005, 127, 5732–5733. [Google Scholar] [CrossRef]
- El Ghandoor, H.; Zidan, H.; Khalil, M.M.; Ismail, M. Synthesis and some physical properties of magnetite (Fe3O4) nanoparticles. Int. J. Electrochem. Sci. 2012, 7, 5734–5745. [Google Scholar]
- Hu, Y.; Xu, B.H.; Xu, J.J.; Shou, D.; Gao, J.Q. Synthesis of mannosylated polyethylenimine and its potential application as cell-targeting non-viral vector for gene therapy. Polymers 2014, 6, 2573–2587. [Google Scholar] [CrossRef] [Green Version]
- Lazarus, G.G.; Singh, M. In vitro cytotoxic activity and transfection efficiency of polyethyleneimine functionalized gold nanoparticles. Colloids Surf. B: Biointerfaces 2016, 145, 906–911. [Google Scholar] [CrossRef] [PubMed]
- Petri-Fink, A.; Steitz, B.; Finka, A.; Salaklang, J.; Hofmann, H. Effect of cell media on polymer coated superparamagnetic iron oxide nanoparticles (SPIONs): Colloidal stability, cytotoxicity, and cellular uptake studies. Eur. J. Pharm. Biopharm. 2008, 68, 129–137. [Google Scholar] [CrossRef] [PubMed]
- Puddu, M.; Broguiere, N.; Mohn, D.; Zenobi-Wong, M.; Stark, W.J.; Grass, R.N. Magnetically deliverable calcium phosphate nanoparticles for localized gene expression. RSC Adv. 2015, 5, 9997–10004. [Google Scholar] [CrossRef]
- Chen, J.; Jiang, C.; Jin, L.; Zhang, X. Regulation of PD-L1: A novel role of pro-survival signalling in cancer. Ann. Oncol. 2016, 27, 409–416. [Google Scholar] [CrossRef]
- Song, M.; Bode, A.M.; Dong, Z.; Lee, M.-H. AKT as a Therapeutic Target for CancerChallenging Cancer Therapy by Targeting AKT. Cancer Res. 2019, 79, 1019–1031. [Google Scholar] [CrossRef] [Green Version]
- Cretella, D.; Digiacomo, G.; Giovannetti, E.; Cavazzoni, A. PTEN alterations as a potential mechanism for tumor cell escape from PD-1/PD-L1 inhibition. Cancers 2019, 11, 1318. [Google Scholar] [CrossRef] [Green Version]
- Bhattacharyya, S.; Ghosh, S.S. Transmembrane TNFα-expressed macrophage membrane-coated chitosan nanoparticles as cancer therapeutics. ACS Omega 2020, 5, 1572–1580. [Google Scholar] [CrossRef]







Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pandey, R.; Yang, F.-S.; Sivasankaran, V.P.; Lo, Y.-L.; Wu, Y.-T.; Chang, C.-Y.; Chiu, C.-C.; Liao, Z.-X.; Wang, L.-F. Comparing the Variants of Iron Oxide Nanoparticle-Mediated Delivery of miRNA34a for Efficiency in Silencing of PD-L1 Genes in Cancer Cells. Pharmaceutics 2023, 15, 215. https://doi.org/10.3390/pharmaceutics15010215
Pandey R, Yang F-S, Sivasankaran VP, Lo Y-L, Wu Y-T, Chang C-Y, Chiu C-C, Liao Z-X, Wang L-F. Comparing the Variants of Iron Oxide Nanoparticle-Mediated Delivery of miRNA34a for Efficiency in Silencing of PD-L1 Genes in Cancer Cells. Pharmaceutics. 2023; 15(1):215. https://doi.org/10.3390/pharmaceutics15010215
Chicago/Turabian StylePandey, Richa, Feng-Shuo Yang, Vyshnav Punnath Sivasankaran, Yu-Lun Lo, Yi-Ting Wu, Chia-Yu Chang, Chien-Chih Chiu, Zi-Xian Liao, and Li-Fang Wang. 2023. "Comparing the Variants of Iron Oxide Nanoparticle-Mediated Delivery of miRNA34a for Efficiency in Silencing of PD-L1 Genes in Cancer Cells" Pharmaceutics 15, no. 1: 215. https://doi.org/10.3390/pharmaceutics15010215
APA StylePandey, R., Yang, F. -S., Sivasankaran, V. P., Lo, Y. -L., Wu, Y. -T., Chang, C. -Y., Chiu, C. -C., Liao, Z. -X., & Wang, L. -F. (2023). Comparing the Variants of Iron Oxide Nanoparticle-Mediated Delivery of miRNA34a for Efficiency in Silencing of PD-L1 Genes in Cancer Cells. Pharmaceutics, 15(1), 215. https://doi.org/10.3390/pharmaceutics15010215