Treated Dentin Matrix in Tissue Regeneration: Recent Advances
Abstract
:1. Introduction
2. The Origin of TDM—Dentin Matrix
3. The Vital Properties of a TDM
3.1. Natural Porous Structure Is Preserved after a Fabrication Process and Shows Open Dentinal Tubules
3.2. A Surface with Proper Hydrophilicity Is Favorable for Cell Adhesion
3.3. Odontogenic/Osteogenic-Related Proteins Are Preserved after a Serial Preparation and Are Similar to Those of a Native Dentin Matrix
3.4. Treated Dentin Matrix and Its Derivatives Promote Cell Proliferation on Most Occasions (Table 1)
| Authors Ref. | Type of Cell | Main Conclusion |
|---|---|---|
| Brunello et al. [41] | DPSC | Both human dentin particulates and deproteinized bovine bone matrix supported cell proliferation equally well. |
| Salehi et al. [42] | OD-21 MDPC-23 | Dose-dependent promotion of cell proliferation with a higher concentration of the dentin matrix components was verified. |
| Horsophonphong et al. [43] | OD-21 | Both human dentin matrix molecules and bovine dentin matrix molecules enhanced cell proliferation. |
| Chen et al. [44] | DPSC | Treated dentin matrix paste significantly promoted cell proliferation. |
| Kulakowski et al. [45] | DPSC | Cells cultured with proanthocyanidin-treated dentin exhibited increased proliferation. |
| Wen et al. [46] | DPSC | Treated dentin matrix extracts combined with dental-pulp-cell-derived small extracellular vesicles suppressed cell proliferation. |
| Xiong et al. [47] | PDLSC | Liquid extracts of fresh/cryopreserved/freeze-dried demineralized dentin matrix slightly inhibited cell proliferation. |
3.5. A Treated Dentin Matrix Possesses Osteogenic and Odontogenic Induction Activity (Table 2)
| Authors Ref. | Type of Cell | Main Conclusion |
|---|---|---|
| Bakhtiar et al. [48] | DPSC | DMP-1 and DSPP expressions of stem cells increased after treated dentin matrix induction. |
| Chang et al. [49] | DPSC | Stem cells were positively stained for DSP and DMP1 in the autoclaved human-treated dentin matrix group. |
| Meng et al. [50] | DPSC | The mRNA expressions of OCN, DSPP, VEGF-1 and Nestin in stem cells were obviously upregulated by a human-treated dentin matrix leaching solution. |
| Chen et al. [44] | DPSC | Treated dentin matrix paste significantly enhanced the expressions of ALP, BSP and DSP. |
| Melling et al. [33] | DPSC | Demineralized dentin matrix liposomes promoted the upregulation of OCN and RUNX2 in stem cells. |
| Kulakowski et al. [45] | DPSC | Proanthocyanidin-treated dentin increased the expressions of RUNX2, BMP2, OCN and DSPP. |
| Jiao et al. [53] | DFC | A cryopreserved dentin matrix extract liquid induced stem cells to highly express BSP, COL-1 and ALP. |
| Yang et al. [27] | DFC | Treated dentin matrix induced stem cells to highly express DMP-1 and BSP. |
| Li et al. [54] | DFC | Porcine-treated dentin matrix can facilitate the odontoblast differentiation of stem cells. |
| Chen et al. [51] | DFC, DPSC and CNCC | With the induction of a treated dentin matrix, DFCs displayed similar expression patterns of neurofilament, tubulin and nestin to DPCs. Meanwhile, DFCs showed more similar protein profiles of COL1, TGF-β1, OPN and DMP-1 to CNCCs than DPCs. |
| Zhang et al. [56] | UCMSC | Liquid extract of a human-treated dentin matrix induced stem cells to express DSPP, DMP-1 and DSP. |
| Yang et al. [57] | BMSC | Human-treated dentin matrix particles promoted the osteogenic differentiation of stem cells. |
| Yang et al. [55] | DFC and HERSC | A treated dentin matrix’s presence and HERSCs’ induction enhanced the osteogenic differentiation of DFCs. |
| Guo et al. [52] | DFC and DPC | A treated dentin matrix induced both DFCs and DPCs to display odontogenic differentiation potential. |
4. Different Methods of Fabricating and Preserving TDM
4.1. Harvesting the Tooth Root from Different Species (Figure 2)

4.2. Special Treatment Procedures (Table 3)
| Authors Ref. | Special Treatment | Main Conclusion |
|---|---|---|
| Bakhtiar et al. [48] | Atelopeptidization with pepsin | Atelopeptidization of demineralized dentin could facilitate preserving collagen structure and reducing the immune reaction. |
| Li et al. [59] | Ethanol/DMA | Treatment using DMA/ethanol solution might be capable of enhancing the mechanical properties of a demineralized dentin matrix. |
| Omar et al. [60] | Plant-based polyphenols | PB-Ps reduced the degradation of dentin extracellular matrixes and improved the apparent elastic modulus. |
| Okamoto et al. [61] | MMPs | Dentin matrix components partially digested by matrix metalloproteinases, especially MMP-20, stimulated tertiary dentin formation in vivo and indicated its potential for wound healing of the dentin–pulp complex. |
| Wang et al. [69] | Freezing | A freeze-dried dentin matrix has similar mechanical and biological properties to those of dentin. |
| Li et al. [58] | Different grinding speeds | An LTDM induced a twice greater expression of DSPP and DMP-1 in stem cells than an HTDM, while an HTDM induced a twice greater expression of BSP in stem cells than an LTDM. Neo-dentin formed on the inner surface of an LTDM and neo-cementum formed on the outer surface of an HTDM. |
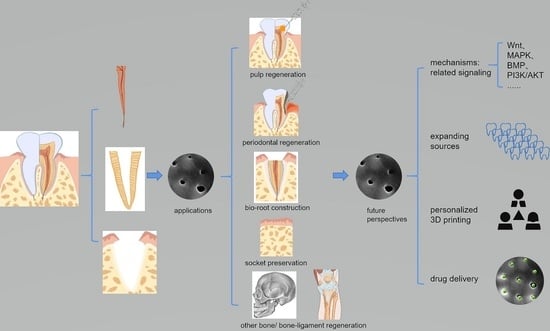
5. TDM Applications
5.1. Dental Pulp/Dentin/Dentin–Pulp Complex Regeneration (Table 4)
| Authors Ref. | Major Composition of the Bio-Material | Main Conclusion |
|---|---|---|
| Chang et al. [49] | Allogenous autoclaved TDM + DPSCs | An allogenous autoclaved treated dentin matrix induced stem cells to develop new dentin pulp-like tissues, dental pulp and cementum periodontal complexes. DSP, βⅢ-tubulin, DMP-1, COL-1 and CAP were positive in toothlike tissue. |
| Liu et al. [70] | TDM + DPSCs | New dentin was found in a rat mandible cultured with a treated dentin matrix and was significantly thicker. |
| Tran et al. [71] | TD + DPSCs | Treated dentin induced stem cells to regenerate dentin-like tissues that expressed DSPP and DMP-1. |
| Melling et al. [33] | DDM + DPSCs | Demineralized dentin matrix liposomes increased stem cells’ mineralization. |
| Wang et al. [69] | FDDM + DPSCs | A freeze-derived dentin matrix supported dentin-pulp-like tissue regeneration, which was positively stained with DSP and ALP. |
| Liu et al. [72] | DDM + DPSCs | A demineralized dentin matrix could induce DPSC to form mineralized tissue, which was stained positive for DSPP. |
| Jiao et al. [53] | CDM + DFCs | A cryopreserved dentin matrix could induce stem cells to regenerate new dentin-pulp-like tissues, including dentinal tubules, predentin, collagen fibers, nerves and blood vessels, which were positive for DSPP, DMP-1, tubulin and COL-1. |
| Li et al. [65] | TDM + DFCs | A human-treated dentin matrix induced complete dentin tissue regeneration that expressed DSP and DMP-1. |
| Holiel et al. [75,76] | TDMH | A treated dentin matrix hydrogel was developed for direct pulp capping. It could contribute to achieving dentin regeneration and conservation of pulp vitality. CBCT showed TDMH-induced superior dentin bridge formation of higher radiodensity and thickness than Biodentine and MTA. Histological analysis showed TDMH induced thicker dentin with layers of well-arranged odontoblasts than Biodentine and MTA. |
| Chen et al. [44] | TDMP | A treated dentin matrix paste was developed for pulp capping. TDMP induced the formation of a continuous reparative dentin bridge that was thicker and denser than calcium hydroxide. TDMP achieved both dentin regeneration and vital pulp conservation. |
| Wen et al. [46] | sEV-TDM | sEV-TDM was developed by combining treated dentin matrix proteins and dental-pulp-cell-derived small extracellular vesicles. It was testified that sEV-TDM promoted the formation of continuous reparative dentin. Odontoblast-like high columnar cells were observed on the pulp side of the dentin bridge. |
| Cunha et al. [77] | DMM | A microparticulate hydrogel supplemented with dentin matrix molecules was developed for dental pulp capping. A microgel + DMM induced more dentin bridge formation and less pulp necrosis than MTA. |
| Fu et al. [73] | TDM + DPEM | Treated dentin matrix combined with a laminin-modified dental pulp extracellular matrix promoted odontogenic differentiation of cells and dental pulp regeneration as shown by the expression of DMP-1 and DSPP and a continuous odontoblastic layer-like structure. |
| Na et al. [74] | TDMF + CSDP + CS | Human-treated dentine matrix fragments combined with a cell sheet and stem-cell sheet-derived pellet induced highly vascularized dental-pulp-like tissue with odontoblast-like cells expressing DSPP, ALP and BSP. |
5.2. Periodontal Tissue Regeneration (Table 5)
| Authors Ref. | Major Composition of the Bio-Material | Main Conclusion |
|---|---|---|
| Chang et al. [49] | Allogenous autoclaved-TDM + DPSCs | An allogenous autoclaved treated dentin matrix induced stem cells to develop cementum periodontal complexes, where COL-1- and CAP-positive stains were produced. |
| Yang et al. [57] | TDMPs + DFC cell sheets | Human treated dentin matrix particles combined with stem cell sheets induced new bone formation and periodontal-like tissues in animal experiments. |
| Li et al. [63] | DDM granules | Demineralized dentin matrix granules prepared at the chairside after extractions showed no significant difference in implant stability quotient values and marginal bone resorption when being applied to guided bone regeneration for immediate implantation in periodontal postextraction sites compared with Bio-Oss. |
| Yang et al. [79] | TDM + DFC TDM + SHED | Treated dentin matrix combined with either DFC or SHED successfully achieved periodontal tissue regeneration, showing periodontal ligament fibers, blood vessels and newly created alveolar bone. |
| Ji et al. [80] | TDM + PRF | A treated dentin matrix combined with autologous platelet-rich fibrin induced cementum and periodontal ligament (PDL)-like tissue regeneration. |
5.3. Bio-Root Construction (Table 6)
| Authors Ref. | Major Composition of the Bio-Material | Main Conclusion |
|---|---|---|
| Meng et al. [50] | TDM + Matrigel + DPSC sheet | A treated dentin matrix/Matrigel/DPSC sheet complex was fabricated for promoting periodontium, dentin and pulp-like tissue regeneration. Periodontium-like dense connective tissue, predentin, odontoblast-like cells, blood vessel-like structures and even nerve-like fibers were observed. |
| Li et al. [66] | pTDM + DFC | A porcine treated dentin matrix induced odontogenesis as observed by the production of pre-dentin, cementum, collagen fibrils, odontoblast-like cells and fibroblasts, even though the xenogeneic implants inevitably initiated Th1 inflammation. |
| Zhang et al. [29] | TDM + DFC + NAC | A treated dentin matrix loaded with antioxidant NAC decreased HO-induced cellular damage, maintained DFCs’ odontogenic differentiation potential and repressed replacement resorption or ankylosis, thus facilitating bio-root regeneration. |
| Sun et al. [32] | TDM + DFC + tBHQ | The scaffold of a tBHQ-treated xenogenic treated dentin matrix with DFCs implanted in vivo showed reduced osteolysis and osteoclastic resorption. |
| Yang et al. [27] | TDM + DFC | A treated dentin matrix induced DFCs to develop new dentin–pulp-like tissues and cementum–periodontal complexes. |
| Guo et al. [82] | TDM + DFC | A treated dentin matrix induced DFCs to form root-like tissues that were stained positive for markers of dental pulp and periodontal tissues. |
| Li et al. [54] | TDM + DFC | A porcine treated dentin matrix combined with DFCs was transplanted into the jaws of rhesus monkeys. Periodontal ligament-like fibers accompanied by macrophage polarization, fibroblasts and blood vessels were observed. Meanwhile, the constructed bio-root possessed biomechanical properties that could endure masticatory forces. |
| Guo et al. [52] | TDM + DFC/DPC | A treated dentin matrix induced both DFCs and DPCs to form pulp–dentin/cementum–periodentium-like tissues. |
| Chen et al. [28] | TDM + DFC + BMP4 + TGF-β1 | A treated dentin matrix combined with TGF-β1, BMP4 and DFCs embodied a spatial interface gradient for functional enthesis formation to promote functional bio-root regeneration. Effectively functional bio-roots made of the composites were successfully constructed, with the presentation of outstanding biomechanical properties and healthy gingiva. |
| Luo et al. [81] | CAD- and FEA-based shape-optimized TDM | Computer-aided design and finite element analysis were used to create shape-optimized treated dentin matrix combined with stem cells that successfully achieved root regeneration and a stable performance of masticatory function. |
| Yuan et al. [67] | TDM + ASC | A porcine treated dentin matrix induced ASC to differentiate toward odontogenesis and promoted dentin-like tissue, pulp-like tissue and periodontal-fiber-like tissue regeneration. |
| Chen et al. [68] | TDM + APES + DPEM | A treated dentin matrix combined with an aligned PLGA/gelatin electrospun sheet and dental pulp extracellular matrix promoted pulp–dentin complex-like tissues and periodontium-complex-like tissue regeneration, presenting columnar odontoblast-like cells, newly formed predentin, blood vessels, cellular cementum and periodontal ligament (PDL)-like tissues. |
| Han et al. [31] | NDM + RSG | A native decellularized matrix made of treated dentin matrix loaded with RSG decreased the expression of IL-1 and TNF-α, increased the expression of IL-10 and TGF-β, induced M2 macrophages to antagonize M1 macrophages using PPARγ, created favorable immunomodulation and promoted ligament-to-bone regeneration. |
| Lan et al. [30] | ECM + RSG | Extracellular matrix made of treated dentin matrix loaded with RSG activated PPAR-γ downregulated the expression of proinflammatory NOS2 + M1 macrophages and ROS to facilitate bio-root regeneration. |
| Li et al. [83] | TDM + PPARγ-primed CD68CD206 M2 phenotype | A treated dentin matrix combined with PPARγ-primed CD68CD206 M2 phenotype alleviated proinflammatory cytokines (TNF-α, IFN-γ) at the inflammation site; decreased CD3CD8 T lymphocytes in the periphery system; immunosuppressed IL-1β, IL-6, TNF-α and MMPs; enabled xenograft escape immune rejection; and promoted a xenogenic bio-root to survive in the host. |
5.4. Bone Regeneration (Table 7)
| Authors Ref. | Major Composition | Main Conclusion |
|---|---|---|
| Moraes et al. [85] | DHDM | Demineralized human dentine matrix contributed to alveolar ridge preservation, as testified by microtomography and histological evaluation showing new bone formation with the slow reabsorption of DHDM. |
| Li et al. [86] | ADDM | Autogenous demineralized dentin matrix exhibited osteogenic effectiveness in bone augmentation, just as Bio-Oss® did for oral bone defects. |
| Murata et al. [62] | pDDM | Partially demineralized dentin/cementum matrix contributed to socket preservation as shown by bone-like radio-opacity in the graft region and newly formed bone connected directly with dentin/cementum area. |
| Um et al. [87] | aDDM | Autogenous demineralized dentin matrix loaded with recombinant human bone morphogenetic 2 contributed to socket preservation. BMP-2 enhanced bone formation effectiveness. |
| Reis-Filho et al. [64] | DHDM | A human demineralized dentine matrix has the potential for osteogenic induction and increases bone tissue formation and vessel tissue formation in sockets. |
6. Future Perspectives
6.1. Underlying Mechanism
6.2. Expanding Sources
6.3. Three-Dimensional Construction for Individual Customization in Clinical Application
6.4. Taking Better Advantage of the Natural Porous Structure for Drug Delivery
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- James, S.L.; Abate, D.; Abate, K.H.; Abay, S.M.; Abbafati, C.; Abbasi, N.; Abbastabar, H.; Abd-Allah, F.; Abdela, J.; Abdelalim, A.; et al. Global, regional, and national incidence, prevalence, and years lived with disability for 354 diseases and injuries for 195 countries and territories, 1990-2017: A systematic analysis for the Global Burden of Disease Study 2017. Lancet 2018, 392, 1789–1858. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Alvarez, E.M.; Force, L.M.; Xu, R.; Compton, K.; Lu, D.; Henrikson, H.J.; Kocarnik, J.M.; Harvey, J.D.; Pennini, A.; Dean, F.E.; et al. The global burden of adolescent and young adult cancer in 2019: A systematic analysis for the Global Burden of Disease Study 2019. Lancet Oncol. 2022, 23, 27–52. [Google Scholar] [CrossRef] [PubMed]
- Murray, C.J.; Ikuta, K.S.; Sharara, F.; Swetschinski, L.; Aguilar, G.R.; Gray, A.; Han, C.; Bisignano, C.; Rao, P.; Wool, E.; et al. Global burden of bacterial antimicrobial resistance in 2019: A systematic analysis. Lancet 2022, 399, 629–655. [Google Scholar] [CrossRef] [PubMed]
- Murray, C. The Global Burden of Disease Study at 30 years. Nat. Med. 2022, 28, 2019–2026. [Google Scholar] [CrossRef]
- Ahmed, M.; Rubio, I.T.; Klaase, J.M.; Douek, M. Surgical treatment of nonpalpable primary invasive and in situ breast cancer. Nat. Rev. Clin. Oncol. 2015, 12, 645–663. [Google Scholar] [CrossRef]
- Saranga-Perry, V.; Ambe, C.; Zager, J.S.; Kudchadkar, R.R. Recent developments in the medical and surgical treatment of melanoma. CA Cancer J. Clin. 2014, 64, 171–185. [Google Scholar] [CrossRef]
- Kempker, R.R.; Vashakidze, S.; Solomonia, N.; Dzidzikashvili, N.; Blumberg, H.M. Surgical treatment of drug-resistant tuberculosis. Lancet Infect. Dis. 2012, 12, 157–166. [Google Scholar] [CrossRef] [Green Version]
- Arfaee, M.; Vis, A.; Kluin, J. Future technologies in total artificial heart development: Can a robot become as good as a donor heart? Eur. Heart J. 2022, 43, 4970–4972. [Google Scholar] [CrossRef]
- Dangas, G.D.; Weitz, J.I.; Giustino, G.; Makkar, R.; Mehran, R. Prosthetic Heart Valve Thrombosis. J. Am. Coll. Cardiol. 2016, 68, 2670–2689. [Google Scholar] [CrossRef]
- Hockel, M.; Dornhofer, N. Vulvovaginal reconstruction for neoplastic disease. Lancet Oncol. 2008, 9, 559–568. [Google Scholar] [CrossRef]
- Guo, S.; Han, Y.; Zhang, X.; Lu, B.; Yi, C.; Zhang, H.; Ma, X.; Wang, D.; Yang, L.; Fan, X.; et al. Human facial allotransplantation: A 2-year follow-up study. Lancet 2008, 372, 631–638. [Google Scholar] [CrossRef] [PubMed]
- Griffith, B.P.; Goerlich, C.E.; Singh, A.K.; Rothblatt, M.; Lau, C.L.; Shah, A.; Lorber, M.; Grazioli, A.; Saharia, K.K.; Hong, S.N.; et al. Genetically Modified Porcine-to-Human Cardiac Xenotransplantation. N. Engl. J. Med. 2022, 387, 35–44. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Song, S.; Ren, X.; Zhang, J.; Lin, Q.; Zhao, Y. Supramolecular Adhesive Hydrogels for Tissue Engineering Applications. Chem. Rev. 2022, 122, 5604–5640. [Google Scholar] [CrossRef] [PubMed]
- Kim, B.S.; Das, S.; Jang, J.; Cho, D.W. Decellularized Extracellular Matrix-based Bioinks for Engineering Tissue- and Organ-specific Microenvironments. Chem. Rev. 2020, 120, 10608–10661. [Google Scholar] [CrossRef] [PubMed]
- De Santis, M.M.; Alsafadi, H.N.; Tas, S.; Bolukbas, D.A.; Prithiviraj, S.; Da, S.I.; Mittendorfer, M.; Ota, C.; Stegmayr, J.; Daoud, F.; et al. Extracellular-Matrix-Reinforced Bioinks for 3D Bioprinting Human Tissue. Adv. Mater. 2021, 33, e2005476. [Google Scholar] [CrossRef]
- Brown, M.; Li, J.; Moraes, C.; Tabrizian, M.; Li-Jessen, N. Decellularized extracellular matrix: New promising and challenging biomaterials for regenerative medicine. Biomaterials 2022, 289, 121786. [Google Scholar] [CrossRef]
- Neishabouri, A.; Soltani, K.A.; Daghigh, F.; Kajbafzadeh, A.M.; Majidi, Z.M. Decellularization in Tissue Engineering and Regenerative Medicine: Evaluation, Modification, and Application Methods. Front. Bioeng. Biotechnol. 2022, 10, 805299. [Google Scholar] [CrossRef]
- Wang, X.; Ma, Y.; Chen, J.; Liu, Y.; Liu, G.; Wang, P.; Wang, B.; Taketo, M.M.; Bellido, T.; Tu, X. A novel decellularized matrix of Wnt signaling-activated osteocytes accelerates the repair of critical-sized parietal bone defects with osteoclastogenesis, angiogenesis, and neurogenesis. Bioact. Mater. 2023, 21, 110–128. [Google Scholar] [CrossRef]
- Philips, C.; Terrie, L.; Thorrez, L. Decellularized skeletal muscle: A versatile biomaterial in tissue engineering and regenerative medicine. Biomaterials 2022, 283, 121436. [Google Scholar] [CrossRef]
- Song, J.J.; Ott, H.C. Organ engineering based on decellularized matrix scaffolds. Trends Mol. Med. 2011, 17, 424–432. [Google Scholar] [CrossRef]
- Barthold, J.E.; Martin, B.M.; Sridhar, S.L.; Vernerey, F.; Schneider, S.E.; Wacquez, A.; Ferguson, V.; Calve, S.; Neu, C.P. Recellularization and Integration of Dense Extracellular Matrix by Percolation of Tissue Microparticles. Adv. Funct. Mater. 2021, 31, 2103355. [Google Scholar] [CrossRef] [PubMed]
- Ohata, K.; Ott, H.C. Human-scale lung regeneration based on decellularized matrix scaffolds as a biologic platform. Surg. Today 2020, 50, 633–643. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.; Min, S.; Choi, Y.S.; Jo, S.H.; Jung, J.H.; Han, K.; Kim, J.; An, S.; Ji, Y.W.; Kim, Y.G.; et al. Tissue extracellular matrix hydrogels as alternatives to Matrigel for culturing gastrointestinal organoids. Nat. Commun. 2022, 13, 1692. [Google Scholar] [CrossRef] [PubMed]
- Sensi, F.; D’Angelo, E.; Biccari, A.; Marangio, A.; Battisti, G.; Crotti, S.; Fassan, M.; Laterza, C.; Giomo, M.; Elvassore, N.; et al. Establishment of a human 3D pancreatic adenocarcinoma model based on a patient-derived extracellular matrix scaffold. Transl. Res. 2022. [Google Scholar] [CrossRef]
- Guo, W.; He, Y.; Zhang, X.; Lu, W.; Wang, C.; Yu, H.; Liu, Y.; Li, Y.; Zhou, Y.; Zhou, J.; et al. The use of dentin matrix scaffold and dental follicle cells for dentin regeneration. Biomaterials 2009, 30, 6708–6723. [Google Scholar] [CrossRef] [PubMed]
- Tabatabaei, F.S.; Tatari, S.; Samadi, R.; Torshabi, M. Surface characterization and biological properties of regular dentin, demineralized dentin, and deproteinized dentin. J. Mater. Sci. Mater. Med. 2016, 27, 164. [Google Scholar] [CrossRef]
- Yang, B.; Chen, G.; Li, J.; Zou, Q.; Xie, D.; Chen, Y.; Wang, H.; Zheng, X.; Long, J.; Tang, W.; et al. Tooth root regeneration using dental follicle cell sheets in combination with a dentin matrix-based scaffold. Biomaterials 2012, 33, 2449–2461. [Google Scholar] [CrossRef]
- Chen, J.; Liao, L.; Lan, T.; Zhang, Z.; Gai, K.; Huang, Y.; Chen, J.; Tian, W.; Guo, W. Treated dentin matrix-based scaffolds carrying TGF-β1/BMP4 for functional bio-root regeneration. Appl. Mater. Today 2020, 20, 100742. [Google Scholar] [CrossRef]
- Zhang, J.; Lan, T.; Han, X.; Xu, Y.; Liao, L.; Xie, L.; Yang, B.; Tian, W.; Guo, W. Improvement of ECM-based bioroot regeneration via N-acetylcysteine-induced antioxidative effects. Stem Cell Res. Ther. 2021, 12, 202. [Google Scholar] [CrossRef]
- Lan, T.; Chen, J.; Zhang, J.; Huo, F.; Han, X.; Zhang, Z.; Xu, Y.; Huang, Y.; Liao, L.; Xie, L.; et al. Xenoextracellular matrix-rosiglitazone complex-mediated immune evasion promotes xenogenic bioengineered root regeneration by altering M1/M2 macrophage polarization. Biomaterials 2021, 276, 121066. [Google Scholar] [CrossRef]
- Han, X.; Liao, L.; Zhu, T.; Xu, Y.; Bi, F.; Xie, L.; Li, H.; Huo, F.; Tian, W.; Guo, W. Xenogeneic native decellularized matrix carrying PPARgamma activator RSG regulating macrophage polarization to promote ligament-to-bone regeneration. Mater. Sci. Eng. C Mater. Biol. Appl. 2020, 116, 111224. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.; Li, J.; Li, H.; Yang, H.; Chen, J.; Yang, B.; Huo, F.; Guo, W.; Tian, W. tBHQ Suppresses Osteoclastic Resorption in Xenogeneic-Treated Dentin Matrix-Based Scaffolds. Adv. Healthc. Mater. 2017, 6, 1700127. [Google Scholar] [CrossRef]
- Melling, G.E.; Colombo, J.S.; Avery, S.J.; Ayre, W.N.; Evans, S.L.; Waddington, R.J.; Sloan, A.J. Liposomal Delivery of Demineralized Dentin Matrix for Dental Tissue Regeneration. Tissue Eng. Part A 2018, 24, 1057–1065. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jing, X.; Xie, B.; Li, X.; Dai, Y.; Nie, L.; Li, C. Peptide decorated demineralized dentin matrix with enhanced bioactivity, osteogenic differentiation via carboxymethyl chitosan. Dent. Mater. 2021, 37, 19–29. [Google Scholar] [CrossRef] [PubMed]
- Grawish, M.E.; Grawish, L.M.; Grawish, H.M.; Grawish, M.M.; Holiel, A.A.; Sultan, N.; El-Negoly, S.A. Demineralized Dentin Matrix for Dental and Alveolar Bone Tissues Regeneration: An Innovative Scope Review. Tissue Eng. Regen. Med. 2022, 19, 687–701. [Google Scholar] [CrossRef]
- Um, I.W.; Kim, Y.K.; Mitsugi, M. Demineralized dentin matrix scaffolds for alveolar bone engineering. J. Indian Prosthodont. Soc. 2017, 17, 120–127. [Google Scholar] [CrossRef] [PubMed]
- Chun, S.Y.; Lee, H.J.; Choi, Y.A.; Kim, K.M.; Baek, S.H.; Park, H.S.; Kim, J.Y.; Ahn, J.M.; Cho, J.Y.; Cho, D.W.; et al. Analysis of the soluble human tooth proteome and its ability to induce dentin/tooth regeneration. Tissue Eng. Part A 2011, 17, 181–191. [Google Scholar] [CrossRef]
- Ravindran, S.; George, A. Dentin Matrix Proteins in Bone Tissue Engineering. Adv. Exp. Med. Biol. 2015, 881, 129–142. [Google Scholar] [CrossRef] [Green Version]
- Murata, M.; Nezu, T.; Takebe, H.; Hirose, Y.; Okubo, N.; Saito, T.; Akazawa, T. Human dentin materials for minimally invasive bone regeneration: Animal studies and clinical cases. J. Oral Biosci. 2022. [Google Scholar] [CrossRef]
- Li, J.; Yang, H.; Lu, Q.; Chen, D.; Zhou, M.; Kuang, Y.; Ying, S.; Song, J. Proteomics and N-glycoproteomics analysis of an extracellular matrix-based scaffold-human treated dentin matrix. J. Tissue Eng. Regen. Med. 2019, 13, 1164–1177. [Google Scholar] [CrossRef] [PubMed]
- Brunello, G.; Zanotti, F.; Scortecci, G.; Sapoznikov, L.; Sivolella, S.; Zavan, B. Dentin Particulate for Bone Regeneration: An In Vitro Study. Int. J. Mol. Sci. 2022, 23, 9283. [Google Scholar] [CrossRef] [PubMed]
- Salehi, S.; Cooper, P.; Smith, A.; Ferracane, J. Dentin matrix components extracted with phosphoric acid enhance cell proliferation and mineralization. Dent. Mater. 2016, 32, 334–342. [Google Scholar] [CrossRef] [PubMed]
- Horsophonphong, S.; Sercia, A.; Franca, C.M.; Tahayeri, A.; Reddy, A.P.; Wilmarth, P.A.; Surarit, R.; Smith, A.J.; Ferracane, J.L.; Bertassoni, L.E. Equivalence of human and bovine dentin matrix molecules for dental pulp regeneration: Proteomic analysis and biological function. Arch. Oral Biol. 2020, 119, 104888. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Cui, C.; Qiao, X.; Yang, B.; Yu, M.; Guo, W.; Tian, W. Treated dentin matrix paste as a novel pulp capping agent for dentin regeneration. J. Tissue Eng. Regen. Med. 2017, 11, 3428–3436. [Google Scholar] [CrossRef] [PubMed]
- Kulakowski, D.; Leme-Kraus, A.A.; Nam, J.W.; McAlpine, J.; Chen, S.N.; Pauli, G.F.; Ravindran, S.; Bedran-Russo, A.K. Oligomeric proanthocyanidins released from dentin induce regenerative dental pulp cell response. Acta Biomater. 2017, 55, 262–270. [Google Scholar] [CrossRef]
- Wen, B.; Huang, Y.; Qiu, T.; Huo, F.; Xie, L.; Liao, L.; Tian, W.; Guo, W. Reparative Dentin Formation by Dentin Matrix Proteins and Small Extracellular Vesicles. J. Endod. 2021, 47, 253–262. [Google Scholar] [CrossRef] [PubMed]
- Xiong, Y.; Shen, T.; Xie, X. Effects of different methods of demineralized dentin matrix preservation on the proliferation and differentiation of human periodontal ligament stem cells. J. Dent. Sci. 2022, 17, 1135–1143. [Google Scholar] [CrossRef]
- Bakhtiar, H.; Mazidi, A.; Mohammadi-Asl, S.; Hasannia, S.; Ellini, M.R.; Pezeshki-Modaress, M.; Ostad, S.N.; Galler, K.; Azarpazhooh, A.; Kishen, A. Potential of Treated Dentin Matrix Xenograft for Dentin-Pulp Tissue Engineering. J. Endod. 2020, 46, 57–64. [Google Scholar] [CrossRef]
- Chang, C.C.; Lin, T.A.; Wu, S.Y.; Lin, C.P.; Chang, H.H. Regeneration of Tooth with Allogenous, Autoclaved Treated Dentin Matrix with Dental Pulpal Stem Cells: An In Vivo Study. J. Endod. 2020, 46, 1256–1264. [Google Scholar] [CrossRef]
- Meng, H.; Hu, L.; Zhou, Y.; Ge, Z.; Wang, H.; Wu, C.T.; Jin, J. A Sandwich Structure of Human Dental Pulp Stem Cell Sheet, Treated Dentin Matrix, and Matrigel for Tooth Root Regeneration. Stem Cells Dev. 2020, 29, 521–532. [Google Scholar] [CrossRef]
- Chen, G.; Sun, Q.; Xie, L.; Jiang, Z.; Feng, L.; Yu, M.; Guo, W.; Tian, W. Comparison of the Odontogenic Differentiation Potential of Dental Follicle, Dental Papilla, and Cranial Neural Crest Cells. J. Endod. 2015, 41, 1091–1099. [Google Scholar] [CrossRef] [PubMed]
- Guo, L.; Li, J.; Qiao, X.; Yu, M.; Tang, W.; Wang, H.; Guo, W.; Tian, W. Comparison of odontogenic differentiation of human dental follicle cells and human dental papilla cells. PLoS ONE 2013, 8, e62332. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jiao, L.; Xie, L.; Yang, B.; Yu, M.; Jiang, Z.; Feng, L.; Guo, W.; Tian, W. Cryopreserved dentin matrix as a scaffold material for dentin-pulp tissue regeneration. Biomaterials 2014, 35, 4929–4939. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Sun, J.; Li, J.; Yang, H.; Luo, X.; Chen, J.; Xie, L.; Huo, F.; Zhu, T.; Guo, W.; et al. Xenogeneic Bio-Root Prompts the Constructive Process Characterized by Macrophage Phenotype Polarization in Rodents and Nonhuman Primates. Adv. Healthc. Mater. 2017, 6, 1601112. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Ge, Y.; Chen, G.; Yan, Z.; Yu, M.; Feng, L.; Jiang, Z.; Guo, W.; Tian, W. Hertwig’s epithelial root sheath cells regulate osteogenic differentiation of dental follicle cells through the Wnt pathway. Bone 2014, 63, 158–165. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Zhang, W.; Li, Y.; Ren, L.; Deng, H.; Yin, X.; Gao, X.; Pan, S.; Niu, Y. Human Umbilical Cord Mesenchymal Stem Cell Differentiation Into Odontoblast-Like Cells and Endothelial Cells: A Potential Cell Source for Dental Pulp Tissue Engineering. Front. Physiol. 2020, 11, 593. [Google Scholar] [CrossRef]
- Yang, H.; Li, J.; Hu, Y.; Sun, J.; Guo, W.; Li, H.; Chen, J.; Huo, F.; Tian, W.; Li, S. Treated dentin matrix particles combined with dental follicle cell sheet stimulate periodontal regeneration. Dent. Mater. 2019, 35, 1238–1253. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Yang, S.; Song, J.; Fu, T.; Liang, P.; Gao, Z.; Tang, J.; Guo, L. Different grinding speeds affect induced regeneration capacity of human treated dentin matrix. J. Biomed. Mater. Res. Part B Appl. Biomater. 2022, 110, 755–767. [Google Scholar] [CrossRef]
- Li, K.; Zhang, Z.; Sun, Y.; Yang, H.; Tsoi, J.; Huang, C.; Yiu, C. In vitro evaluation of the anti-proteolytic and cross-linking effect of mussel-inspired monomer on the demineralized dentin matrix. J. Dent. 2021, 111, 103720. [Google Scholar] [CrossRef]
- Omar, H.; Gao, F.; Yoo, H.; Bim, O.J.; Garcia, C.; LePard, K.J.; Mitchell, J.C.; Agostini-Walesch, G.; Carrilho, M.R. Changes to dentin extracellular matrix following treatment with plant-based polyphenols. J. Mech. Behav. Biomed. Mater. 2022, 126, 105055. [Google Scholar] [CrossRef]
- Okamoto, M.; Takahashi, Y.; Komichi, S.; Cooper, P.R.; Hayashi, M. Dentinogenic effects of extracted dentin matrix components digested with matrix metalloproteinases. Sci. Rep. 2018, 8, 10690. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Murata, M.; Kabir, M.A.; Hirose, Y.; Ochi, M.; Okubo, N.; Akazawa, T.; Kashiwazaki, H. Histological Evidences of Autograft of Dentin/Cementum Granules into Unhealed Socket at 5 Months after Tooth Extraction for Implant Placement. J. Funct. Biomater. 2022, 13, 66. [Google Scholar] [CrossRef] [PubMed]
- Li, P.; Zhu, H.; Huang, D. Autogenous DDM versus Bio-Oss granules in GBR for immediate implantation in periodontal postextraction sites: A prospective clinical study. Clin. Implant. Dent. Relat. Res. 2018, 20, 923–928. [Google Scholar] [CrossRef] [PubMed]
- Reis-Filho, C.R.; Silva, E.R.; Martins, A.B.; Pessoa, F.F.; Gomes, P.V.; de Araujo, M.S.; Miziara, M.N.; Alves, J.B. Demineralised human dentine matrix stimulates the expression of VEGF and accelerates the bone repair in tooth sockets of rats. Arch. Oral Biol. 2012, 57, 469–476. [Google Scholar] [CrossRef] [PubMed]
- Li, R.; Guo, W.; Yang, B.; Guo, L.; Sheng, L.; Chen, G.; Li, Y.; Zou, Q.; Xie, D.; An, X.; et al. Human treated dentin matrix as a natural scaffold for complete human dentin tissue regeneration. Biomaterials 2011, 32, 4525–4538. [Google Scholar] [CrossRef]
- Li, H.; Ma, B.; Yang, H.; Qiao, J.; Tian, W.; Yu, R. Xenogeneic dentin matrix as a scaffold for biomineralization and induced odontogenesis. Biomed. Mater. 2021, 16, 045020. [Google Scholar] [CrossRef] [PubMed]
- Yuan, Y.; Zhang, X.; Zhan, Y.; Tang, S.; Deng, P.; Wang, Z.; Li, J. Adipose-derived stromal/stem cells are verified to be potential seed candidates for bio-root regeneration in three-dimensional culture. Stem Cell Res. Ther. 2022, 13, 234. [Google Scholar] [CrossRef]
- Chen, G.; Chen, J.; Yang, B.; Li, L.; Luo, X.; Zhang, X.; Feng, L.; Jiang, Z.; Yu, M.; Guo, W.; et al. Combination of aligned PLGA/Gelatin electrospun sheets, native dental pulp extracellular matrix and treated dentin matrix as substrates for tooth root regeneration. Biomaterials 2015, 52, 56–70. [Google Scholar] [CrossRef]
- Wang, F.; Xie, C.; Ren, N.; Bai, S.; Zhao, Y. Human Freeze-dried Dentin Matrix as a Biologically Active Scaffold for Tooth Tissue Engineering. J. Endod. 2019, 45, 1321–1331. [Google Scholar] [CrossRef]
- Liu, S.; Sun, J.; Yuan, S.; Yang, Y.; Gong, Y.; Wang, Y.; Guo, R.; Zhang, X.; Liu, Y.; Mi, H.; et al. Treated dentin matrix induces odontogenic differentiation of dental pulp stem cells via regulation of Wnt/beta-catenin signaling. Bioact. Mater. 2022, 7, 85–97. [Google Scholar] [CrossRef]
- Tran, H.B.; Doan, V.N. Human dental pulp stem cells cultured onto dentin derived scaffold can regenerate dentin-like tissue in vivo. Cell Tissue Bank. 2015, 16, 559–568. [Google Scholar] [CrossRef] [PubMed]
- Liu, G.; Xu, G.; Gao, Z.; Liu, Z.; Xu, J.; Wang, J.; Zhang, C.; Wang, S. Demineralized Dentin Matrix Induces Odontoblastic Differentiation of Dental Pulp Stem Cells. Cells Tissues Organs 2016, 201, 65–76. [Google Scholar] [CrossRef]
- Fu, J.; Chen, J.; Li, W.; Yang, X.; Yang, J.; Quan, H.; Huang, H.; Chen, G. Laminin-Modified Dental Pulp Extracellular Matrix for Dental Pulp Regeneration. Front. Bioeng. Biotechnol. 2020, 8, 595096. [Google Scholar] [CrossRef] [PubMed]
- Na, S.; Zhang, H.; Huang, F.; Wang, W.; Ding, Y.; Li, D.; Jin, Y. Regeneration of dental pulp/dentine complex with a three-dimensional and scaffold-free stem-cell sheet-derived pellet. J. Tissue Eng. Regen. Med. 2016, 10, 261–270. [Google Scholar] [CrossRef] [PubMed]
- Holiel, A.A.; Mahmoud, E.M.; Abdel-Fattah, W.M.; Kawana, K.Y. Histological evaluation of the regenerative potential of a novel treated dentin matrix hydrogel in direct pulp capping. Clin. Oral Investig. 2021, 25, 2101–2112. [Google Scholar] [CrossRef] [PubMed]
- Holiel, A.A.; Mahmoud, E.M.; Abdel-Fattah, W.M. Tomographic evaluation of direct pulp capping using a novel injectable treated dentin matrix hydrogel: A 2-year randomized controlled clinical trial. Clin. Oral Investig. 2021, 25, 4621–4634. [Google Scholar] [CrossRef] [PubMed]
- Cunha, D.; Souza, N.; Moreira, M.; Rodrigues, N.; Silva, P.; Franca, C.; Horsophonphong, S.; Sercia, A.; Subbiah, R.; Tahayeri, A.; et al. 3D-printed microgels supplemented with dentin matrix molecules as a novel biomaterial for direct pulp capping. Clin. Oral Investig. 2022. [Google Scholar] [CrossRef]
- Bi, F.; Tang, H.; Zhang, Z.; Lyu, Y.; Huo, F.; Chen, G.; Guo, W. Hertwig’s epithelial root sheath cells show potential for periodontal complex regeneration. J. Periodontol. 2022. [Google Scholar] [CrossRef]
- Yang, X.; Ma, Y.; Guo, W.; Yang, B.; Tian, W. Stem cells from human exfoliated deciduous teeth as an alternative cell source in bio-root regeneration. Theranostics 2019, 9, 2694–2711. [Google Scholar] [CrossRef]
- Ji, B.; Sheng, L.; Chen, G.; Guo, S.; Xie, L.; Yang, B.; Guo, W.; Tian, W. The combination use of platelet-rich fibrin and treated dentin matrix for tooth root regeneration by cell homing. Tissue Eng. Part A 2015, 21, 26–34. [Google Scholar] [CrossRef]
- Luo, X.; Yang, B.; Sheng, L.; Chen, J.; Li, H.; Xie, L.; Chen, G.; Yu, M.; Guo, W.; Tian, W. CAD based design sensitivity analysis and shape optimization of scaffolds for bio-root regeneration in swine. Biomaterials 2015, 57, 59–72. [Google Scholar] [CrossRef] [PubMed]
- Guo, W.; Gong, K.; Shi, H.; Zhu, G.; He, Y.; Ding, B.; Wen, L.; Jin, Y. Dental follicle cells and treated dentin matrix scaffold for tissue engineering the tooth root. Biomaterials 2012, 33, 1291–1302. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Sun, J.; Yang, H.; Han, X.; Luo, X.; Liao, L.; Yang, B.; Zhu, T.; Huo, F.; Guo, W.; et al. Recruited CD68+ CD206+ macrophages orchestrate graft immune tolerance to prompt xenogeneic-dentin matrix-based tooth root regeneration. Bioact. Mater. 2021, 6, 1051–1072. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Li, Q.; Lu, H.; Liu, Z.; Wu, Y.; Mao, J.; Gong, S. Effects of the Combined Application of Trimethylated Chitosan and Carbodiimide on the Biostability and Antibacterial Activity of Dentin Collagen Matrix. Polymers 2022, 14, 3166. [Google Scholar] [CrossRef]
- Moraes, G.F.; Caetano, R.O.; Prochnow, F.; Pupo, Y.M.; Schussel, J.L.; Schwartz-Filho, H.O. Demineralized human dentin matrix for alveolar ridge preservation using a volumetric and histologic analyses in rats. Braz. Dent. J. 2022, 33, 82–91. [Google Scholar] [CrossRef]
- Li, Y.; Zhou, W.; Li, P.; Luo, Q.; Li, A.; Zhang, X. Comparison of the osteogenic effectiveness of an autogenous demineralised dentin matrix and Bio-Oss® in bone augmentation: A systematic review and meta-analysis. Br. J. Oral Maxillofac. Surg. 2022, 60, 868–876. [Google Scholar] [CrossRef]
- Um, I.W.; Kim, Y.K.; Park, J.C.; Lee, J.H. Clinical application of autogenous demineralized dentin matrix loaded with recombinant human bone morphogenetic-2 for socket preservation: A case series. Clin. Implant. Dent. Relat. Res. 2019, 21, 4–10. [Google Scholar] [CrossRef]
- Han, J.; Jeong, W.; Kim, M.K.; Nam, S.H.; Park, E.K.; Kang, H.W. Demineralized Dentin Matrix Particle-Based Bio-Ink for Patient-Specific Shaped 3D Dental Tissue Regeneration. Polymers 2021, 13, 1294. [Google Scholar] [CrossRef]
- Ozdemir, O.; Kopac, T. Cytotoxicity and biocompatibility of root canal sealers: A review on recent studies. J. Appl. Biomater. Funct. Mater. 2022, 20, 1597430629. [Google Scholar] [CrossRef]
- Chen, H.; Zhang, Y.; Yu, T.; Song, G.; Xu, T.; Xin, T.; Lin, Y.; Han, B. Nano-Based Drug Delivery Systems for Periodontal Tissue Regeneration. Pharmaceutics 2022, 14, 2250. [Google Scholar] [CrossRef]
- Ozdemir, O.; Kopac, T. Recent Progress on the Applications of Nanomaterials and Nano-Characterization Techniques in Endodontics: A Review. Materials 2022, 15, 5109. [Google Scholar] [CrossRef] [PubMed]
- Wilson, C.J.; Clegg, R.E.; Leavesley, D.I.; Pearcy, M.J. Mediation of biomaterial-cell interactions by adsorbed proteins: A review. Tissue Eng. 2005, 11, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Kopac, T. Protein corona, understanding the nanoparticle-protein interactions and future perspectives: A critical review. Int. J. Biol. Macromol. 2021, 169, 290–301. [Google Scholar] [CrossRef] [PubMed]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bi, F.; Zhang, Z.; Guo, W. Treated Dentin Matrix in Tissue Regeneration: Recent Advances. Pharmaceutics 2023, 15, 91. https://doi.org/10.3390/pharmaceutics15010091
Bi F, Zhang Z, Guo W. Treated Dentin Matrix in Tissue Regeneration: Recent Advances. Pharmaceutics. 2023; 15(1):91. https://doi.org/10.3390/pharmaceutics15010091
Chicago/Turabian StyleBi, Fei, Zhijun Zhang, and Weihua Guo. 2023. "Treated Dentin Matrix in Tissue Regeneration: Recent Advances" Pharmaceutics 15, no. 1: 91. https://doi.org/10.3390/pharmaceutics15010091
APA StyleBi, F., Zhang, Z., & Guo, W. (2023). Treated Dentin Matrix in Tissue Regeneration: Recent Advances. Pharmaceutics, 15(1), 91. https://doi.org/10.3390/pharmaceutics15010091