Design, Development, Evaluation, and In Vivo Performance of Buccal Films Embedded with Paliperidone-Loaded Nanostructured Lipid Carriers
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Quantification of Paliperidone
2.3. NLC Formulation Preliminary Studies
2.4. Preparation of NLCs
2.5. Optimization of NLCs by Box–Behnken Method
2.6. Characterization of NLCs
2.6.1. Particle Characterization
2.6.2. Entrapment Efficiency (EE) and Drug Loading
2.6.3. Drug Release
2.7. Morphology
2.7.1. Optical Microscopy
2.7.2. Transmission Electron Microscopy (TEM)
2.8. Preparation of Buccal Mucoadhesive Film
2.9. Characterization of Buccal Mucoadhesive Film
2.10. Tensile Strength
2.11. Mucoadhesive Strength
2.12. Degree of Crystallinity
2.13. Spectral Analysis
2.14. Paliperidone Release from Films
2.15. Drug Permeation
2.16. Pharmacokinetics in Rabbits
2.17. Data Assessment
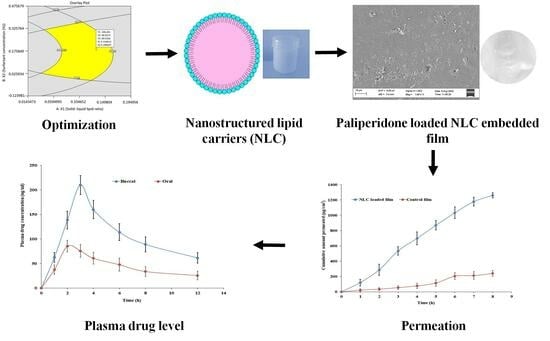
3. Results and Discussion
3.1. Selection of Lipids
3.2. Preliminary Batches of NLCs
3.3. Box-Behnken Design for Optimization of NLCs
3.3.1. Effect on Particle Size
3.3.2. Effect on EE
3.3.3. Effect on Drug Release
3.3.4. Optimization and Point Prediction
3.4. Characterization of NLCs
3.4.1. Particle Characterization
3.4.2. Zeta Potential
3.4.3. Morphology
3.5. Characterization of NLC-Loaded Buccal Mucoadhesive Film
3.6. Degree of Crystallinity
3.7. Spectral Analysis
3.8. Drug Release from Films
3.9. Drug Permeation
3.10. In Vivo Study
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lobo, M.C.; Whitehurst, T.S.; Kaar, S.J.; Howes, O.D. New and emerging treatments for schizophrenia: A narrative review of their pharmacology, efficacy and side effect profile relative to established antipsychotics. Neurosci. Biobehav. Rev. 2022, 132, 324–361. [Google Scholar] [CrossRef] [PubMed]
- Fellner, C. New Schizophrenia Treatments Address Unmet Clinical Needs. P T Peer-Rev. J. Formul. Manag. 2017, 42, 130–134. [Google Scholar]
- Devrimci-Ozguven, H.; Atmaca, M.; Baran, Z.; Cengisiz, C.; Çinar, C.; Erol, A.; Genç, Y.; Karadağ, H.; Karakülah, K.; Karasu, U.; et al. Efficacy and Safety of Paliperidone Palmitate Treatment in Patients with Schizophrenia: A Real-World Multicenter, Retrospective, Mirror-Image Study. J. Clin. Psychopharmacol. 2019, 39, 604–610. [Google Scholar] [CrossRef] [PubMed]
- Rehman, S.; Nabi, B.; Javed, A.; Khan, T.; Iqubal, A.; Ansari, M.J.; Baboota, S.; Ali, J. Unraveling enhanced brain delivery of paliperidone-loaded lipid nanoconstructs: Pharmacokinetic, behavioral, biochemical, and histological aspects. Drug Deliv. 2022, 29, 1409–1422. [Google Scholar] [CrossRef]
- Mauri, M.C.; Reggiori, A.; Paletta, S.; Di Pace, C.; Altamura, A.C. Paliperidone for the treatment of schizophrenia and schizoaffective disorders—A drug safety evaluation. Expert Opin. Drug Saf. 2017, 16, 365–379. [Google Scholar] [CrossRef]
- Wesołowska, A.; Jastrzębska-Więsek, M.; Cios, A.; Partyka, A. The preclinical discovery and development of paliperidone for the treatment of schizophrenia. Expert Opin. Drug Discov. 2020, 15, 279–292. [Google Scholar] [CrossRef]
- Mali, S.; Oza, N. Formulation and optimization of Paliperidone palmitate biodegradable injectable microspheres using Box-Behnken design. J. Drug Deliv. Sci. Technol. 2022, 74, 103609. [Google Scholar] [CrossRef]
- Rehman, S.; Nabi, B.; Baboota, S.; Ali, J. Tailoring lipid nanoconstructs for the oral delivery of paliperidone: Formulation, optimization and in vitro evaluation. Chem. Phys. Lipids 2021, 234, 105005. [Google Scholar] [CrossRef]
- Patel, M.R.; Patel, R.B.; Bhatt, K.K.; Patel, B.G.; Gaikwad, R.V. Paliperidone microemulsion for nose-to-brain targeted drug delivery system: Pharmacodynamic and pharmacokinetic evaluation. Drug Deliv. 2016, 23, 346–354. [Google Scholar] [CrossRef]
- Thimmasetty, J.; Ghosh, T.; Nayak, N.S.; Raheem, A. Oral bioavailability enhancement of paliperidone by the use of cocrystalization and precipitation inhibition. J. Pharm. Innov. 2021, 16, 160–169. [Google Scholar] [CrossRef]
- Jee, J.P.; Kim, Y.H.; Lee, J.H.; Min, K.A.; Jang, D.J.; Jin, S.G.; Cho, K.H. Paliperidone-Cation Exchange Resin Complexes of Different Particle Sizes for Controlled Release. Pharmaceutics 2023, 15, 932. [Google Scholar] [CrossRef] [PubMed]
- Raval, S.; Jani, P.; Patil, P.; Thakkar, P.; Sawant, K. Enhancement of bioavailability through transdermal drug delivery of paliperidone palmitate-loaded nanostructured lipid carriers. Ther. Deliv. 2021, 12, 583–596. [Google Scholar] [CrossRef] [PubMed]
- Deruyver, L.; Rigaut, C.; Gomez-Perez, A.; Lambert, P.; Haut, B.; Goole, J. In vitro Evaluation of Paliperidone Palmitate Loaded Cubosomes Effective for Nasal-to-Brain Delivery. Int. J. Nanomed. 2023, 18, 1085–1106. [Google Scholar] [CrossRef] [PubMed]
- Jacob, S.; Nair, A.B.; Boddu, S.H.S.; Gorain, B.; Sreeharsha, N.; Shah, J. An updated overview of the emerging role of patch and film-based buccal delivery systems. Pharmaceutics 2021, 13, 1206. [Google Scholar] [CrossRef]
- Tzanova, M.M.; Hagesaether, E.; Tho, I. Solid lipid nanoparticle-loaded mucoadhesive buccal films—Critical quality attributes and in vitro safety & efficacy. Int. J. Pharm. 2021, 592, 120100. [Google Scholar] [CrossRef]
- Montero-Padilla, S.; Velaga, S.; Morales, J.O. Buccal Dosage Forms: General Considerations for Pediatric Patients. AAPS PharmSciTech 2017, 18, 273–282. [Google Scholar] [CrossRef]
- Shipp, L.; Liu, F.; Kerai-Varsani, L.; Okwuosa, T.C. Buccal films: A review of therapeutic opportunities, formulations & relevant evaluation approaches. J. Control. Release Off. J. Control. Release Soc. 2022, 352, 1071–1092. [Google Scholar] [CrossRef]
- Al-Dhubiab, B.E.; Nair, A.B.; Kumria, R.; Attimarad, M.; Harsha, S. Development and evaluation of buccal films impregnated with selegiline-loaded nanospheres. Drug Deliv. 2016, 23, 2154–2162. [Google Scholar] [CrossRef]
- Al-Dhubiab, B.E.; Nair, A.B.; Kumria, R.; Attimarad, M.; Harsha, S. Formulation and evaluation of nano based drug delivery system for the buccal delivery of acyclovir. Colloids Surf. B Biointerfaces 2015, 136, 878–884. [Google Scholar] [CrossRef]
- Beloqui, A.; Solinís, M.; Rodríguez-Gascón, A.; Almeida, A.J.; Préat, V. Nanostructured lipid carriers: Promising drug delivery systems for future clinics. Nanomed. Nanotechnol. Biol. Med. 2016, 12, 143–161. [Google Scholar] [CrossRef]
- Fonseca-Santos, B.; Silva, P.B.; Rigon, R.B.; Sato, M.R.; Chorilli, M. Formulating SLN and NLC as Innovative Drug Delivery Systems for Non-Invasive Routes of Drug Administration. Curr. Med. Chem. 2020, 27, 3623–3656. [Google Scholar] [CrossRef] [PubMed]
- Basahih, T.S.; Alamoudi, A.A.; El-Say, K.M.; Alhakamy, N.A.; Ahmed, O.A.A. Improved Transmucosal Delivery of Glimepiride via Unidirectional Release Buccal Film Loaded with Vitamin E TPGS-Based Nanocarrier. Dose-Response A Publ. Int. Hormesis Soc. 2020, 18, 1559325820945164. [Google Scholar] [CrossRef] [PubMed]
- Kraisit, P.; Limmatvapirat, S.; Luangtana-Anan, M.; Sriamornsak, P. Buccal administration of mucoadhesive blend films saturated with propranolol loaded nanoparticles. Asian J. Pharm. Sci. 2018, 13, 34–43. [Google Scholar] [CrossRef] [PubMed]
- Tetyczka, C.; Griesbacher, M.; Absenger-Novak, M.; Fröhlich, E.; Roblegg, E. Development of nanostructured lipid carriers for intraoral delivery of Domperidone. Int. J. Pharm. 2017, 526, 188–198. [Google Scholar] [CrossRef]
- Kamboj, S.; Bala, S.; Nair, A.B. Solid lipid nanoparticles: An effective lipid based technology for poorly water soluble drugs. Int. J. Pharm. Sci. Rev. Res. 2010, 5, 78–90. [Google Scholar]
- Van, N.H.; Vy, N.T.; Van Toi, V.; Dao, A.H.; Lee, B.-J. Nanostructured lipid carriers and their potential applications for versatile drug delivery via oral administration. OpenNano 2022, 8, 100064. [Google Scholar]
- Zhu, Z.; Zhai, Y.; Zhang, N.; Leng, D.; Ding, P. The development of polycarbophil as a bioadhesive material in pharmacy. Asian J. Pharm. Sci. 2013, 8, 218–227. [Google Scholar] [CrossRef]
- Fonseca-Santos, B.; Chorilli, M. An overview of polymeric dosage forms in buccal drug delivery: State of art, design of formulations and their in vivo performance evaluation. Mater. Sci. Eng. C Mater. Biol. Appl. 2018, 86, 129–143. [Google Scholar] [CrossRef]
- Rudragangaiah, S.; Bhatta, R.G.; Kotappa, S.B.B. Stability-Indicating RP-HPLC Method for the Quantification of Paliperidone in Bulk and Solid Dosage Form to Establish Validation and Stability Indicating Parameters. Order 2019, 3, 5. [Google Scholar] [CrossRef]
- Mahmood, A.; Rapalli, V.K.; Gorantla, S.; Waghule, T.; Singhvi, G. Dermatokinetic assessment of luliconazole-loaded nanostructured lipid carriers (NLCs) for topical delivery: QbD-driven design, optimization, and in vitro and ex vivo evaluations. Drug Deliv. Transl. Res. 2022, 12, 1118–1135. [Google Scholar] [CrossRef]
- Gomaa, E.; Fathi, H.A.; Eissa, N.G.; Elsabahy, M. Methods for preparation of nanostructured lipid carriers. Methods 2022, 199, 3–8. [Google Scholar] [CrossRef]
- Wu, K.W.; Sweeney, C.; Dudhipala, N.; Lakhani, P.; Chaurasiya, N.D.; Tekwani, B.L.; Majumdar, S. Primaquine Loaded Solid Lipid Nanoparticles (SLN), Nanostructured Lipid Carriers (NLC), and Nanoemulsion (NE): Effect of Lipid Matrix and Surfactant on Drug Entrapment, in vitro Release, and ex vivo Hemolysis. AAPS PharmSciTech 2021, 22, 240. [Google Scholar] [CrossRef]
- Subramaniam, B.; Siddik, Z.H.; Nagoor, N.H. Optimization of nanostructured lipid carriers: Understanding the types, designs, and parameters in the process of formulations. J. Nanoparticle Res. 2020, 22, 141. [Google Scholar] [CrossRef]
- Wang, W.; Chen, L.; Huang, X.; Shao, A. Preparation and Characterization of Minoxidil Loaded Nanostructured Lipid Carriers. AAPS PharmSciTech 2017, 18, 509–516. [Google Scholar] [CrossRef]
- Shete, M.B.; Deshpande, A.S.; Shende, P. Enhancement of in-vitro anti-oral cancer activities of silymarin using dispersion of nanostructured lipid carrier in mucoadhesive in-situ gel. Int. J. Pharm. 2023, 636, 122860. [Google Scholar] [CrossRef] [PubMed]
- Nair, A.B.; Al-Dhubiab, B.E.; Shah, J.; Vimal, P.; Attimarad, M.; Harsha, S. Development and evaluation of palonosetron loaded mucoadhesive buccal films. J. Drug Deliv. Sci. Technol. 2018, 47, 351–358. [Google Scholar] [CrossRef]
- Ammar, H.O.; Ghorab, M.M.; Felton, L.A.; Gad, S.; Fouly, A.A. Effect of Antiadherents on the Physical and Drug Release Properties of Acrylic Polymeric Films. AAPS PharmSciTech 2016, 17, 682–692. [Google Scholar] [CrossRef]
- Nair, A.B.; Al-Dhubiab, B.E.; Shah, J.; Jacob, S.; Saraiya, V.; Attimarad, M.; SreeHarsha, N.; Akrawi, S.H.; Shehata, T.M. Mucoadhesive buccal film of almotriptan improved therapeutic delivery in rabbit model. Saudi Pharm. J. 2020, 28, 201–209. [Google Scholar] [CrossRef]
- Jug, M.; Hafner, A.; Lovrić, J.; Kregar, M.L.; Pepić, I.; Vanić, Ž.; Cetina-Čižmek, B.; Filipović-Grčić, J. An overview of in vitro dissolution/release methods for novel mucosal drug delivery systems. J. Pharm. Biomed. Anal. 2018, 147, 350–366. [Google Scholar] [CrossRef]
- Nair, A.; Vyas, H.; Shah, J.; Kumar, A. Effect of permeation enhancers on the iontophoretic transport of metoprolol tartrate and the drug retention in skin. Drug Deliv. 2011, 18, 19–25. [Google Scholar] [CrossRef]
- Anroop, B.; Ghosh, B.; Parcha, V.; Kumar, A.; Khanam, J. Synthesis and comparative skin permeability of atenolol and propranolol esters. J. Drug Deliv. Sci. Technol. 2005, 15, 187–190. [Google Scholar] [CrossRef]
- Jacob, S.; Nair, A.B.; Morsy, M.A. Dose conversion between animals and humans: A practical solution. Indian J. Pharm. Educ. Res. 2022, 56, 600–607. [Google Scholar] [CrossRef]
- Persson, L.C.; Porter, C.J.; Charman, W.N.; Bergström, C.A. Computational prediction of drug solubility in lipid based formulation excipients. Pharm. Res. 2013, 30, 3225–3237. [Google Scholar] [CrossRef] [PubMed]
- Marathe, S.; Shadambikar, G.; Mehraj, T.; Sulochana, S.P.; Dudhipala, N.; Majumdar, S. Development of α-Tocopherol Succinate-Based Nanostructured Lipid Carriers for Delivery of Paclitaxel. Pharmaceutics 2022, 14, 1034. [Google Scholar] [CrossRef]
- Izza, N.; Suga, K.; Okamoto, Y.; Watanabe, N.; Bui, T.T.; Wibisono, Y.; Fadila, C.R.; Umakoshi, H. Systematic Characterization of Nanostructured Lipid Carriers from Cetyl Palmitate/Caprylic Triglyceride/Tween 80 Mixtures in an Aqueous Environment. Langmuir ACS J. Surf. Colloids 2021, 37, 4284–4293. [Google Scholar] [CrossRef]
- Cirri, M.; Maestrini, L.; Maestrelli, F.; Mennini, N.; Mura, P.; Ghelardini, C.; Di Cesare Mannelli, L. Design, characterization and in vivo evaluation of nanostructured lipid carriers (NLC) as a new drug delivery system for hydrochlorothiazide oral administration in pediatric therapy. Drug Deliv. 2018, 25, 1910–1921. [Google Scholar] [CrossRef]
- Kraisit, P.; Sarisuta, N. Development of Triamcinolone Acetonide-Loaded Nanostructured Lipid Carriers (NLCs) for Buccal Drug Delivery Using the Box-Behnken Design. Molecules 2018, 23, 982. [Google Scholar] [CrossRef]
- Zhang, K.; Lv, S.; Li, X.; Feng, Y.; Li, X.; Liu, L.; Li, S.; Li, Y. Preparation, characterization, and in vivo pharmacokinetics of nanostructured lipid carriers loaded with oleanolic acid and gentiopicrin. Int. J. Nanomed. 2013, 8, 3227–3239. [Google Scholar] [CrossRef]
- Elmowafy, M.; Shalaby, K.; Ali, H.M.; Alruwaili, N.K.; Salama, A.; Ibrahim, M.F.; Akl, M.A.; Ahmed, T.A. Impact of nanostructured lipid carriers on dapsone delivery to the skin: In vitro and in vivo studies. Int. J. Pharm. 2019, 572, 118781. [Google Scholar] [CrossRef]
- Thapa, C.; Ahad, A.; Aqil, M.; Imam, S.S.; Sultana, Y. Formulation and optimization of nanostructured lipid carriers to enhance oral bioavailability of telmisartan using Box–Behnken design. J. Drug Deliv. Sci. Technol. 2018, 44, 431–439. [Google Scholar] [CrossRef]
- Javed, S.; Mangla, B.; Almoshari, Y.; Sultan, M.H.; Ahsan, W. Nanostructured lipid carrier system: A compendium of their formulation development approaches, optimization strategies by quality by design, and recent applications in drug delivery. Nanotechnol. Rev. 2022, 11, 1744–1777. [Google Scholar] [CrossRef]
- Agrawal, M.; Saraf, S.; Pradhan, M.; Patel, R.J.; Singhvi, G.; Ajazuddin; Alexander, A. Design and optimization of curcumin loaded nano lipid carrier system using Box-Behnken design. Biomed. Pharmacother. Biomed. Pharmacother. 2021, 141, 111919. [Google Scholar] [CrossRef] [PubMed]
- Qadir, A.; Aqil, M.; Ali, A.; Warsi, M.H.; Mujeeb, M.; Ahmad, F.J.; Ahmad, S.; Beg, S. Nanostructured lipidic carriers for dual drug delivery in the management of psoriasis: Systematic optimization, dermatokinetic and preclinical evaluation. J. Drug Deliv. Sci. Technol. 2020, 57, 101775. [Google Scholar] [CrossRef]
- Gordillo-Galeano, A.; Mora-Huertas, C.E. Solid lipid nanoparticles and nanostructured lipid carriers: A review emphasizing on particle structure and drug release. Eur. J. Pharm. Biopharm. Off. J. Arbeitsgemeinschaft Fur Pharm. Verfahrenstechnik e.V 2018, 133, 285–308. [Google Scholar] [CrossRef] [PubMed]
- Sneha, K.; Kumar, A. Nanoemulsions: Techniques for the preparation and the recent advances in their food applications. Innov. Food Sci. Emerg. Technol. 2022, 76, 102914. [Google Scholar]
- Khosa, A.; Reddi, S.; Saha, R.N. Nanostructured lipid carriers for site-specific drug delivery. Biomed. Pharmacother. Biomed. Pharmacother. 2018, 103, 598–613. [Google Scholar] [CrossRef]
- Gordillo-Galeano, A.; Mora-Huertas, C.E. Hydrodynamic diameter and zeta potential of nanostructured lipid carriers: Emphasizing some parameters for correct measurements. Colloids Surf. A Physicochem. Eng. Asp. 2021, 620, 126610. [Google Scholar] [CrossRef]
- Veider, F.; Akkuş-Dağdeviren, Z.B.; Knoll, P.; Bernkop-Schnürch, A. Design of nanostructured lipid carriers and solid lipid nanoparticles for enhanced cellular uptake. Int. J. Pharm. 2022, 624, 122014. [Google Scholar] [CrossRef]
- Varela-Fernández, R.; García-Otero, X.; Díaz-Tomé, V.; Regueiro, U.; López-López, M.; González-Barcia, M.; Isabel Lema, M.; Javier Otero-Espinar, F. Lactoferrin-loaded nanostructured lipid carriers (NLCs) as a new formulation for optimized ocular drug delivery. Eur. J. Pharm. Biopharm. Off. J. Arbeitsgemeinschaft Fur Pharm. Verfahrenstechnik e.V 2022, 172, 144–156. [Google Scholar] [CrossRef]
- Kumria, R.; Nair, A.B.; Al-Dhubiab, B.E. Loratidine buccal films for allergic rhinitis: Development and evaluation. Drug Dev. Ind. Pharm. 2014, 40, 625–631. [Google Scholar] [CrossRef]
- Okafor, N.I.; Ngoepe, M.; Noundou, X.S.; Krause, R.W.M. Nano-enabled liposomal mucoadhesive films for enhanced efavirenz buccal drug delivery. J. Drug Deliv. Sci. Technol. 2019, 54, 101312. [Google Scholar] [CrossRef]
- Kraisit, P.; Limmatvapirat, S.; Nunthanid, J.; Sriamornsak, P.; Luangtana-Anan, M. Preparation and Characterization of Hydroxypropyl Methylcellulose/Polycarbophil Mucoadhesive Blend Films Using a Mixture Design Approach. Chem. Pharm. Bull. 2017, 65, 284–294. [Google Scholar] [CrossRef] [PubMed]
- Elkomy, M.H.; Elmowafy, M.; Shalaby, K.; Azmy, A.F.; Ahmad, N.; Zafar, A.; Eid, H.M. Development and machine-learning optimization of mucoadhesive nanostructured lipid carriers loaded with fluconazole for treatment of oral candidiasis. Drug Dev. Ind. Pharm. 2021, 47, 246–258. [Google Scholar] [CrossRef] [PubMed]
- Ho, H.N.; Le, H.H.; Le, T.G.; Duong, T.H.A.; Ngo, V.Q.T.; Dang, C.T.; Nguyen, V.M.; Tran, T.H.; Nguyen, C.N. Formulation and characterization of hydroxyethyl cellulose-based gel containing metronidazole-loaded solid lipid nanoparticles for buccal mucosal drug delivery. Int. J. Biol. Macromol. 2022, 194, 1010–1018. [Google Scholar] [CrossRef]
- Zewail, M.B.; Asaad, G.F.; Swellam, S.M.; Abd-Allah, S.M.; Hosny, S.K.; Sallah, S.K.; Eissa, J.E.; Mohamed, S.S.; El-Dakroury, W.A. Design, characterization and in vivo performance of solid lipid nanoparticles (SLNs)-loaded mucoadhesive buccal tablets for efficient delivery of Lornoxicam in experimental inflammation. Int. J. Pharm. 2022, 624, 122006. [Google Scholar] [CrossRef]
- Na, Y.G.; Huh, H.W.; Kim, M.K.; Byeon, J.J.; Han, M.G.; Lee, H.K.; Cho, C.W. Development and evaluation of a film-forming system hybridized with econazole-loaded nanostructured lipid carriers for enhanced antifungal activity against dermatophytes. Acta Biomater. 2020, 101, 507–518. [Google Scholar] [CrossRef]
- Arunprasert, K.; Pornpitchanarong, C.; Piemvuthi, C.; Siraprapapornsakul, S.; Sripeangchan, S.; Lertsrimongkol, O.; Opanasopit, P.; Patrojanasophon, P. Nanostructured lipid carrier-embedded polyacrylic acid transdermal patches for improved transdermal delivery of capsaicin. Eur. J. Pharm. Sci. Off. J. Eur. Fed. Pharm. Sci. 2022, 173, 106169. [Google Scholar] [CrossRef]
- Corsaro, C.; Neri, G.; Mezzasalma, A.M.; Fazio, E. Weibull Modeling of Controlled Drug Release from Ag-PMA Nanosystems. Polymers 2021, 13, 2897. [Google Scholar] [CrossRef]
- Azadi, S.; Ashrafi, H.; Azadi, A. Mathematical modeling of drug release from swellable polymeric nanoparticles. J. Appl. Pharm. Sci. 2017, 7, 125–133. [Google Scholar]
- Hosny, K.M.; Sindi, A.M.; Ali, S.; Alharbi, W.S.; Hajjaj, M.S.; Bukhary, H.A.; Badr, M.Y.; Mushtaq, R.Y.; Murshid, S.S.A.; Almehmady, A.M.; et al. Development, optimization, and evaluation of a nanostructured lipid carrier of sesame oil loaded with miconazole for the treatment of oral candidiasis. Drug Deliv. 2022, 29, 254–262. [Google Scholar] [CrossRef]














| Factors | Actual (Coded) Values | ||
|---|---|---|---|
| Low (−1) | Medium (0) | High (+1) | |
| Independent variables | |||
| X1 = Solid–liquid lipid ratio | 7:3 | 7.5:2.5 | 8:2 |
| X2 = Surfactant concentration (%) | 1 | 2 | 3 |
| X3 = Ultrasonication time (min) | 10 | 15 | 20 |
| Dependent variable | Targets | ||
| Y1 = Particle size (nm) | Minimum | ||
| Y2 = EE % | Maximum | ||
| Y3 = Drug release (%) | Maximum | ||
| Lipid/Surfactant | Amount of Paliperidone Dissolved (µg/mg) |
|---|---|
| Glycerol monostearate | 29.83 ± 0.98 |
| Precirol ATO 5 | 15.78 ± 0.67 |
| Compritol 888 ATO | 19.44 ± 1.06 |
| Dynasan 114 | 20.11 ± 0.74 |
| Stearic acid | 14.24 ± 0.85 |
| Oleic acid | 2.23 ± 0.64 |
| Labrafil® M 2125 CS | 0.94 ± 0.53 |
| Labrafil® M 1944 CS | 1.67 ± 0.38 |
| Labrafac® CC | 1.45 ± 0.68 |
| Tween 80 | 45.58 ± 1.72 |
| Batches | Composition | Entrapment Efficiency (%) | ||
|---|---|---|---|---|
| Paliperidone (%, w/w) | Glycerol Monostearate: Oleic Acid Ratio | Tween 80 (%, w/w) | ||
| PB1 | 1 | 9:1 | 2 | 75.43 |
| PB2 | 1 | 8:2 | 2 | 80.23 |
| PB3 | 1 | 7:3 | 2 | 88.45 |
| PB4 | 1 | 6:4 | 2 | 80.65 |
| PB5 | 1 | 5:5 | 2 | 75.73 |
| PB6 | 1 | 4:6 | 2 | 70.12 |
| PB7 | 1 | 3:7 | 2 | 63.56 |
| PB8 | 1 | 2:8 | 2 | 55.98 |
| PB9 | 1 | 1:9 | 2 | 45.30 |
| PB10 | 1 | 7:3 | 0.5 | 58.46 |
| PB11 | 1 | 7:3 | 1 | 75.33 |
| PB12 | 1 | 7:3 | 3 | 68.93 |
| PB13 | 1 | 7:3 | 4 | 63.47 |
| Formulation | Actual Values | Response Values | PDI | Zeta Potential (mV) | ||||
|---|---|---|---|---|---|---|---|---|
| Solid–Liquid Lipid Ratio, X1 | Surfactant Concentration (%), X2 | Ultrasonication Time (min), X3 | Particle Size (nm), Y1 | Entrapment Efficiency (%), Y2 | Drug Release (%), Y3 | |||
| NL1 | 7:3 | 2 | 20 | 357.24 ± 43.66 | 68.34 ± 3.28 | 65.25 ± 3.35 | 0.65 | −54.6 |
| NL2 | 8:2 | 2 | 20 | 366.56 ± 38.24 | 73.75 ± 3.95 | 68.32 ± 4.08 | 0.75 | −48.2 |
| NL3 | 7:3 | 2 | 10 | 306.57 ± 29.88 | 66.42 ± 3.50 | 67.31 ± 3.82 | 0.39 | −56.2 |
| NL4 | 7.5:2.5 | 1 | 20 | 394.78 ± 47.61 | 61.29 ± 3.08 | 63.68 ± 5.04 | 0.44 | −52.4 |
| NL5 | 7.5:2.5 | 2 | 15 | 130.53 ± 27.32 | 86.42 ± 4.22 | 87.58 ± 4.66 | 0.37 | −49.2 |
| NL6 | 7.5:2.5 | 3 | 20 | 262.91 ± 30.64 | 61.24 ± 3.34 | 70.37 ± 4.12 | 0.57 | −37.8 |
| NL7 | 7.5:2.5 | 1 | 10 | 380.71 ± 35.82 | 73.48 ± 3.94 | 66.73 ± 3.06 | 0.51 | −53.6 |
| NL8 | 7.5:2.5 | 2 | 15 | 146.08 ± 25.68 | 86.65 ± 4.71 | 90.79 ± 4.28 | 0.48 | −66.3 |
| NL9 | 8:2 | 3 | 15 | 491.87 ± 44.20 | 81.43 ± 4.30 | 79.48 ± 4.37 | 0.69 | −45.7 |
| NL10 | 7.5:2.5 | 3 | 10 | 304.83 ± 37.08 | 71.47 ± 2.97 | 72.62 ± 4.16 | 0.45 | −31.4 |
| NL11 | 7.5:2.5 | 2 | 15 | 196.45 ± 33.18 | 86.35 ± 4.15 | 85.32 ± 4.72 | 0.26 | −41.4 |
| NL12 | 7:3 | 1 | 15 | 269.07 ± 39.27 | 67.34 ± 3.01 | 74.54 ± 3.97 | 0.45 | −53.1 |
| NL13 | 7.5:2.5 | 2 | 15 | 230.69 ± 28.16 | 89.32 ± 4.64 | 89.52 ± 4.60 | 0.51 | −43.8 |
| NL14 | 7.5:2.5 | 2 | 15 | 194.15 ± 30.42 | 89.45 ± 4.28 | 89.57 ± 4.49 | 0.20 | −53.3 |
| NL15 | 8:2 | 1 | 15 | 455.32 ± 35.61 | 73.56 ± 3.65 | 65.43 ± 3.88 | 0.59 | −34.7 |
| NL16 | 8:2 | 2 | 10 | 388.94 ± 39.37 | 79.31 ± 3.44 | 83.64 ± 4.14 | 0.67 | −39.5 |
| NL17 | 7:3 | 1 | 15 | 437.86 ± 48.94 | 76.99 ± 3.90 | 64.36 ± 3.90 | 0.54 | −40.6 |
| Y1 (Particle Size) nm | Y2 (EE) % | Y3 (Drug Release) % | |||
|---|---|---|---|---|---|
| Estimated | Observed | Estimated | Observed | Estimated | Observed |
| 186.40 | 186.33 | 88.03 | 88.18 | 89.63 | 89.19 |
| Parameter | Paliperidone NLC-Loaded Film (Mean ± SD) | Paliperidone Suspension (Mean ± SD) |
|---|---|---|
| Cmax (ng/mL) | 209.75 ± 19.27 * | 85.87 ± 11.87 |
| Tmax (h) | 3 | 2 |
| AUC0–12 (ng. h/mL) | 1266.94 ± 103.66 * | 536.17 ± 60.33 |
| Relative bioavailability (%) | 236 | 100 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
AlMulhim, F.M.; Nair, A.B.; Aldhubiab, B.; Shah, H.; Shah, J.; Mewada, V.; Sreeharsha, N.; Jacob, S. Design, Development, Evaluation, and In Vivo Performance of Buccal Films Embedded with Paliperidone-Loaded Nanostructured Lipid Carriers. Pharmaceutics 2023, 15, 2530. https://doi.org/10.3390/pharmaceutics15112530
AlMulhim FM, Nair AB, Aldhubiab B, Shah H, Shah J, Mewada V, Sreeharsha N, Jacob S. Design, Development, Evaluation, and In Vivo Performance of Buccal Films Embedded with Paliperidone-Loaded Nanostructured Lipid Carriers. Pharmaceutics. 2023; 15(11):2530. https://doi.org/10.3390/pharmaceutics15112530
Chicago/Turabian StyleAlMulhim, Fahad Mohammed, Anroop B. Nair, Bandar Aldhubiab, Hiral Shah, Jigar Shah, Vivek Mewada, Nagaraja Sreeharsha, and Shery Jacob. 2023. "Design, Development, Evaluation, and In Vivo Performance of Buccal Films Embedded with Paliperidone-Loaded Nanostructured Lipid Carriers" Pharmaceutics 15, no. 11: 2530. https://doi.org/10.3390/pharmaceutics15112530
APA StyleAlMulhim, F. M., Nair, A. B., Aldhubiab, B., Shah, H., Shah, J., Mewada, V., Sreeharsha, N., & Jacob, S. (2023). Design, Development, Evaluation, and In Vivo Performance of Buccal Films Embedded with Paliperidone-Loaded Nanostructured Lipid Carriers. Pharmaceutics, 15(11), 2530. https://doi.org/10.3390/pharmaceutics15112530