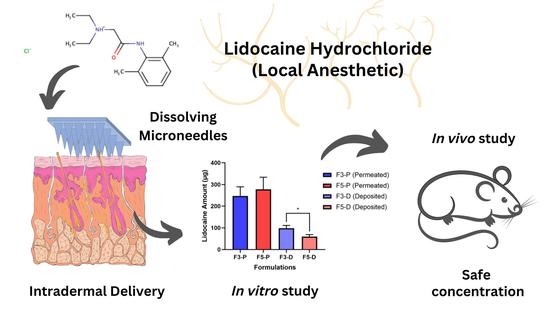
Enhancing Intradermal Delivery of Lidocaine by Dissolving Microneedles: Comparison between Hyaluronic Acid and Poly(Vinyl Pyrrolidone) Backbone Polymers
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
2.2.1. Fabrication and Physical Evaluation of Lidocaine Hydrochloride-Loaded Dissolving Microneedles (LiH-DMNs)
2.2.2. Mechanical Strength of LiH-Loaded DMNs
2.2.3. Loss of Mass
2.2.4. Insertion Study
2.2.5. Ex Vivo Skin-Dissolution Study
2.2.6. Determination of Drug Content in the Needles
2.2.7. Stability Study of LiH in DMN
2.2.8. In Vitro Permeation Study
2.2.9. In Vivo Permeation Study
2.2.10. Analytical Method of LiH and Chromatographic Condition
2.2.11. Statistical Analysis
3. Results and Discussion
3.1. Fabrication and Physical Evaluation of Lidocaine Hydrochloride-Loaded Dissolving Microneedles (LiH-DMNs)
3.2. Mechanical Strength of LiH-Loaded DMNs
3.3. Loss of Mass
3.4. Skin Simulation Insertion Study
3.5. Ex Vivo Skin-Dissolution Study
3.6. Determination of Drug Content in the Needles
3.7. Stability Study of Lidocaine
3.8. In Vitro Permeation Study
3.9. In Vivo Permation Study
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gahalaut, P.; Mishra, N.; Chauhan, S.; Rastogi, M.K. Comparison of Topical Anesthetics for Radiofrequency Ablation of Achrocordons: Eutectic Mixture of Lignocaine/Prilocaine versus Lidocaine/Tetracaine. ISRN Dermatol. 2014, 2014, 43027. [Google Scholar] [CrossRef] [PubMed]
- Gaskin, D.J.; Richard, P. The economic costs of pain in the United States. J. Pain 2012, 13, 715–724. [Google Scholar] [CrossRef] [PubMed]
- Dusek, J.A.; Griffin, K.H.; Finch, M.D.; Rivard, R.L.; Watson, D. Cost Savings from Reducing Pain Through the Delivery of Integrative Medicine Program to Hospitalized Patients. J. Altern Complement Med. 2018, 24, 557–563. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, H.; Kang, G.; Jang, M.; Um, D.J.; Shin, J.; Kim, H.; Hong, J.; Jung, H.; Ahn, H.; Gong, S.; et al. Development of lidocaine-loaded dissolving microneedle for rapid and efficient local anesthesia. Pharmaceutics 2020, 12, 1067. [Google Scholar] [CrossRef] [PubMed]
- Lin, E.J.; Jacob, S.E. Lidocaine. J. Dermatol. Nurses Assoc. 2016, 8, 394–396. [Google Scholar] [CrossRef]
- Chamaraux-Tran, T.N.; Piegeler, T. The amide local anesthetic lidocaine in cancer surgery-potential antimetastatic effects and preservation of immune cell function? A narrative review. Front Med. 2017, 4, 235. [Google Scholar] [CrossRef] [Green Version]
- Kottke, D.; Majid, H.; Breitkreutz, J.; Burckhardt, B.B. Ex-vivo permeation studies to facilitate the development of a buccal child-appropriate dosage form by using lidocaine minitablets. In Proceedings of the 12th International Conference and Workshop on Biological Barriers, Saarbrücken, Germany, 27–29 August 2018; Volume 2005, p. 402240. [Google Scholar]
- FDA. Xylocaine (lidocaine HCl Injection, USP). 2010; 5. Available online: https://www.accessdata.fda.gov/drugsatfda_docs/label/2010/006488s074lbl.pdf (accessed on 17 April 2022).
- Ramadon, D.; Permana, A.D.; Courtenay, A.J.; McCrudden, M.T.C.; Tekko, I.A.; McAlister, E.; Anjani, Q.K.; Utomo, E.; McCarthy, H.O.; Donnelly, R.F. Development, Evaluation, and Pharmacokinetic Assessment of Polymeric Mi-croarray Patches for Transdermal Delivery of Vancomycin Hydrochloride. Mol. Pharm. 2020, 17, 3353–3368. [Google Scholar] [CrossRef]
- Ramadon, D.; McCrudden, M.T.C.; Courtenay, A.J.; Donnelly, R.F. Enhancement strategies for transdermal drug delivery systems: Current trends and applications. Drug Deliv. Transl. Res. 2021, 12, 758–791. [Google Scholar] [CrossRef]
- Williams, D.G. Drugs and Poisons. In Renal Disease: An Illustrated Guide; Springer: Dordrecht, The Netherlands, 1981; pp. 73–76. [Google Scholar] [CrossRef]
- Mehra, P.; Caiazzo, A.; Maloney, P. Lidocaine Toxicity. Anesth. Prog. 1998, 45, 38–41. [Google Scholar] [CrossRef]
- Kumar, M.; Chawla, R.; Goyal, M. Topical anesthesia. J. Anaesthesiol. Clin. Pharmacol. 2015, 31, 450–456. [Google Scholar] [CrossRef]
- Leite-Silva, V.R.; De Almeida, M.M.; Fradin, A.; Grice, J.E.; Roberts, M.S. Delivery of drugs applied topically to the skin. Expert Rev. Dermatol. 2012, 7, 383–397. [Google Scholar] [CrossRef]
- Gorzelanny, C.; Mess, C.; Schneider, S.W.; Huck, V.; Brandner, J.M. Skin barriers in dermal drug delivery: Which barriers have to be overcome and how can we measure them? Pharmaceutics 2020, 12, 684. [Google Scholar] [CrossRef]
- Zhang, Y.; Brown, K.; Siebenaler, K.; Determan, A.; Dohmeier, D.; Hansen, K. Development of lidocaine-coated microneedle product for rapid, safe, and prolonged local analgesic action. Pharm. Res. 2012, 29, 170–177. [Google Scholar] [CrossRef]
- Matsumoto, T.; Chaki, T.; Hirata, N.; Yamakage, M. The eutectic mixture local anesthetics (EMLA) cream is more effective on venipuncture pain compared with lidocaine tape in the same patients. JA Clin. Rep. 2018, 4, 73. [Google Scholar] [CrossRef] [PubMed]
- Kumar Mandal, U.; Chatterjee, B.; Pauzi, F.H.B. A Review on Transdermal Spray: Formulation Aspect. Rev. Transdermal. Spray Formul. Asp. M. J. Phar. 2016, 2, 6. [Google Scholar]
- Mohammadi-Samani, S.; Jamshidzadeh, A.; Montaseri, H.; Rangbar-Zahedani, M.; Kianrad, R. The effects of some permeability enhancers on the percutaneous absorption of lidocaine. Pak. J. Pharm. Sci. 2010, 23, 83–88. [Google Scholar] [PubMed]
- Donnelly, R.F.; Singh, T.R.R.; Larrañeta, E.; McCrudden, M.T.C. Microneedles for Drug and Vaccine Delivery. Adv. Drug Deliv. Rev. 2018, 64, 1547–1568. [Google Scholar] [CrossRef] [Green Version]
- Peng, K.; Vora, L.K.; Tekko, I.A.; Permana, A.D.; Domínguez-Robles, J.; Ramadon, D.; Chambers, P.; McCarthy, H.O.; Larrañeta, E.; Donnelly, R.F. Dissolving microneedle patches loaded with amphotericin B microparticles for localised and sustained intradermal delivery: Potential for enhanced treatment of cutaneous fungal infections. J. Control. Release 2021, 339, 361–380. [Google Scholar] [CrossRef]
- Waghule, T.; Singhvi, G.; Dubey, S.K.; Pandey, M.M.; Gupta, G.; Singh, M.; Dua, K. Microneedles: A smart approach and increasing potential for transdermal drug delivery system. Biomed. Pharm. 2019, 109, 1249–1258. [Google Scholar] [CrossRef]
- Kurakula, M.; Rao, G.S.N.K. Pharmaceutical assessment of polyvinylpyrrolidone (PVP): As excipient from conventional to controlled delivery systems with a spotlight on COVID-19 inhibition. J. Drug Deliv. Sci. Technol. 2020, 60, 102046. [Google Scholar] [CrossRef]
- Harrer, D.; Armengol, E.S.; Friedl, J.D.; Jalil, A.; Jelkmann, M.; Leichner, C.; Laffleur, F. Is hyaluronic acid the perfect excipient for the pharmaceutical need? Int. J. Pharm. 2021, 601, 120589. [Google Scholar] [CrossRef]
- Huang, D.; Li, J.; Li, T.; Wang, Z.; Wang, Q.; Li, Z. Recent advances on fabrication of microneedles on the flexible substrate. J. Micromech. Microeng. 2021, 31, 073001. [Google Scholar] [CrossRef]
- Putri, H.E.; Utami, R.N.; Wahyudin, E.; Oktaviani, W.W.; Mudjahid, M.; Permana, A.D. Dissolving Microneedle Formulation of Ceftriaxone: Effect of Polymer Concentrations on Characterisation and Ex Vivo Permeation Study. J. Pharm. Innov. 2021, 17, 1176–1188. [Google Scholar] [CrossRef]
- Anjani, Q.K.; Sabri AHBin Utomo, E.; Domínguez-Robles, J.; Donnelly, R.F. Elucidating the Impact of Surfactants on the Performance of Dissolving Microneedle Array Patches. Mol. Pharm. 2022, 19, 1191–1208. [Google Scholar] [CrossRef] [PubMed]
- Larrañeta, E.; Moore, J.; Vicente-Pérez, E.M.; González-Vázquez, P.; Lutton, R.; Woolfson, A.D.; Donnelly, R.F. A proposed model membrane and test method for microneedle insertion studies. Int. J. Pharm. 2014, 472, 65–73. [Google Scholar] [CrossRef] [Green Version]
- Overmyer, K.A.; Thonusin, C.; Qi, N.R.; Burant, C.F.; Evans, C.R. Impact of anesthesia and euthanasia on metabolomics of mammalian tissues: Studies in a C57BL/6J mouse model. PLoS ONE 2015, 10, e0117232. [Google Scholar] [CrossRef]
- EMA. Guideline on Bioanalytical Method Validation Guideline on Bioanalytical Method Validation Table of Contents. Eur. Med. Agency 2011, 44, 510. [Google Scholar]
- FDA. Analytical Method Validation. In New Drug Development: Regulatory Paradigms for Clinical Pharmacology and Biopharmaceutics; CRC Press: Boca Raton, FL, USA, 2016; pp. 138–159. [Google Scholar] [CrossRef]
- Anjani, Q.K.; Sabri, A.H.B.; Moreno-Castellanos, N.; Utomo, E.; Cárcamo-Martínez, Á.; Domínguez-Robles, J.; Hari Wardoyo, A.L.; Donnelly, R.F. Soluplus®-based dissolving microarray patches loaded with colchicine: Towards a minimally invasive treatment and management of gout. Biomater Sci. 2022, 10, 5838–5855. [Google Scholar] [CrossRef]
- Zhao, Z.Q.; Zhang, B.L.; Chu, H.Q.; Liang, L.; Chen, B.Z.; Zheng, H.; Guo, X.D. A high-dosage microneedle for programmable lidocaine delivery and enhanced local long-lasting analgesia. Mater Sci. Eng. C 2021, 133, 112620. [Google Scholar] [CrossRef]
- Bhusal, P.; Sharma, M.; Harrison, J.; Procter, G.; Andrews, G.; Jones, D.S.; Hill, A.G.; Svirskis, D. Development, Validation and Application of a Stability Indicating HPLC Method to Quantify Lidocaine from Polyethylene-co-Vinyl Acetate (EVA) Matrices and Biological Fluids. J. Chromatogr. Sci. 2017, 55, 832–838. [Google Scholar] [CrossRef]
- Schleusener, J.; Salazar, A.; von Lademann, H.J.J.; Darvin, M.E. Retaining skin barrier function properties of the stratum corneum with components of the natural moisturizing factor—A randomized, placebo-controlled double-blind in vivo study. Molecules 2021, 26, 1649. [Google Scholar] [CrossRef] [PubMed]
- Osseiran, S.; Cruz JDela Jeong, S.; Wang, H.; Fthenakis, C.; Evans, C.L. Characterizing stratum corneum structure, barrier function, and chemical content of human skin with coherent Raman scattering imaging. Biomed. Opt. Express. 2018, 9, 6425. [Google Scholar] [CrossRef] [PubMed]
- Dąbrowska, A.K.; Spano, F.; Derler, S.; Adlhart, C.; Spencer, N.D.; Rossi, R.M. The relationship between skin function, barrier properties, and body-dependent factors. Ski. Res. Technol. 2018, 24, 165–174. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shim, W.S.; Hwang, Y.M.; Park, S.G.; Lee, C.K.; Kang, N.G. Role of Polyvinylpyrrolidone in Dissolving Microneedle for Efficient Transdermal Drug Delivery: In vitro and Clinical Studies. Bull. Korean Chem. Soc. 2018, 39, 789–793. [Google Scholar] [CrossRef]
- Dovedytis, M.; Liu, Z.J.; Bartlett, S. Hyaluronic acid and its biomedical applications: A review. Eng. Regen. 2020, 1, 102–113. [Google Scholar] [CrossRef]
- Wang, Q.L.; Ren, J.W.; Chen, B.Z.; Jin, X.; Zhang, C.Y.; Guo, X.D. Effect of humidity on mechanical properties of dissolving microneedles for transdermal drug delivery. J. Ind. Eng. Chem. 2018, 59, 251–258. [Google Scholar] [CrossRef]
- Liu, Y.; Cheng, M.; Zhao, J.; Zhang, X.; Huang, Z.; Zang, Y.; Ding, Y. Transdermal Delivery of Lidocaine-Loaded Elastic. Biomedicines 2021, 9, 592. [Google Scholar] [CrossRef]
- Lee, I.C.; Wu, Y.C.; Tsai, S.W.; Chen, C.H.; Wu, M.H. Fabrication of two-layer dissolving polyvinylpyrrolidone microneedles with different molecular weights for: In vivo insulin transdermal delivery. RSC Adv. 2017, 7, 5067–5075. [Google Scholar] [CrossRef] [Green Version]
- Necas, J.; Bartosikova, L.; Brauner, P.; Kolar, J. Hyaluronic acid (hyaluronan): A review. Vet. Med. 2008, 53, 397–411. [Google Scholar] [CrossRef] [Green Version]
- How, K.N.; Yap, W.H.; Lim, C.L.H.; Goh, B.H.; Lai, Z.W. Hyaluronic Acid-Mediated Drug Delivery System Targeting for Inflammatory Skin Diseases: A Mini Review. Front. Pharmacol. 2020, 11, 1105. [Google Scholar] [CrossRef]
- Harmita. Analisis Fisikokimia: Kromatografi; EGC: Jakarta, Indonesia, 2014. [Google Scholar]
- Wei, Y. Masking the Bitter Taste of Injectable Lidocaine HCl Formulation for Dental Procedures. Aaps Pharmscitech 2015, 16, 455–465. [Google Scholar] [CrossRef] [Green Version]
- Yang, X.; Wei, X.; Mu, Y.; Li, Q.; Liu, J. A review of the mechanism of the central analgesic effect of lidocaine. Medicine 2020, 99, e19898. [Google Scholar] [CrossRef] [PubMed]
- Cherobin, A.C.F.P.; Tavares, G.T. Safety of local anesthetics. An. Bras. De Dermatol. 2020, 95, 82–90. [Google Scholar] [CrossRef]
- Draelos, Z.D.; Diaz, I.; Namkoong, J.; Wu, J.; Boyd, T. Efficacy Evaluation of a Topical Hyaluronic Acid Serum in Facial Photoaging. Dermatol. Ther. 2021, 11, 1385–1394. [Google Scholar] [CrossRef] [PubMed]
- Saha, I.; Rai, V.K. Hyaluronic acid based microneedle array: Recent applications in drug delivery and cosmetology. Carbohydr. Polym. 2021, 267, 118168. [Google Scholar] [CrossRef] [PubMed]






| Formulations | Composition (% w/w) | |||
|---|---|---|---|---|
| LiH | HA | PVP-K30 | Water | |
| F1 | 5 | 2.5 | - | Ad 100 |
| F2 | 5 | 5 | - | Ad 100 |
| F3 | 5 | 10 | - | Ad 100 |
| F4 | 5 | - | 20 | Ad 100 |
| F5 | 5 | - | 25 | Ad 100 |
| F6 | 5 | - | 30 | Ad 100 |
| Formulations | Physical Appearance Parameter | Decision | |||||
|---|---|---|---|---|---|---|---|
| Air Bubbles | Drug Precipitation | Breaking | Flat Baseplate | Optimal Needles Filling | Optimal Needle Heights | ||
| F1 | ☓ | ✓ | ✓ | ☓ | ✓ | ☓ | Discarded |
| F2 | ✓ | ☓ | ✓ | ☓ | ✓ | ☓ | Discarded |
| F3 | ☓ | ☓ | ☓ | ☓ | ✓ | ✓ | Selected |
| F4 | ☓ | ☓ | ☓ | ✓ | ✓ | ✓ | Selected |
| F5 | ☓ | ☓ | ☓ | ✓ | ✓ | ✓ | Selected |
| F6 | ☓ | ☓ | ☓ | ✓ | ✓ | ✓ | Selected |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ramadon, D.; Sutrisna, L.F.P.; Harahap, Y.; Putri, K.S.S.; Ulayya, F.; Hartrianti, P.; Anjani, Q.K.; Donnelly, R.F. Enhancing Intradermal Delivery of Lidocaine by Dissolving Microneedles: Comparison between Hyaluronic Acid and Poly(Vinyl Pyrrolidone) Backbone Polymers. Pharmaceutics 2023, 15, 289. https://doi.org/10.3390/pharmaceutics15010289
Ramadon D, Sutrisna LFP, Harahap Y, Putri KSS, Ulayya F, Hartrianti P, Anjani QK, Donnelly RF. Enhancing Intradermal Delivery of Lidocaine by Dissolving Microneedles: Comparison between Hyaluronic Acid and Poly(Vinyl Pyrrolidone) Backbone Polymers. Pharmaceutics. 2023; 15(1):289. https://doi.org/10.3390/pharmaceutics15010289
Chicago/Turabian StyleRamadon, Delly, Lissa Florencia Putri Sutrisna, Yahdiana Harahap, Kurnia Sari Setio Putri, Fathin Ulayya, Pietradewi Hartrianti, Qonita Kurnia Anjani, and Ryan F. Donnelly. 2023. "Enhancing Intradermal Delivery of Lidocaine by Dissolving Microneedles: Comparison between Hyaluronic Acid and Poly(Vinyl Pyrrolidone) Backbone Polymers" Pharmaceutics 15, no. 1: 289. https://doi.org/10.3390/pharmaceutics15010289
APA StyleRamadon, D., Sutrisna, L. F. P., Harahap, Y., Putri, K. S. S., Ulayya, F., Hartrianti, P., Anjani, Q. K., & Donnelly, R. F. (2023). Enhancing Intradermal Delivery of Lidocaine by Dissolving Microneedles: Comparison between Hyaluronic Acid and Poly(Vinyl Pyrrolidone) Backbone Polymers. Pharmaceutics, 15(1), 289. https://doi.org/10.3390/pharmaceutics15010289