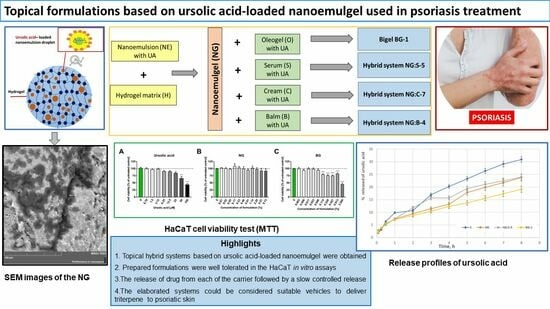
Topical Formulations Based on Ursolic Acid-Loaded Nanoemulgel with Potential Application in Psoriasis Treatment
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Cytotoxicity Test of Formulation Components
2.2.1. Cell Culture
2.2.2. Cell Viability Assay
2.3. Preparation of Formulations
2.3.1. Preparation of the Nanoemulgel
2.3.2. Preparation of the Macroemulsions (Cream, Serum, Body Balm)
2.3.3. Preparation of the Oleogel
2.3.4. Preparation of Hybrid Systems (Nanoemulgel–Macroemulsions; Nanoemulgel–Oleogel)
2.4. Physicochemical Analysis of Formulations
2.4.1. DLS Analysis
2.4.2. Microscopic Analysis
2.4.3. SEM Analysis
2.4.4. TEM Analysis
2.4.5. Rheology Analysis
2.4.6. pH Analysis
2.4.7. Stability Analysis
2.4.8. Texture Analysis
2.5. Kinetic Study of Ursolic Acid Release from the Obtained Systems
Evaluation of the Release Kinetics
- Qt—the amount of drug released in time t;
- Q0—the initial amount of drug;
- K0—zero-order kinetic constant;
- K1—first-order kinetic constant;
- KH—Higuchi kinetic constant;
- KKP—Korsmeyer–Peppas release constant;
- n—diffusional release exponent;
- t—time.
2.6. Statistical Analysis
3. Results
3.1. Screening of Formulation Components
3.2. Composition and Characterization of Formulations
3.2.1. Morphology of Formulations
3.2.2. Physicochemical Properties of the Obtained Formulations
3.2.3. Texture Profile
3.2.4. Cytotoxicity Test of Formulations
3.2.5. Ursolic Acid Release Studies
Kinetic Analysis of Ursolic Acid Release
4. Discussion
4.1. Composition and Characterization of Formulations
4.1.1. Physicochemical Properties of Formulations
The pH of the Formulations
Average Particle Size and Viscosity of the Formulation
Stability of Formulations
4.1.2. Texture Profile
4.2. Cytotoxicity Test of Formulations
4.3. Release of Ursolic Acid from Formulation and Kinetic Analysis
5. Conclusions
6. Patents
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| B | balm |
| C | cream |
| DMEM | Dulbecco’s modified Eagle’s medium |
| DLS | dynamic light scattering |
| FBS | fetal bovine serum |
| ME | macroemulsion |
| NE | nanoemulsion |
| NG | nanoemulgel |
| O | oleogel |
| PBS | phosphate-buffered saline |
| PDI | polydispersity index |
| S | serum |
| SEM | scanning electron microscope |
| TPA | profile texture analysis |
| TSI | Turbiscan stability index |
| UA | ursolic acid |
References
- Griffiths, C.E.M.; Barker, J.N.W.N. Psoriasis 1. Pathogenesis and clinical features of psoriasis. Lancet 2007, 370, 263–271. [Google Scholar] [CrossRef]
- Rendon, A.; Schäkel, K. Psoriasis pathogenesis and treatment. Int. J. Mol. Sci. 2019, 20, 1475. [Google Scholar] [CrossRef]
- Zagórska-Dziok, M.; Sobczak, M. Hydrogel-based active substance release systems for cosmetology and dermatology application: A review. Pharmaceutics 2020, 12, 396. [Google Scholar] [CrossRef]
- Reich, K. Łuszczyca Jako Układowa Choroba Zapalna: Implikacje Dla Postępowania. Dermatol. Po Dyplomie 2012, 3, 4–15. [Google Scholar]
- Parisi, R.; Symmons, D.P.M.; Griffiths, C.E.M.; Ashcroft, D.M. Global epidemiology of psoriasis: A systematic review of incidence and prevalence. J. Investig. Dermatol. 2013, 133, 377–385. [Google Scholar] [CrossRef]
- Kulawik-Pióro, A.; Miastkowska, M. Polymeric gels and their application in the treatment of psoriasis vulgaris: A review. Int. J. Mol. Sci. 2021, 22, 5124. [Google Scholar] [CrossRef]
- Furue, K.; Ito, T.; Furue, M. Differential efficacy of biologic treatments targeting the TNF-α/IL-23/IL-17 axis in psoriasis and psoriatic arthritis. Cytokine 2018, 111, 182–188. [Google Scholar] [CrossRef]
- van der Fits, L.; Mourits, S.; Voerman, J.S.A.; Kant, M.; Boon, L.; Laman, J.D.; Cornelissen, F.; Mus, A.-M.; Florencia, E.; Prens, E.P.; et al. Imiquimod-Induced Psoriasis-Like Skin Inflammation in Mice Is Mediated via the IL-23/IL-17 Axis. J. Immunol. 2009, 182, 5836–5845. [Google Scholar] [CrossRef]
- Sumida, H.; Yanagida, K.; Kita, Y.; Abe, J.; Matsushima, K.; Nakamura, M.; Ishii, S.; Sato, S.; Shimizu, T. Interplay between CXCR2 and BLT1 Facilitates Neutrophil Infiltration and Resultant Keratinocyte Activation in a Murine Model of Imiquimod-Induced Psoriasis. J. Immunol. 2014, 192, 4361–4369. [Google Scholar] [CrossRef]
- Kim, W.B.; Jerome, D.; Yeung, D.J. Diagnosis and management of psoriasis. Can. Fam. Physician 2017, 63, 278–285. [Google Scholar]
- Strzałka-Mrozik, B.; Krzaczyński, J. Pharmacological and non-pharmacological methods of psoriasis therapy with particular emphasis on biopharmaceuticals. Farm. Pol. 2020, 76, 333–343. [Google Scholar] [CrossRef]
- Bos, J.D.; Spuls, P.I. Topical treatments in psoriasis: Today and tomorrow. Clin. Dermatol. 2008, 26, 432–437. [Google Scholar] [CrossRef]
- Fluhr, J.W.; Cavallotti, C.; Berardesca, E. Emollients, moisturizers, and keratolytic agents in psoriasis. Clin. Dermatol. 2008, 26, 380–386. [Google Scholar] [CrossRef]
- Dattola, A.; Silvestri, M.; Bennardo, L.; Passante, M.; Rizzuto, F.; Dastoli, S.; Patruno, C.; Bianchi, L.; Nisticò, S.P. A novel vehicle for the treatment of psoriasis. Dermatol. Ther. 2020, 33, 13185. [Google Scholar] [CrossRef]
- Kaszuba, A.; Adamski, Z.; Szepietowski, J. Dermatologia Geriatryczna, 1st ed.; Wydawnictwo Czelej: Warszawa, Poland, 2016. [Google Scholar]
- Uva, L.; Miguel, D.; Pinheiro, C.; Antunes, J.; Cruz, D.; Ferreira, J.; Filipe, P. Mechanisms of action of topical corticosteroids in psoriasis. Int. J. Endocrinol. 2012, 2012, 561018. [Google Scholar] [CrossRef]
- Mygind, N.; Dahl, R. The rationale for use of topical corticosteroids in allergic rhinitis. Allergy 1996, 26, 2–10. [Google Scholar] [CrossRef]
- Schäcke, H.; Döcke, W.-D.; Asadullah, K. Mechanisms involved in the side effects of glucocorticoids. Pharmacol. Ther. 2002, 96, 23–43. [Google Scholar] [CrossRef]
- Teixeira, A.; Vasconcelos, V.; Teixeira, M.; Almeida, V.; Azevedo, R.; Torres, T.; Sousa Lobo, J.M.; Costa, P.C.; Almeida, I.F. Mechanical Properties of Topical Anti-Psoriatic Medicines: Implications for Patient Satisfaction with Treatment. AAPS Pharm. Sci. Tech. 2019, 20, 36. [Google Scholar] [CrossRef]
- Zang, L.L.; Wu, B.N.; Lin, Y.; Wang, J.; Fu, L.; Tang, Z.Y. Research progress of ursolic acid’s anti-tumor actions. Chin. J. Integr. Med. 2014, 20, 72–79. [Google Scholar] [CrossRef]
- Banno, N.; Akihisa, T.; Tokuda, H.; Yasukawa, K.; Higashihara, H.; Ukiya, M.; Watanabe, K.; Kimura, Y.; Hasegawa, J.-I.; Nishino, H. Triterpene Acids from the Leaves of Perilla frutescens and Their Anti-inflammatory and Antitumor-promoting Effects. Biosci. Biotechnol. Biochem. 2004, 68, 85–90. [Google Scholar] [CrossRef]
- Xu, T.; Wang, X.; Zhong, B.; Nurieva, R.I.; Ding, S.; Dong, C. Ursolic acid suppresses interleukin-17 (IL-17) production by selectively antagonizing the function of RORγt protein. J. Biol. Chem. 2011, 286, 22707–22710. [Google Scholar] [CrossRef]
- Ahmad, A.; Abuzinadah, M.F.; Alkreathy, H.M.; Banaganapalli, B.; Mujeeb, M. Ursolic acid rich Ocimum sanctum L. leaf extract loaded nanostructured lipid carriers ameliorate adjuvant induced arthritis in rats by inhibition of COX-1, COX-2, TNF-α and IL-1: Pharmacological and docking studies. PLoS ONE 2018, 13, e0193451. [Google Scholar] [CrossRef]
- Huang, Y.; Nikolic, D.; Pendland, S.; Doyle, B.J.; Locklear, T.D.; Mahady, G.B. Effects of cranberry extracts and ursolic acid derivatives on P-fimbriated Escherichia coli, COX-2 activity, pro-inflammatory cytokine release and the NF-κβ transcriptional response in vitro. Pharm. Biol. 2009, 47, 18–25. [Google Scholar] [CrossRef]
- Akhtar, N.; Verma, A.; Pathak, K. Exploring preclinical and clinical effectiveness of nanoformulations in the treatment of atopic dermatitis: Safety aspects and patent reviews. Bull. Fac. Pharm. Cairo Univ. 2017, 55, 1–10. [Google Scholar] [CrossRef]
- Lovelyn, C.; Attama, A.A. Current State of Nanoemulsions in Drug Delivery. J. Biomater. Nanobiotechnol. 2011, 2, 626–639. [Google Scholar] [CrossRef]
- Mishra, B.; Patel, B.B.; Tiwari, S. Colloidal nanocarriers: A review on formulation technology, types and applications toward targeted drug delivery. Nanomedicine 2010, 6, 9–24. [Google Scholar] [CrossRef]
- Mudshinge, S.R.; Deore, A.B.; Patil, S.; Bhalgat, C.M. Nanoparticles: Emerging carriers for drug delivery. Saudi Pharm. J. 2011, 19, 129–141. [Google Scholar] [CrossRef]
- Wilczewska, A.Z.; Niemirowicz, K.; Markiewicz, K.H.; Car, H. Nanoparticles as drug delivery systems. Pharmacol. Rep. 2012, 64, 1020–1037. [Google Scholar] [CrossRef]
- Algahtani, M.S.; Ahmad, M.Z.; Ahmad, J. Nanoemulgel for improved topical delivery of retinyl palmitate: Formulation design and stability evaluation. Nanomaterials 2020, 10, 848. [Google Scholar] [CrossRef]
- Aithal, G.C.; Nayak, U.Y.; Mehta, C.; Narayan, R.; Gopalkrishna, P.; Pandiyan, S.; Garg, S. Localized in situ nanoemulgel drug delivery system of quercetin for periodontitis: Development and computational simulations. Molecules 2018, 23, 1363. [Google Scholar] [CrossRef]
- Miastkowska, M.; Kulawik-Pióro, A.; Szczurek, M. Nanoemulsion gel formulation optimization for burn wounds: Analysis of rheological and sensory properties. Processes 2020, 8, 1416. [Google Scholar] [CrossRef]
- Nowak, K.; Kulawik-Pióro, A.; Lasoń, E.; Malinowska, M.; Miastkowska, M.; Sikora, E.; Śliwa, K. A Method of Producing a Nanogel Composition with Care, Cosmetic and Therapeutic Properties and a Nanogel Composition with Care, Cosmetic and Healing Properties. Patent 441627, 1 July 2022. [Google Scholar]
- Dash, S.; Murthy, P.N.; Nath, L.; Chowdhury, P. Kinetic modeling on drug release from controlled drug delivery systems. Acta Pol. Pharm. 2010, 67, 217–233. [Google Scholar]
- Cojocaru, V.; Ranetti, A.E.; Hinescu, L.G.; Ionescu, M.; Cosmescu, C.; Poștoarcă, A.G.; Cinteză, L.O. Formulation and evaluation of in vitro release kinetics of Na3CaDTPA decorporation agent embedded in microemulsion-based hydrogel formulation for topical delivery. Farmacia 2015, 63, 656–664. [Google Scholar]
- Miastkowska, M.; Konieczna, M.; Lasoń, E.; Tabaszewska, M.; Sikora, E.; Ogonowski, J. The Release of Perillyl Alcohol from the Different Kind of Vehicles. Curr. Pharm. Biotechnol. 2018, 19, 573–580. [Google Scholar] [CrossRef]
- Yang, M.; Gu, Y.; Yang, D.; Tang, X.; Liu, J. Development of triptolide-nanoemulsion gels for percutaneous administration: Physicochemical, transport, pharmacokinetic and pharmacodynamic characteristics. J. Nanobiotechnol. 2017, 15, 88. [Google Scholar] [CrossRef]
- Nawaz, A.; Latif, M.S.; Alnuwaiser, M.A.; Ullah, S.; Iqbal, M.; Alfatama, M.; Lim, V. Synthesis and Characterization of Chitosan-Decorated Nanoemulsion Gel of 5-Fluorouracil for Topical Delivery. Gels 2022, 8, 412412. [Google Scholar] [CrossRef]
- Mao, Y.; Chen, X.; Xu, B.; Shen, Y.; Ye, Z.; Chaurasiya, B.; Liu, L.; Li, Y.; Xing, X.; Chen, D. Eprinomectin nanoemulgel for transdermal delivery against endoparasites and ectoparasites: Preparation, in vitro and in vivo evaluation. Drug Deliv. 2019, 26, 1104–1114. [Google Scholar] [CrossRef]
- Vartak, R.; Menon, S.; Patki, M.; Billack, B.; Patel, K. Ebselen nanoemulgel for the treatment of topical fungal infection. Eur. J. Pharm. Sci. 2020, 148, 105323. [Google Scholar] [CrossRef]
- Hashemnejad, S.M.; Badruddoza, A.Z.M.; Zarket, B.; Castaneda, C.R.; Doyle, P.S. Thermoresponsive nanoemulsion-based gel synthesized through a low-energy process. Nat. Commun. 2019, 10, 2749. [Google Scholar] [CrossRef]
- Blumlein, A.; McManus, J.J. Bigels formed via spinodal decomposition of unfolded protein. J. Mater. Chem. B 2015, 3, 3429–3435. [Google Scholar] [CrossRef]
- Paul, S.R.; Qureshi, D.; Yogalakshmi, Y.; Nayak, S.K.; Singh, V.K.; Syed, I.; Sarkar, P.; Pal, K. Development of Bigels Based on Stearic Acid–Rice Bran Oil Oleogels and Tamarind Gum Hydrogels for Controlled Delivery Applications. J. Surfactants Deterg. 2018, 21, 17–29. [Google Scholar] [CrossRef]
- Zhang, J.; Ge, D.; Wang, X.; Wang, W.; Cui, D.; Yuan, G.; Wang, K.; Zhang, W. Influence of Surfactant and Weak-Alkali Concentrations on the Stability of O/W Emulsion in an Alkali-Surfactant-Polymer Compound System. ACS Omega 2021, 6, 5001–5008. [Google Scholar] [CrossRef] [PubMed]
- Ren, Y.; Zheng, J.; Xu, Z.; Zhang, Y.; Zheng, J. Application of Turbiscan LAB to study the influence of lignite on the static stability of PCLWS. Fuel 2018, 214, 446–456. [Google Scholar] [CrossRef]
- Celia, C.; Trapasso, E.; Cosco, D.; Paolino, D.; Fresta, M. Turbiscan Lab® Expert analysis of the stability of ethosomes® and ultradeformable liposomes containing a bilayer fluidizing agent. Colloids Surf. B Biointerfaces 2009, 72, 155–160. [Google Scholar] [CrossRef]
- Wiśniewska, M. Influences of polyacrylic acid adsorption and temperature on the alumina suspension stability. Powder Technol. 2010, 198, 258–266. [Google Scholar] [CrossRef]
- Xu, D.; Zhang, J.; Cao, Y.; Wang, J.; Xiao, J. Influence of microcrystalline cellulose on the microrheological property and freeze-thaw stability of soybean protein hydrolysate stabilized curcumin emulsion. LWT-Food Sci. Technol. 2016, 66, 590–597. [Google Scholar] [CrossRef]
- Kowalska, M.; Turek, P.; Żbikowska, A.; Babut, M.; Szakiel, J. The quality of emulsions with new synthetized lipids stabilized by xanthan gum. Biomolecules 2021, 11, 213. [Google Scholar] [CrossRef]
- Zalewska, A.; Kowalik, J.; Grubecki, I. Application of turbiscan lab to study the effect of emulsifier content on the stability of plant origin dispersion. Chem. Process Eng. 2019, 40, 399–409. [Google Scholar] [CrossRef]
- Danila, A.; Ibanescu, S.A.; Zaharia, C.; Muresan, E.I.; Popescu, A.; Danu, M.; Rotaru, V. Eco-friendly O/W emulsions with potential application in skincare products. Colloids Surf. A Physicochem. Eng. Asp. 2021, 612, 125969. [Google Scholar] [CrossRef]
- Kim, K.P.; Jeon, S.; Kim, M.J.; Cho, Y. Borage oil restores acidic skin pH by up-regulating the activity or expression of filaggrin and enzymes involved in epidermal lactate, free fatty acid, and acidic free amino acid metabolism in essential fatty acid-deficient Guinea pigs. Nutr. Res. 2018, 58, 26–35. [Google Scholar] [CrossRef]
- Kulawik-Pióro, A.; Drabczyk, A.K.; Kruk, J.; Wróblewska, M.; Winnicka, K.; Tchórzewska, J. Thiolated silicone oils as new components of protective creams in the prevention of skin diseases. Materials 2021, 14, 4723. [Google Scholar] [CrossRef]
- Mou, D.; Chen, H.; Du, D.; Mao, C.; Wan, J.; Xu, H.; Yang, X. Hydrogel-thickened nanoemulsion system for topical delivery of lipophilic drugs. Int. J. Pharm. 2008, 353, 270–276. [Google Scholar] [CrossRef] [PubMed]
- Kulawik-Pióro, A.; Ptaszek, A.; Kruk, J. Effective tool for assessment of the quality of barrier creams—Relationships between rheological, textural and sensory properties. Regul. Toxicol. Pharmacol. 2019, 103, 113–123. [Google Scholar] [CrossRef] [PubMed]
- Garg, A.; Aggarwal, D.; Garg, S.; Singla, A.K. Spreading of Semisolid Formulations an Update. Pharm. Technol. 2002, 26, 84–105. [Google Scholar]
- Savary, G.; Grisel, M.; Picard, C. Impact of emollients on the spreading properties of cosmetic products: A combined sensory and instrumental characterization. Colloids Surf. B Biointerfaces 2013, 102, 371–378. [Google Scholar] [CrossRef] [PubMed]
- Gore, E.; Picard, C.; Savary, G. Spreading behavior of cosmetic emulsions: Impact of the oil phase. Biotribology 2018, 16, 17–24. [Google Scholar] [CrossRef]
- Kulawik-Pióro, A.; Gibas, K.; Osak, E. Consistency as a quality parameter of hydrophobic skin protection preparations. Polish. J. Cosmetol. 2020, 23, 27–34. [Google Scholar]
- Gore, E.; Picard, C.; Savary, G. Complementary approaches to understand the spreading behavior on skin of O/W emulsions containing different emollients. Colloids Surf. B Biointerfaces 2020, 193, 111132. [Google Scholar] [CrossRef]
- Manca, M.L.; Matricardi, P.; Cencetti, C.; Peris, J.E.; Melis, V.; Carbone, C.; Escribano, E.; Zaru, M.; Fadda, A.M.; Manconi, M. Combination of argan oil and phospholipids for the development of an effective liposome-like formulation able to improve skin hydration and allantoin dermal delivery. Int. J. Pharm. 2016, 505, 204–211. [Google Scholar] [CrossRef]
- Śliwkowska, P. Badanie Właściwości Fizykochemicznych i Aplikacyjnych Formulacji Kosmetycznych Zawierających Jasmonidy wraz z oceną Kinetyki ich Przenikania przez Bariery Imitujące Skórę. Ph.D. Thesis, Adam Mickiewicz University, Poznań, Poland, 2017. [Google Scholar]
- Huang, K.; Liu, R.; Zhang, Y.; Guan, X. Characteristics of two cedarwood essential oil emulsions and their antioxidant and antibacterial activities. Food Chem. 2021, 346, 128970. [Google Scholar] [CrossRef]
- Nizioł-Łukaszewska, Z.; Wasilewski, T.; Gaweł-Bęben, T.; Osika, P.; Czerwonka, D. Cornus mas L. extract as a multifunctional material for manufacturing cosmetic emulsions. Chin. J. Nat. Med. 2018, 16, 284–0292. [Google Scholar] [CrossRef]
- Klimaszewska, E.; Seweryn, A.; Małysa, A.; Zięba, M.; Lipińska, J. The effect of chamomile extract obtained in supercritical carbon dioxide conditions on physicochemical and usable properties of pharmaceutical ointments. Pharm. Dev. Technol. 2018, 23, 780–786. [Google Scholar] [CrossRef]
- McMullen, R.L.; Gorcea, M.; Chen, S. Emulsions and their characterization by texture profile analysis. In Handbook of Formulating Dermal Applications; Dayan, N., Ed.; Scrivener Publishing LLC.: Beverly, MA, USA, 2017; pp. 129–153. [Google Scholar] [CrossRef]
- Tai, A.; Bianchini, R.; Jachowicz, J. Texture analysis of cosmetic/pharmaceutical raw materials and formulations. Int. J. Cosmet. Sci. 2014, 36, 291–304. [Google Scholar] [CrossRef]
- Peleg, M. The instrumental texture profile analysis revisited. J. Texture Stud. 2019, 50, 362–368. [Google Scholar] [CrossRef]
- Huynh, A.; Garcia, A.G.; Young, L.K.; Szoboszlai, M.; Liberatore, M.W.; Baki, G. Measurements meet perceptions: Rheology–texture–sensory relations when using green, bio-derived emollients in cosmetic emulsions. Int. J. Cosmet. Sci. 2021, 43, 11–19. [Google Scholar] [CrossRef]
- Moldovan, M.; Lahmar, A.; Bogdan, C.; Parauan, S.; Tomuţă, I.; Crişan, M. Formulation and evaluation of a water-in-oil cream containing herbal active ingredients and ferulic acid. Clujul Med. 2017, 90, 212–219. [Google Scholar] [CrossRef]
- Yener, G.; Dal, Ö.; Üner, M. Effect of Vehicles on Release of Meloxicam from Various Topical Formulations. Open Drug Deliv. J. 2009, 3, 19–23. [Google Scholar] [CrossRef]
- Trucillo, P. Drug Carriers: A Review on the Most Used Mathematical Models for Drug Release. Processes 2022, 10, 1094. [Google Scholar] [CrossRef]
- Hung, C.F.; Fang, C.L.; Liao, M.H.; Fang, Y.J. The effect of oil components on the physicochemical properties and drug delivery of emulsions: Tocol emulsion versus lipid emulsion. Int. J. Pharm. 2007, 335, 193–202. [Google Scholar] [CrossRef]
- Chung, H.; Kim, W.; Kwon, M.; Kwon, C.; Jeong, S.Y. Oil components modulate physical characteristics and function of the natural oil emulsions as drug or gene delivery system. J. Control Release 2001, 71, 339–350. [Google Scholar] [CrossRef]
- Miastkowska, M.; Śliwa, P. Influence of Terpene Type on the Release from an O/W Nanoemulsion: Experimental and Theoretical Studies. Molecules 2020, 25, 2747. [Google Scholar] [CrossRef]
- Özsoy, Y.; Güngör, S.; Cevher, E. Vehicle effects on in vitro release of tiaprofenic acid from different topical formulations. Il Farmaco 2004, 59, 563–566. [Google Scholar] [CrossRef] [PubMed]
- Macoon, R.; Robey, M.; Chauhan, A. In vitro release of hydrophobic drugs by oleogel rods with biocompatible gelators. Eur. J. Pharm. Sci. 2020, 152, 105413. [Google Scholar] [CrossRef]
- Li, J.; Mooney, D.J. Designing hydrogels for controlled drug delivery. Nat. Rev. Mater. 2016, 1, 16071. [Google Scholar] [CrossRef] [PubMed]
- Andonova, V.; Peneva, P.; Georgiev, G.S.; Toncheva, V.T.; Apostolova, E.; Peychev, Z.; Dimitrova, S.; Katsarova, M.; Petrova, N.; Kassarova, M. Ketoprofen-loaded polymer carriers in bigel formulation: An approach to enhancing drug photostability in topical application forms. Int. J. Nanomed. 2017, 12, 6221–6238. [Google Scholar] [CrossRef]
- Soares, J.S.; Zunino, P. A mixture model for water uptake, degradation, erosion and drug release from polydisperse polymeric networks. Biomaterials 2010, 31, 3032–3042. [Google Scholar] [CrossRef] [PubMed]
- Khan, M.A.; Shafeeq, T. Role of Mathematical Modeling in Controlled Drug Delivery. J. Sci. Res. 2009, 1, 539–550. [Google Scholar] [CrossRef]
- Grassi, M.; Grassi, G. Mathematical Modelling and Controlled Drug Delivery: Matrix Systems. Curr. Drug Deliv. 2005, 2, 97–116. [Google Scholar] [CrossRef]
- Raza, S.N.; Khan, N.A. Role of mathematical modelling in controlled release drug delivery. Int. J. Med. Res. Pharm. Sci. 2017, 4, 84–95. [Google Scholar] [CrossRef]
- Zhang, Y.; Huo, M.; Zhou, J.; Zou, A.; Li, W.; Yao, C.; Xie, S. DDSolver: An add-in program for modeling and comparison of drug dissolution profiles. AAPS J. 2010, 12, 263–271. [Google Scholar] [CrossRef]
- Arifin, D.Y.; Lee, L.Y.; Wang, C.H. Mathematical modeling and simulation of drug release from microspheres: Implications to drug delivery systems. Adv. Drug Deliv. Rev. 2006, 58, 1274–1325. [Google Scholar] [CrossRef] [PubMed]
- Siepmann, J.; Siepmann, F. Mathematical modeling of drug delivery. Int. J. Pharm. 2008, 364, 328–343. [Google Scholar] [CrossRef] [PubMed]
- Siepmann, J.; Siegel, R.A.; Siepmann, F. Diffusion Controlled Drug Delivery Systems. In Fundamentals and Applications of Controlled Release Drug Delivery; Siepmann, J., Siegel, R.A., Rathbone, M.J., Eds.; Springer: New York, NY, USA; Dordrecht, The Netherlands; Heidelberg, Germany; London, UK, 2012; pp. 127–152. [Google Scholar]
- Mircioiu, C.; Voicu, V.; Anuta, V.; Tudose, A.; Celia, C.; Paolino, D.; Fresta, M.; Sandulovici, R.; Mircioiu, I. Mathematical modeling of release kinetics from supramolecular drug delivery systems. Pharmaceutics 2019, 11, 140. [Google Scholar] [CrossRef]
- Christensen, J.M.; Chuong, M.C.; Le, H.; Pham, L.; Bendas, E. Hydrocortisone Diffusion through Synthetic Membrane, Mouse Skin, and EpidermTM Cultured Skin. Arch. Drug Inf. 2011, 4, 10–21. [Google Scholar] [CrossRef]
- Ciurba, A.; Todoran, N.; Tăurean, A.; Antonoaea, P.; Hancu, G.; Moisei, A.; Sipos, E. Kinetic analysis of in vitro drug release from valproic acid and sodium valproate suppositories. Farmacia 2014, 62, 1143–1156. [Google Scholar]
- Azevedo De Mello, V.; Ricci-Júnior, E. Encapsulation of naproxen in nanostructured system: Structural characterization and in vitro release studies. Quim. Nova 2011, 34, 933–939. [Google Scholar] [CrossRef]
- Alvarado, H.L.; Abrego, G.; Souto, E.B.; Garduño-Ramirez, M.L.; Clares, B.; García, M.L.; Calpena, A.C. Nanoemulsions for dermal controlled release of oleanolic and ursolic acids: In vitro, ex vivo and in vivo characterization. Colloids Surf. B Biointerfaces 2015, 130, 40–47. [Google Scholar] [CrossRef]
- Alfei, S.; Schito, A.M.; Zuccari, G. Considerable improvement of ursolic acid water solubility by its encapsulation in dendrimer nanoparticles: Design, synthesis and physicochemical characterization. Nanomaterials 2021, 11, 2196. [Google Scholar] [CrossRef]












| Component Groups | Commercial Name | INCI Name | Suppliers |
|---|---|---|---|
| Emulsifiers | Crodesta SL 40 | Aqua (and) sucrose cocoate and alcohol | Croda Cracow Poland |
| Emulgade PL 68/50 | Cetearyl glucoside (and) cetearyl alcohol | BASF Warsaw Poland | |
| Olivatis 18 | Olive oil polyglyceryl-6 esters, sodium stearoyl lactylate, cetearyl alcohol | Alfa Sagittarius Cracow Poland | |
| Olivem 1000 | Cetearyl olivate, sorbitan olivate. | Hallstar HSH Chemie Sp.z o.o Warsaw Poland | |
| Emulgin SML 20 | Polysorbate 20 | BASF Warsaw Poland | |
| Plantacer 2000 UP | Decyl glucoside | BASF Warsaw Poland | |
| Hydrogelators | Carbopol ETD 2050 | Carbomer | Lubrizol Warsaw Poland |
| Sodium hyaluroniate 1.5–2.0 MDa high molecular weight | Sodium hyaluroniate | Alfa Saggittarius Cracow Poland | |
| Oleogelators | Span 60 | Sorbitan stearate | Croda Cracow Poland |
| Span 80 | Sorbitan oleate | Croda Cracow Poland | |
| Tween 80 | Polysorbate 80 | Croda Cracow Poland | |
| Aerosil 200 | Silica | Evonik Warsaw Poland | |
| Aerosil R 816 | Silica cetyl silylate | Evonik Warsaw Poland | |
| Plant extracts | Aloe vera juice | Aloe vera barbadensis leaf juice | Provital Cracow Poland |
| Horse chestnut seed extract | Aesculus hippocastanum (Horse chestnut) seed extract | ||
| Prunus Persica fruit extract | Glycerin (and) water (and) Prunus Persica (peach) fruit extract (and) sodium benzoate (and) potassium sorbate | ||
| Chia seed extract | Salvia hispanica seed extract | ||
| Liquid lipids | Raspberry seed oil | Rubus idaeus (raspberry) seed oil | Ol’Vita Marcinowice Poland |
| Borage seed oil | Borago officinalis (borage) seed oil | ||
| Tamanu seed oil | Calophyllum inophyllum (tamanu) seed oil | ||
| Sweet almond oil | Prunus amygdalus dulcis (sweet almond) oil | ||
| Safflower oil | Carthamus tinctorius (safflower) oil | ||
| Rice bran oil | Oryza sativa (rice) bran oil | ||
| Coconut oil | Cocos nucifera (coconut) oil | ||
| Cannabis sativa seed oil | Cannabis sativa seed oil | ||
| Carrot seed oil | Daucus carota sativa (carrot) seed oil | ||
| Sunflower oil | Helianthus annus (sunflower) seed oil | ||
| Myritol 318 | Caprylic/capric triglyceride | Croda Cracow Poland | |
| Semisolid lipids | Shea butter | Butyrospermum Parkii butter | Croda Cracow Poland |
| Cocoa butter | Theobroma cacao (cocoa) seed butter | Croda Cracow Poland | |
| Murumuru butter | Astrocaryum murumuru seed butter | Alfa Sagittarius Cracow Poland | |
| Solid lipids | Cutina CBS | Glyceryl stearate (and) cetearyl alcohol (and) cetyl palmitate (and) cocoglycerides | BASF Warsaw Poland |
| Preservatives | Dermosoft 1388 | Aqua (and) glycerin (and) sodium levulinate (and) sodium anisate | Evonik Warsaw Poland |
| Geogard Ultra® | Gluconolactone, sodium benzoate | Arxada Basel, Switzerland | |
| Active ingredient | Ursolic acid | Ursolic acid | Merck Warsaw Poland |
| Phase | Component | Concentration (% wt.) |
|---|---|---|
| A | Aqua | 30.0 |
| Hydrogelator | 1.0 | |
| B | Emollient | 10.0 |
| Emulsifier | 4.0 | |
| Ursolic acid | 0.01–0.5 | |
| C | Preservative | 2.5 |
| Aqua | up to 100 |
| Phase | Component | Serum | Face Cream | Body Balm |
|---|---|---|---|---|
| Concentration (% wt.) | ||||
| A | Aqua | Up to 100 | Up to 100 | Up to 100 |
| Plant extracts | 10.0 | 15.0 | 5.0 | |
| Preservative | 2.5 | 2.5 | 2.5 | |
| B | Liquid lipid (vegetable oils, medium-chain triglycerides) | 20.0 | 15.0 | 10.0 |
| Semisolid lipids (vegetable butters) | 5.0 | 7.0 | 10.0 | |
| Solid lipids (natural waxes, fatty alcohols) | − | 4.0 | − | |
| Emulsifier | 4.0 | 4.0 | 4.0 | |
| Ursolic acid | 0.01–0.5 | 0.01–0.5 | 0.01–0.5 | |
| Phase | Component | Concentration (% wt.) |
|---|---|---|
| A | Oleogelator 1 | 6.0 |
| Oleogelator 2 | 6.0 | |
| Liquid lipids | 88.0 | |
| Ursolic acid | 0.01–0.5 |
| Nanoemulgel (NG) | Formulation (F) | |||
|---|---|---|---|---|
| Serum (S) | Cream (C) | Balm (B) | Oleogel (O) | |
| Ratios of NG:F (w/w) | NG:S | NG:C | NG:B | BG |
| 5:95 | − | − | − | BG-1 |
| 10:90 | NG:S-1 | NG:C-1 | NG:B-1 | BG-2 |
| 20:80 | NG:S-2 | NG:C-2 | NG:B-2 | BG-3 |
| 30:70 | NG:S-3 | NG:C-3 | NG:B-3 | − |
| 40:60 | NG:S-4 | NG:C-4 | NG:B-4 | − |
| 50:50 | NG:S-5 | NG:C-5 | NG:B-5 | − |
| 60:40 | NG:S-6 | NG:C-6 | NG:B-6 | − |
| 70:30 | NG:S-7 | NG:C-7 | NG:B-7 | − |
| 80:20 | NG:S-8 | NG:C-8 | NG:B-8 | − |
| 90:10 | NG:S-9 | NG:C-9 | NG:B-9 | − |
| Sample | pH | Average Particle Size (μm) | Viscosity (Pa∙s) for γ = 50 s−1, T = 25 °C | |||
|---|---|---|---|---|---|---|
| t = 0 | t = 3 Months | t = 0 | t = 3 Months | t = 0 | t = 3 Months | |
| NG | 5.00 ± 0.0 | 5.05 ± 0.0 | 101·10−3 ± 0.002 | 110·10−3 ± 0.003 | 1.20 ± 0.01 | 1.25 ± 0.01 |
| S | 6.00 ± 0.0 | 6.00 ± 0.0 | 1.25 ± 0.04 | 1.15 ± 0.03 | 2.50 ± 0.17 | 2.55 ± 0.15 |
| NG:S-4 | 5.09 ± 0.1 | 5.20 ± 0.0 | 1.25 ± 0.05 | 1.10 ± 0.06 | 2.26 ± 0.09 | 2.30 ± 0.10 |
| NG:S-5 | 5.00 ± 0.1 | 5.10 ± 0.0 | 1.26 ± 0.08 | 1.23 ± 0.03 | 2.15 ± 0.08 | 2.20 ± 0.06 |
| NG:S-6 | 5.40 ± 0.0 | 5.50 ± 0.0 | 1.39 ± 0.03 | 1.21 ± 0.08 | 2.00 ± 0.06 | 2.10 ± 0.04 |
| C | 6.00 ± 0.1 | 6.05 ± 0.0 | 2.61 ± 0.10 | 3.34 ± 0.18 | 2.98 ± 0.09 | 2.72 ± 0.10 |
| NG:C-6 | 5.00 ± 0.0 | 5.25 ± 0.0 | 2.71 ± 0.16 | 3.06 ± 0.12 | 2.45 ± 0.09 | 2.50 ± 0.10 |
| NG:C-7 | 5.00 ± 0.1 | 5.10 ± 0.0 | 2.68 ± 0.08 | 3.12 ± 0.09 | 2.39 ± 0.04 | 2.40 ± 0.03 |
| B | 6.05 ± 0.1 | 6.11 ± 0.1 | 2.23 ± 0.11 | 2.86 ± 0.10 | 2.90 ± 0.08 | 2.80 ± 0.11 |
| NG:B-4 | 4.76 ± 0.1 | 4.88 ± 0.1 | 2.16 ± 0.05 | 2.95 ± 0.03 | 2.60 ± 0.10 | 2.70 ± 0.12 |
| NG:B-5 | 4.94 ± 0.0 | 4.99 ± 0.0 | 1.70 ± 0.09 | 1.03 ± 0.04 | 2.34 ± 0.07 | 2.40 ± 0.15 |
| O | 4.05 ± 0.0 | 4.10 ± 0.0 | − | − | 3.36 ± 0.06 | 3.47 ± 0.08 |
| BG-1 | 4.69 ± 0.1 | 4.73 ± 0.1 | 34.35 ± 1.73 | 35.75 ± 2.15 | 3.28 ± 0.17 | 3.97 ± 0.16 |
| Sample | IC50% (UA Concentration µM) | IC25% (UA Concentration µM) |
|---|---|---|
| Ursolic acid | -(>50 µM) | -(>25 µM) |
| NG | >9.13% (>200 µM) | >9.13% (>200 µM) |
| BG | >0.91% (>20 µM) | >0.05% (>1 µM) |
| B | >18.27% (>400 µM) | >2.28% (>50 µM) |
| S | >1.14% (>25 µM) | >0.23% (>5 µM) |
| C | >18.27% (>400 µM) | >9.13% (>200 µM) |
| NG:S-5 | >9.13% (>200 µM) | >2.28% (>50 µM) |
| NG:B-4 | >18.27% (>400 µM) | >9.13% (>200 µM) |
| NG:C-7 | >18.27% (>400 µM) | >18.27% (>400 µM) |
| Model | Parameter | Formulation | |||
|---|---|---|---|---|---|
| S | NG | NG:S | BG | ||
| Zero order | R2 | 0.9743 | 0.9844 | 0.9569 | 0.9741 |
| K0 (mg/h) | 0.1089 | 0.0793 | 0.0803 | 0.0582 | |
| First order | R2 | 0.9851 | 0.9883 | 0.9674 | 0.9804 |
| K1 (h−1) | 0.04398 | 0.03063 | 0.0311 | 0.02189 | |
| Higuchi | R2 | 0.9867 | 0.9754 | 0.9919 | 0.9878 |
| KH (mg/h1/2) | 0.3481 | 0.2506 | 0.2597 | 0.1859 | |
| Korsmeyer–Peppas | R2 | 0.9884 | 0.9899 | 0.9823 | 0.9912 |
| KHP (h−n) | 8.5349 | 7.2443 | 8.3772 | 6.9374 | |
| n | 0.6119 | 0.5313 | 0.4932 | 0.4485 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Miastkowska, M.; Kulawik-Pióro, A.; Lasoń, E.; Śliwa, K.; Malinowska, M.A.; Sikora, E.; Kantyka, T.; Bielecka, E.; Maksylewicz, A.; Klimaszewska, E.; et al. Topical Formulations Based on Ursolic Acid-Loaded Nanoemulgel with Potential Application in Psoriasis Treatment. Pharmaceutics 2023, 15, 2559. https://doi.org/10.3390/pharmaceutics15112559
Miastkowska M, Kulawik-Pióro A, Lasoń E, Śliwa K, Malinowska MA, Sikora E, Kantyka T, Bielecka E, Maksylewicz A, Klimaszewska E, et al. Topical Formulations Based on Ursolic Acid-Loaded Nanoemulgel with Potential Application in Psoriasis Treatment. Pharmaceutics. 2023; 15(11):2559. https://doi.org/10.3390/pharmaceutics15112559
Chicago/Turabian StyleMiastkowska, Małgorzata, Agnieszka Kulawik-Pióro, Elwira Lasoń, Karolina Śliwa, Magdalena Anna Malinowska, Elżbieta Sikora, Tomasz Kantyka, Ewa Bielecka, Anna Maksylewicz, Emilia Klimaszewska, and et al. 2023. "Topical Formulations Based on Ursolic Acid-Loaded Nanoemulgel with Potential Application in Psoriasis Treatment" Pharmaceutics 15, no. 11: 2559. https://doi.org/10.3390/pharmaceutics15112559
APA StyleMiastkowska, M., Kulawik-Pióro, A., Lasoń, E., Śliwa, K., Malinowska, M. A., Sikora, E., Kantyka, T., Bielecka, E., Maksylewicz, A., Klimaszewska, E., Ogorzałek, M., Tabaszewska, M., Skoczylas, Ł., & Nowak, K. (2023). Topical Formulations Based on Ursolic Acid-Loaded Nanoemulgel with Potential Application in Psoriasis Treatment. Pharmaceutics, 15(11), 2559. https://doi.org/10.3390/pharmaceutics15112559