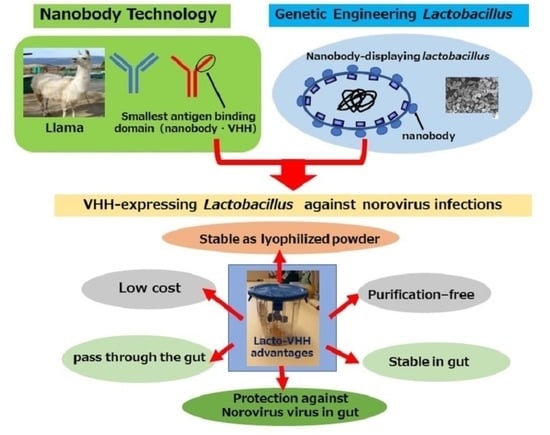
Lactobacilli as a Vector for Delivery of Nanobodies against Norovirus Infection
Abstract
:1. Introduction
2. Materials and Methods
2.1. Bacterial Strains, Plasmids, and Growth Conditions
2.2. Construction of Recombinant L. paracasei BL23 Expressing VHH 1E4
2.3. Western Blot Analysis
2.4. ELISA Using Whole Bacteria in Suspension to Confirm Cell-Surface Display of VHH
2.5. Plasmid Stability According to Plate Count Assay
2.6. Neutralization Assay of Human Norovirus
2.7. Oral Administration, Isolation, and Culture of L. paracasei BL23 Strains
2.8. Flow Cytometric Analysis of Lactobacilli
2.9. Statistical Analysis
3. Results
3.1. Production of 1E4-VHH-Displaying Lactobacilli
3.2. Norovirus Neutralization by L. paracasei BL23 Expressing VHH 1E4 Using an In Vitro Propagation Assay with Human iPSC-Derived IECs
3.3. In Vitro Norovirus Neutralization by VHH-Displaying Lactobacillus Cells Isolated from the Feces of Germ-Free Mice after Their Oral Administration
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Vinjé, J. Advances in Laboratory Methods for Detection and Typing of Norovirus. J. Clin. Microbiol. 2015, 53, 373–381. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lopman, B.A.; Steele, D.; Kirkwood, C.D.; Parashar, U.D. The Vast and Varied Global Burden of Norovirus: Prospects for Prevention and Control. PLoS Med. 2016, 13, e1001999. [Google Scholar] [CrossRef] [PubMed]
- Matsushima, Y.; Ishikawa, M.; Shimizu, T.; Komane, A.; Kasuo, S.; Shinohara, M.; Nagasawa, K.; Kimura, H.; Ryo, A.; Okabe, N.; et al. Genetic Analyses of GII.17 Norovirus Strains in Diarrheal Disease Outbreaks from December 2014 to March 2015 in Japan Reveal a Novel Polymerase Sequence and Amino Acid Substitutions in the Capsid Region. Eurosurveillance 2015, 20, 21173. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, L.; Lin, K.; Kasparek, K.; Gottlieb, K.; Liebowitz, D.; Trager, G.; Garg, S.J.; Tucker, S.N.; Kim, L.; Liebowitz, D.; et al. Safety and Immunogenicity of an Oral Tablet Norovirus Vaccine, a Phase I Randomized, Placebo-Controlled Trial. JCI Insight 2018, 3, e121077. [Google Scholar] [CrossRef]
- Treanor, J.; Sherwood, J.; Cramer, J.P.; le Cam Bouveret, N.; Lin, S.; Baehner, F.; Borkowski, A. A Phase 2 Study of the Bivalent VLP Norovirus Vaccine Candidate in Older Adults; Impact of MPL Adjuvant or a Second Dose. Vaccine 2020, 38, 5842–5850. [Google Scholar] [CrossRef] [PubMed]
- Vesikari, T.; Saez-Llorens, X.; Blazevic, V.; Lopez, P.; Lopez, E.; Masuda, T.; Mendelman, P.M.; Liu, M.; Sherwood, J.; Baehner, F.; et al. Immunogenicity of a Bivalent Virus-like Particle Norovirus Vaccine in Children from 1 to 8 Years of Age: A Phase 2 Randomized, Double-Blind Study. Vaccine 2022, 40, 3588–3596. [Google Scholar] [CrossRef]
- Hamers-Casterman, C.; Atarhouch, T.; Muyldermans, S.; Robinson, G.; Hammers, C.; Songa, E.B.; Bendahman, N.; Hammers, R. Naturally Occurring Antibodies Devoid of Light Chains. Nature 1993, 363, 446–448. [Google Scholar] [CrossRef]
- Dutt, T.; Shaw, R.J.; Stubbs, M.; Yong, J.; Bailiff, B.; Cranfield, T.; Crowley, M.P.; Desborough, M.; Eyre, T.A.; Gooding, R.; et al. Real-World Experience with Caplacizumab in the Management of Acute TTP. Blood 2021, 137, 1731–1740. [Google Scholar] [CrossRef]
- Sarker, S.A.; Jäkel, M.; Sultana, S.; Alam, N.H.; Bardhan, P.K.; Chisti, M.J.; Salam, M.A.; Theis, W.; Hammarström, L.; Frenken, L.G.J. Anti-Rotavirus Protein Reduces Stool Output in Infants with Diarrhea: A Randomized Placebo-Controlled Trial. Gastroenterology 2013, 145, 740–748. [Google Scholar] [CrossRef]
- Abe, M.; Yuki, Y.; Kurokawa, S.; Mejima, M.; Kuroda, M.; Park, E.J.; Scheller, J.; Nakanishi, U.; Kiyono, H. A Rice-Based Soluble Form of a Murine TNF-Specific Llama Variable Domain of Heavy-Chain Antibody Suppresses Collagen-Induced Arthritis in Mice. J. Biotechnol. 2014, 175, 45–52. [Google Scholar] [CrossRef]
- Tokuhara, D.; Álvarez, B.; Mejima, M.; Hiroiwa, T.; Takahashi, Y.; Kurokawa, S.; Kuroda, M.; Oyama, M.; Kozuka-Hata, H.; Nochi, T.; et al. Rice-Based Oral Antibody Fragment Prophylaxis and Therapy against Rotavirus Infection. J. Clin. Investig. 2013, 123, 3829–3838. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sasou, A.; Yuki, Y.; Kurokawa, S.; Sato, S.; Goda, Y.; Uchida, M.; Matsumoto, N.; Sagara, H.; Watanabe, Y.; Kuroda, M.; et al. Development of Antibody-Fragment–Producing Rice for Neutralization of Human Norovirus. Front. Plant Sci. 2021, 12, 639953. [Google Scholar] [CrossRef] [PubMed]
- Pant, N.; Hultberg, A.; Zhao, Y.; Svensson, L.; Pan-Hammarström, Q.; Johansen, K.; Pouwels, P.H.; Ruggeri, F.M.; Hermans, P.; Frenken, L.; et al. Lactobacilli Expressing Variable Domain of Llama Heavy-Chain Antibody Fragments (Lactobodies) Confer Protection against Rotavirus-Induced Diarrhea. J. Infect. Dis. 2006, 194, 1580–1588. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kalusche, S.; Vanshylla, K.; Kleipass, F.; Gruell, H.; Müller, B.; Zeng, Z.; Koch, K.; Stein, S.; Marcotte, H.; Klein, F.; et al. Lactobacilli Expressing Broadly Neutralizing Nanobodies against HIV-1 as Potential Vectors for HIV-1 Prophylaxis? Vaccines 2020, 8, 758. [Google Scholar] [CrossRef] [PubMed]
- Andersen, K.K.; Strokappe, N.M.; Hultberg, A.; Truusalu, K.; Smidt, I.; Mikelsaar, R.H.; Mikelsaar, M.; Verrips, T.; Hammarström, L.; Marcotte, H. Neutralization of Clostridium Difficile Toxin B Mediated by Engineered Lactobacilli That Produce Single-Domain Antibodies. Infect. Immun. 2016, 84, 395–406. [Google Scholar] [CrossRef] [Green Version]
- Sato, S.; Hisaie, K.; Kurokawa, S.; Suzuki, A.; Sakon, N.; Uchida, Y.; Yuki, Y.; Kiyono, H. Human Norovirus Propagation in Human Induced Pluripotent Stem Cell–Derived Intestinal Epithelial Cells. CMGH 2019, 7, 686–688.e5. [Google Scholar] [CrossRef] [Green Version]
- Pérez-Arellano, I.; Zúñiga, M.; Pérez-Martínez, G. Construction of Compatible Wide-Host-Range Shuttle Vectors for Lactic Acid Bacteria, and Escherichia Coli. Plasmid 2001, 46, 106–116. [Google Scholar] [CrossRef]
- Yuki, Y.; Kurokawa, S.; Sato, S.; Sasou, A.; Matsumoto, N.; Suzuki, A.; Sakon, N.; Goda, Y.; Takeyama, N.; Miyoshi, T.; et al. A Heterodimeric Antibody Fragment for Passive Immunotherapy against Norovirus Infection. J. Infect. Dis. 2020, 222, 470–478. [Google Scholar] [CrossRef]
- Martín, M.C.; Pant, N.; Ladero, V.; Günaydin, G.; Andersen, K.K.; Álvarez, B.; Martínez, N.; Alvarez, M.A.; Hammarström, L.; Marcotte, H. Integrative Expression System for Delivery of Antibody Fragments by Lactobacilli. Appl. Environ. Microbiol. 2011, 77, 2174–2179. [Google Scholar] [CrossRef] [Green Version]
- Acedo-Félix, E.; Pérez-Martínez, G. Significant Differences between Lactobacillus Casei Subsp. Casei ATCC 393 T and a Commonly Used Plasmid-Cured Derivative Revealed by a Polyphasic Study. Int. J. Syst. Evol. Microbiol. 2003, 53, 67–75. [Google Scholar] [CrossRef]
- de Keersmaecker, S.C.J.; Braeken, K.; Verhoeven, T.L.A.; Vélez, M.P.; Lebeer, S.; Vanderleyden, J.; Hols, P. Flow Cytometric Testing of Green Fluorescent Protein-Tagged Lactobacillus Rhamnosus GG for Response to Defensins. Appl. Environ. Microbiol. 2006, 72, 4923–4930. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Andersen, K.K.; Marcotte, H.; Álvarez, B.; Boyaka, P.N.; Hammarström, L. In Situ Gastrointestinal Protection against Anthrax Edema Toxin by Single-Chain Antibody Fragment Producing Lactobacilli. BMC Biotechnol. 2011, 11, 126. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Navarre, W.W.; Schneewind, O. Surface Proteins of Gram-Positive Bacteria and Mechanisms of Their Targeting to the Cell Wall Envelope. Microbiol. Mol. Biol. Rev. 1999, 63, 174–229. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sato, S.; Matsumoto, N.; Hisaie, K.; Uematsu, S. Alcohol Abrogates Human Norovirus Infectivity in a PH-Dependent Manner. Sci. Rep. 2020, 10, 15878. [Google Scholar] [CrossRef] [PubMed]
- Bartsch, S.M.; Lopman, B.A.; Ozawa, S.; Hall, A.J.; Lee, B.Y. Global Economic Burden of Norovirus Gastroenteritis. PLoS ONE 2016, 11, e0151219. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Todd, K.V.; Tripp, R.A. Human Norovirus: Experimental Models of Infection. Viruses 2019, 11, 151. [Google Scholar] [CrossRef] [Green Version]
- Ettayebi, K.; Crawford, S.E.; Murakami, K.; Broughman, J.R.; Karandikar, U.; Tenge, V.R.; Neill, F.H.; Blutt, S.E.; Zeng, X.L.; Qu, L.; et al. Replication of Human Noroviruses in Stem Cell-Derived Human Enteroids. Science 2016, 353, 1387–1393. [Google Scholar] [CrossRef] [Green Version]
- Czakó, R.; Atmar, R.L.; Opekun, A.R.; Gilger, M.A.; Graham, D.Y.; Estesa, M.K. Serum Hemagglutination Inhibition Activity Correlates with Protection from Gastroenteritis in Persons Infected with Norwalk Virus. Clin. Vaccine Immunol. 2012, 19, 284–287. [Google Scholar] [CrossRef] [Green Version]
- Lin, Y.; Krogh-Andersen, K.; Hammarström, L.; Marcotte, H. Lactobacillus Delivery of Bioactive Interleukin-22. Microb Cell Fact. 2017, 16, 148. [Google Scholar] [CrossRef] [Green Version]
- Alander, M.; Satokari, R.; Korpela, R.; Saxelin, M.; Vilpponen-salmela, T.; Mattila-sandholm, T.; von Wright, A. Persistence of Colonization of Human Colonic Mucosa by a Probiotic Strain, Lactobacillus Rhamnosus GG, after Oral Consumption. Appl. Environ. Microbiol 1999, 65, 351–354. [Google Scholar] [CrossRef]
- Yue, Y.; Xu, X.; Yang, B.; Lu, J.; Zhang, S.; Liu, L.; Nassar, K.; Zhang, C.; Zhang, M.; Pang, X.; et al. Stable Colonization of Orally Administered Lactobacillus Casei SY13 Alters the Gut Microbiota. Biomed. Res. Int. 2020, 2020, 5281639. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zuo, F.; Zeng, Z.; Hammarström, L.; Marcotte, H. Inducible Plasmid Self-Destruction (IPSD) Assisted Genome Engineering in Lactobacilli and Bifidobacteria. ACS Synth. Biol. 2019, 8, 1723–1729. [Google Scholar] [CrossRef] [PubMed]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yuki, Y.; Zuo, F.; Kurokawa, S.; Uchida, Y.; Sato, S.; Sakon, N.; Hammarström, L.; Kiyono, H.; Marcotte, H. Lactobacilli as a Vector for Delivery of Nanobodies against Norovirus Infection. Pharmaceutics 2023, 15, 63. https://doi.org/10.3390/pharmaceutics15010063
Yuki Y, Zuo F, Kurokawa S, Uchida Y, Sato S, Sakon N, Hammarström L, Kiyono H, Marcotte H. Lactobacilli as a Vector for Delivery of Nanobodies against Norovirus Infection. Pharmaceutics. 2023; 15(1):63. https://doi.org/10.3390/pharmaceutics15010063
Chicago/Turabian StyleYuki, Yoshikazu, Fanglei Zuo, Shiho Kurokawa, Yohei Uchida, Shintaro Sato, Naomi Sakon, Lennart Hammarström, Hiroshi Kiyono, and Harold Marcotte. 2023. "Lactobacilli as a Vector for Delivery of Nanobodies against Norovirus Infection" Pharmaceutics 15, no. 1: 63. https://doi.org/10.3390/pharmaceutics15010063
APA StyleYuki, Y., Zuo, F., Kurokawa, S., Uchida, Y., Sato, S., Sakon, N., Hammarström, L., Kiyono, H., & Marcotte, H. (2023). Lactobacilli as a Vector for Delivery of Nanobodies against Norovirus Infection. Pharmaceutics, 15(1), 63. https://doi.org/10.3390/pharmaceutics15010063