Permeation Protection by Waterproofing Mucosal Membranes
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
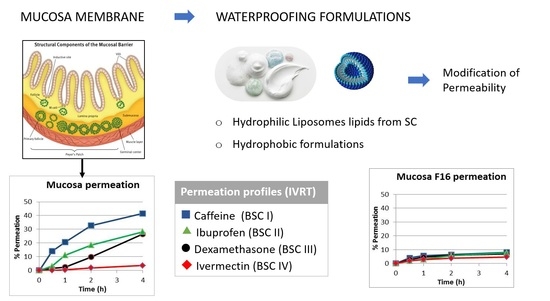
2.2. Waterproofing Formulations
2.3. Water Permeability Test
2.4. In Vitro Release Assay
2.5. HPLC/DAD Analytical Measurements
2.6. Statistical Analysis
3. Results
3.1. Water Permeability Test (TMWL)
3.2. In Vitro Drug Release Test
4. Discussion
4.1. Water Permeability Test (TMWL)
4.2. In Vitro Drug Release Test
4.3. Rank Order Penetration of Drugs
5. Conclusions
6. Patents
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wertz, P.W. Roles of lipids in the permeability barriers of skin and oral mucosa. Int. J. Mol. Sci. 2021, 22, 5229. [Google Scholar] [CrossRef]
- Wertz, P.W.; Squier, C.A. Cellular and molecular basis of barrier function in oral epithelium. Crit. Rev. Ther. Drug Carr. Syst. 1991, 8, 237–269. [Google Scholar]
- Kontogiannidou, E.; Andreadis, D.A.; Zografos, A.L.; Nazar, H.; Klepetsanis, P.; van der Merwe, S.M.; Fatouros, D.G. Ex vivo drug delivery of ropinirole hydrochloride in the presence of permeation enhancers: The effect of charge. Pharm. Dev. Technol. 2017, 22, 1017–1021. [Google Scholar] [CrossRef]
- Lee, J.; Lww, S.K.; Choi, Y.W. The effect of storage conditions on the permeability of porcine buccal mucosa. Arch. Pharmacal Res. 2002, 25, 546–549. [Google Scholar] [CrossRef]
- White, S.H.; Mirejovsky, D.; King, G.I. Structure of Lamellar Lipid Domains and Corneocyte Envelopes of Murine Stratum Corneum. An X-ray Diffraction Study. Biochemistry 1998, 27, 3725–3732. [Google Scholar] [CrossRef]
- Bouwstra, J.; Pilgram, G.; Gooris, G.; Koerten, H.; Ponec, M. New aspects of the skin barrier organization. Skin Pharmacol. Physiol. 2001, 14 (Suppl. S1), 52–62. [Google Scholar] [CrossRef]
- Janůšová, B.; Zbytovská, J.; Lorenc, P.; Vavrysová, H.; Palát, K.; Hrabálek, A.; Vávrová, K. Effect of ceramide acyl chain length on skin permeability and thermotropic phase behavior of model stratum corneum lipid membranes. Biochim. Biophys. Acta 2011, 1811, 129–137. [Google Scholar] [CrossRef]
- Kessner, D.; Ruettinger, A.; Kiselev, M.A.; Wartewig, S.; Neubert, R.H.H. Properties of ceramides and their impact on the stratum corneum structure: A review—Part 2: Stratum corneum lipid model systems. Skin Pharmacol. Physiol. 2008, 21, 58–74. [Google Scholar] [CrossRef]
- Harding, C.R. The stratum corneum: Structure and function in health and disease. Dermatol. Ther. 2004, 17 (Suppl. S1), 6–15. [Google Scholar] [CrossRef]
- Lai-cheong, J.E.; McGrath, J.A. Structure and function of skin, hair and nails. Medicine 2013, 41, 317–320. [Google Scholar] [CrossRef]
- Shao, M.; Hussain, Z.; Thu, H.E.; Khan, S.; Katas, H.; Ahmed, T.A.; Tripathy, M.; Leng, J.; Qin, H.L.; Bukhari, S.N.A. Drug nanocarrier, the future of atopic diseases: Advanced drug delivery systems and smart management of disease. Colloids Surf. B 2016, 147, 475–491. [Google Scholar] [CrossRef]
- Wolf, R.; Wolf, D. Abnormal epidermal barrier in the pathogenesis of atopic dermatitis. Clin. Dermatol. 2012, 30, 329–334. [Google Scholar] [CrossRef]
- Abdelgawad, R.; Nasr, M.; Moftah, N.H.; Hamza, M.Y. Phospholipid membrane tubulation using ceramide doping “Cerosomes”: Characterization and clinical application in psoriasis treatment. Eur. J. Pharm. Sci. 2017, 101, 258–268. [Google Scholar] [CrossRef]
- Khazanov, E.; Priev, A.; Shillemans, J.P.; Barenholz, Y. Physicochemical and biological characterization of ceramide-containing liposomes: Paving the way to ceramide therapeutic application. Langmuir 2008, 24, 6965–6980. [Google Scholar] [CrossRef]
- van Smeden, J.; Janssens, M.; Gooris, G.S.; Bouwstra, J.A. The important role of stratum corneum lipids for the cutaneous barrier function. Biochim. Biophys. Acta (BBA)-Mol. Cell Biol. Lipids 2014, 1841, 295–313. [Google Scholar] [CrossRef]
- Shojael, A.H. Buccal mucosa as a route for systemic drug delivery: A Review. J. Pharm. Pharm. Sci. 1988, 1, 15–30. [Google Scholar]
- Bhati, R.; Nagrajan, R.K. A detailed review on oral mucosal drug delivery system. Int. J. Pharm. Sci. Res. 2012, 3, 6597. [Google Scholar]
- Meckfessel, M.H.; Brandt, S. The structure, function, and importance of ceramides in skin and their use as therapeutic agents in skin-care products. J. Am. Acad. Dermatol. 2014, 71, 177–184. [Google Scholar] [CrossRef]
- Draelos, Z.D. The effect of ceramide-containing skin care products on eczema resolution duration. Cutis 2008, 81, 87–91. [Google Scholar] [PubMed]
- Zeichner, J.A.; Del Rosso, J.Q. Multivesicular emulsion ceramide-containing moisturizers: An evaluation of their role in the management of common skin disorders. J. Clin. Aesthetic Dermatol. 2016, 9, 26–32. [Google Scholar]
- Coderch, L.; López, O.; de la Maza, A.; Parra, J.L. Ceramides and skin function. Am. J. Clin. Dermatol. 2003, 4, 107–129. [Google Scholar] [CrossRef]
- van Smeden, J.; Hoppel, L.; van der Heijden, R.; Hankemeier, T.; Vreeken, R.J.; Bouwstra, J.A. LC/MS analysis of stratum corneum lipids: Ceramide profiling and discovery. J. Lipid Res. 2011, 52, 1211–1221. [Google Scholar] [CrossRef]
- Rabionet, M.; Gorgas, K.; Sandhoff, R. Ceramide synthesis in the epidermis. Biochim. Biophys. Acta-Mol. Cell Biol. Lipids 2014, 1841, 422–434. [Google Scholar] [CrossRef]
- Vovesná, A.; Zhigunov, A.; Balouch, M.; Zbytovská, J. Ceramide liposomes for skin barrier recovery: A novel formulation based on natural skin lipids. Int. J. Pharm. 2021, 596, 120264. [Google Scholar] [CrossRef]
- Ganem-Quintanar, A.; Falson-Rieg, F.; Buri, P. Contribution of lipid components to the permeability barrier of oral mucosa. Eur. J. Pharm. Biopharm. 1997, 44, 107–120. [Google Scholar] [CrossRef]
- Squier, C.A.; Cox, P.; Wertz, P.W. Lipid content and water permeability of skin and oral mucosa. J. Investig. Dermatol. 1991, 96, 123–126. [Google Scholar] [CrossRef]
- Selvaratnam, L.; Cruchley, A.T.; Navsaria, H.; Wertz, P.W.; Hagi-Pavli, E.P.; Leigh, I.M.; Squier, C.A.; Williams, D.M. Permeability barrier properties of oral keratinocyte cultures: A model of intact human oral mucosa. Oral Dis. 2001, 7, 252–258. [Google Scholar] [CrossRef]
- Kinikoglu, B.; Damour, O.; Hasirci, V. Tissue engineering of oral mucosa: A shared concept with skin. J. Artif. Organs 2015, 18, 8–19. [Google Scholar] [CrossRef]
- Winning, T.A.; Townsend, G.C. Oral mucosal embryology and histology. Clin. Dermatol. 2000, 18, 499–511. [Google Scholar] [CrossRef] [PubMed]
- Alonso, C.; Martí, M.; Ramos, A.; Calpena, A.C.; Clares-Naveros, B.; Coderch, L. Synthetic Model of the Mucosa for Oral Penetration Studies. Preprints 2023, 2023100270. [Google Scholar]
- European Medicines Agency. Assessment Report Pursuant to Article 29(4) of Directive 2001/83/EC, as Amended. Available online: https://www.ema.europa.eu/en/documents/referral/dexamethasone-alapis-article-29-referral-assessment-report_en.pdf (accessed on 17 April 2023).
- Alonso, C.; Martí, M.; Coderch, L.; Calpena, A.C.; Mallandrich, M.; Pérez, L.; Clares, B.; Pérez, N. Lipophilic-Based Composition. Patent N. Sol: EP23382737.7 (2023) N. Ref: ES1641.1822. CSIC, UB, UGR, 19 June 2023. [Google Scholar]
- Alonso, C.; Martí, M.; Coderch, L.; Calpena, A.C.; Pérez, L.; Clares, B. Liposomal-Based Composition. Patent N. de Sol: EP23382651.0 (2023) N. Ref: ES1641.1823. CSIC, UB, UGR, 26 June 2023. [Google Scholar]
- Thakker, K.D.; Chern, W.H. Development and validation of in vitro release tests for semisolid dosage forms—Case study. Dissolution Technol. 2003, 10, 10–15. [Google Scholar] [CrossRef]
- Mallandrich, M.; Fernández-Campos, F.; Clares, B.; Halbaut, L.; Alonso, C.; Coderch, L.; Garduño-Ramírez, M.L.; Andrade, B.; Del Pozo, A.; Lane, M.E.; et al. Developing Transdermal Applications of Ketorolac Tromethamine Entrapped in Stimuli Sensitive Block Copolymer Hydrogels. Pharm. Res. 2017, 34, 1728–1740. [Google Scholar] [CrossRef] [PubMed]
- ICH Guideline. Validation of Analytical Procedures: Text and Methodology. In Proceedings of the International Conference on Harmonization (ICH), Q2(R1). 2005, pp. 1–17. Available online: https://database.ich.org/sites/default/files/Q2%28R1%29%20Guideline.pdf (accessed on 27 July 2022).
- Rubio, L.; Alonso, C.; Lopez, O.; Rodríguez, G.; Coderch, L.; Notario, J.; de la Maza, A.; Parra, J.L. Barrier function of intact and impaired skin: Percutaneous penetration of caffeine and salicylic acid. Int. J. Dermatol. 2011, 50, 881–889. [Google Scholar] [CrossRef] [PubMed]
- Ku, M.S. Use of the Biopharmaceutical Classification System in early drug development. AAPS J. 2008, 10, 208–212. [Google Scholar] [CrossRef] [PubMed]
- Antonov, D.; Schliemann, S.; Elsner, P. Methods for the Assessment of Barrier Function. In Skin Barrier Function; Agner, T., Ed.; Current Problems in Dermatology; Karger: Basel, Switzerland, 2016; Volume 49, pp. 61–70. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions, and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions, or products referred to in the content. |


| Active Substances (API) | pKa | LogP (pH7.4) | MW | BSC Group |
|---|---|---|---|---|
| Caffeine (CAF) | 10.4 | −0.07 | 194.2 | 1 |
| Ibuprofen (IBU) | 5.30 | 3.97 | 206.3 | 2 |
| Dexamethasone (DEX) | 12.1 | 1.74 | 392.5 | 1/3 [31] |
| Ivermectin (IVE) | 12.47 | 5.83 | 875.1 | 4 |
| Formulation Number | Formulation Name | Composition |
|---|---|---|
| 1 | Tea tree oil mouthwash | Glycerin 15%, sorbitol 4.5%, Lauryl sulfate sodium 3%, ethanol 10% (Merck, Darmstadt, Germany) and Tea tree oil (Acofarma, Terrassa, Spain) |
| 2 | Semi-solid anhydrous absorption base | Lecithin 50% in liquid Vaseline |
| 3 | Lipophilic base MI | Isopropyl myristate 10% in Filant Vaseline |
| 4 | Lipophilic base TGM | Propyl glycol 10%, medium chain triglycerides 10% in Filant Vaseline |
| 5 | Fluid anhydrous absorption base | Soy lecithin 50% in Isopropyl palmitate |
| Formulation Number | Formulation Name | Composition |
|---|---|---|
| 6 | SCMC gel 4% | Sodium carboxymethylcellulose 4%, Glycerin 10% in water |
| 7 | SHYL gel 2% | Sodium hyaluronate 2% in water |
| 8 | CHIT gel 2% | Chitosan 2%, lactic acid 1% in water |
| 9 | ALG gel 4% | Alginate sodium 4%, calcium chloride 4% in water |
| 10 | PLX-CBP gel | Poloxamer 26%, Carbopol 940 1% in water |
| Formulation Number | Formulation Name | Composition |
|---|---|---|
| 11 | PC 10% | Soy Phosphatidylcholine 10% (Lipoid Ludwigshafen, Ludwigshafen am Rhein, Germany) |
| 12 | HPC 10% | Soy Hydrogenated Phosphatidylcholine 10% (Lipoid Ludwigshafen, Germany) |
| 13 | Cer3 1% | Ceramide3 46.9% (Evonik, Essen, Germany), Cholesterol 30.8%, Palmitic acid 22.4%. Total lipid concentration 1% |
| 14 | Cer3 10% | Ceramide3 46.8% (Evonik, Essen, Germany), Cholesterol 31.6%, Palmitic acid 23.0%. Total lipid concentration 10% |
| 15 | Cer3Cer6 1% | Ceramide3 24.6% (Evonik, Essen, Germany), Ceramide6 26.5% (Evonik, Essen, Germany), Cholesterol 35.5%, Palmitic acid 22.8%. Total lipid concentration 1% |
| 16 | Cer3Cer6 10% | Ceramide3 23.7% (Evonik, Essen, Germany), Ceramide6 24.0% (Evonik, Essen, Germany), Cholesterol 31.6%, Palmitic acid 23.0%. Total lipid concentration 10% |
| Caffeine (CAF) | Ibuprofen (IBU) | Dexamethasone (DEX) | Ivermectin (IVE) | |
|---|---|---|---|---|
| Linear Reg. Eq. (R2) | ) | A = | ||
| LoD/LoQ (µg/mL) | 0.82/2.49 | 0.50/1.52 | 0.23/0.70 | 0.55/1.66 |
| Formulations | Nuclepore TMWL 1 h (g/h·m2) | Sublingual Mucosa TMWL 1 h (g/h·m2) |
|---|---|---|
| Nuclepore control | 80.8 | -- |
| Sublingual mucosa control | -- | 72.4 |
| Hydrophobic formulations | ||
| F1 Tea tree mouthwash | 71.3 | 58.5 |
| F2 Semi-solid anhydrous absorption base | 15.98 | 23.6 |
| F3 Lipophilic base MI | 2.6 | 6.5 |
| F4 Lipophilic base TGCM | 3.6 | 3.0 |
| F5 Fluid anhydrous absorption base | 22.5 | 34.2 |
| Hydrophilic formulations | ||
| F6 SCMC gel 4% | 64.6 | 53.8 |
| F7 SHYL gel 2% | 75.4 | 59.3 |
| F8 CHIT gel 2% | 75.2 | 63.0 |
| F9 ALG gel 4% | 74.5 | 62.9 |
| F10 PLX-CBP gel | 74.8 | 57.0 |
| Liposomal formulations | ||
| F11 PC 10% | 57.8 | 66.1 |
| F12 HPC 10% | 68.0 | 63.7 |
| F13 Cer3 1% | 59.3 | 65.6 |
| F14 Cer3 10% | 57.2 | 60.8 |
| F15 Cer3Cer6 1% | 49.2 | 47.2 |
| F16 Cer3Cer6 10% | 50.4 | 45.1 |
| Sample | Amount Applied | TMWL (g/m2/h) |
|---|---|---|
| Skin | --- | 13.68 ± 1.22 |
| Sublingual mucosa | --- | 84.72 ± 4.44 |
| Sublingual mucosa + F3 (Lipophilic base MI) | 51.87 mg | 4.17 ± 1.73 |
| Sublingual mucosa + F6 (SCMC gel 4%) | 70 µL | 57.63 ± 4.72 |
| Sublingual mucosa + F16 (Cer3Cer6 10%) | 70 µL | 45.05 ± 2.35 |
| API | Parameter | SKIN | MUCOSA | MUCOSA F3 | MUCOSA F6 | MUCOSA F16 |
|---|---|---|---|---|---|---|
| CAF | AUC ((mg/cm2)·h) | 0.41 ± 0.27 | 2.19 ± 0.93 | 0.49 ± 0.13 | 2.51 ± 0.58 | 0.45 ± 0.09 |
| Flux. J (µg/cm2/h) | 75.09 ± 51.32 | 428.40 ± 81.41 | 102.73 ± 30.73 | 611.08 ± 85.12 | 78.93 ± 11.05 | |
| Permeability Coef. Kp (10−3 cm/h) | 6.06 ± 4.12 | 34.55 ± 0.01 | 8.28 ± 2.48 | 49.28 ± 0.01 | 6.37 ± 0.89 | |
| Lag Time. TL (h) | 0.65 ± 0.24 | 0.04 ± 0.34 | 0.10 ± 0.08 | 0.28 ± 0.08 | −0.18 ± 0.03 | |
| Maximal Conc. Cmax (µg/mL) | 107.23 ± 57.73 | 514.46 ± 119.97 | 118.25 ± 34.84 | 719.67 ± 92.99 | 99.33 ± 8.49 | |
| IBU | AUC ((mg/cm2)·h) | 0.10 ± 0.01 | 1.27 ± 0.49 | 1.32 ± 0.16 | 1.68 ± 0.47 | 0.37 ± 0.0002 |
| Flux. J (µg/cm2/h) | 24.79 ± 2.75 | 346.91± 5.87 | 498.25 ± 49.45 | 451.01 ± 98.04 | 74.41 ± 0.16 | |
| Permeability Coef. Kp (10−3 cm/h) | 2.05 ± 0.23 | 28.67 ± 0.49 | 41.18 ± 4.09 | 37.27 ± 8.10 | 6.15 ± 0.001 | |
| Lag Time. TL (h) | −0.24 ± 0.19 | 0.17 ± 0.07 | 0.23 ± 0.01 | 0.35 ± 0.06 | 0.15 ± 0.003 | |
| Maximal Conc. Cmax (µg/mL) | 21.93 ± 1.98 | 340.76 ± 63.62 | 406.04 ± 36.48 | 545.68 ± 114.46 | 96.52 ± 0.08 | |
| DEX | AUC ((mg/cm2)·h) | 0.03 ± 0.01 | 0.83 ± 0.19 | 1.10 ± 0.18 | 1.38 ± 0.55 | 0.38 ± 0.06 |
| Flux. J (µg/cm2/h) | 5.84 ± 3.46 | 335.25 ± 61.25 | 330.43 ± 11.68 | 289.38 ± 99.51 | 68.26 ± 1.67 | |
| Permeability Coef. Kp (10−3 cm/h) | 0.49 ± 0.29 | 28.17 ± 5.15 | 27.77 ± 0.98 | 24.32 ± 8.36 | 5.74 ± 0.14 | |
| Lag Time. TL (h) | 0.62 ± 0.16 | 0.31 ± 0.13 | 0.36 ± 0.04 | 0.64 ± 0.36 | −0.11 ± 0.27 | |
| Maximal Conc. Cmax (µg/mL) | 67.41 ± 95.05 | 315.34 ± 87.38 | 363.51 ± 41.15 | 493.36 ± 131.99 | 82.14 ± 9.84 | |
| IVE | AUC ((mg/cm2)·h) | 0.026 ± 0.002 | 0.13 ± 0.02 | 0.17 ± 0.01 | 0.31 ± 0.24 | 0.27 ± 0.24 |
| Flux. J (µg/cm2/h) | 3.13 ± 0.86 | 63.75 ± 1.57 | 87.23 ± 21.83 | 144.86 ± 29.93 | 41.70 ± 5.67 | |
| Permeability Coef. Kp (10−3 cm/h) | 0.25 ± 0.07 | 5.06± 0.13 | 6.92 ± 1.73 | 11.50 ± 2.38 | 3.17 ± 1.62 | |
| Lag Time. TL (h) | 0.59 ± 0.28 | 0.24 ± 0.001 | 0.28 ± 0.02 | 0.32 ± 0.10 | −0.09 ± 0.46 | |
| Maximal Conc. Cmax (µg/mL) | 7.38 ± 0.13 | 44.45 ± 1.68 | 69.66 ± 18.61 | 134.92 ± 79.36 | 59.13 ± 40.84 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Coderch, L.; Alonso, C.; Calpena, A.C.; Pérez-García, M.L.; Clares-Naveros, B.; Ramos, A.; Martí, M. Permeation Protection by Waterproofing Mucosal Membranes. Pharmaceutics 2023, 15, 2698. https://doi.org/10.3390/pharmaceutics15122698
Coderch L, Alonso C, Calpena AC, Pérez-García ML, Clares-Naveros B, Ramos A, Martí M. Permeation Protection by Waterproofing Mucosal Membranes. Pharmaceutics. 2023; 15(12):2698. https://doi.org/10.3390/pharmaceutics15122698
Chicago/Turabian StyleCoderch, Luisa, Cristina Alonso, Ana Cristina Calpena, Maria Luisa Pérez-García, Beatriz Clares-Naveros, Anderson Ramos, and Meritxell Martí. 2023. "Permeation Protection by Waterproofing Mucosal Membranes" Pharmaceutics 15, no. 12: 2698. https://doi.org/10.3390/pharmaceutics15122698
APA StyleCoderch, L., Alonso, C., Calpena, A. C., Pérez-García, M. L., Clares-Naveros, B., Ramos, A., & Martí, M. (2023). Permeation Protection by Waterproofing Mucosal Membranes. Pharmaceutics, 15(12), 2698. https://doi.org/10.3390/pharmaceutics15122698