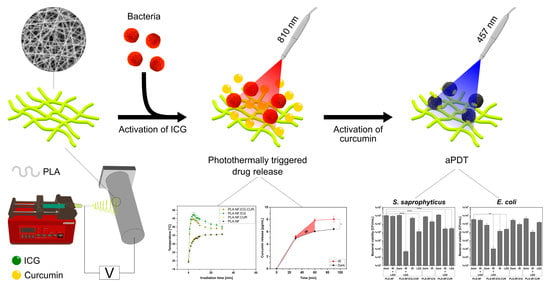
Photothermally Controlled Drug Release of Poly(d,l-lactide) Nanofibers Loaded with Indocyanine Green and Curcumin for Efficient Antimicrobial Photodynamic Therapy
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Bacterial Strains and Media
2.3. Cell Lines and Media
2.4. Light Source
2.5. Preparation of Nanofibers
2.6. Scanning Electron Microscopy (SEM) of the Fibers
2.7. Drug Loading
2.8. Photothermal Activity
2.9. In Vitro Drug Release Studies
2.10. Antimicrobial Photodynamic Activity
2.10.1. Planktonic Bacteria
2.10.2. Adhered Bacteria
2.10.3. Qualitative Assessment of Surface Eradication of the Bacteria via SEM
2.11. Biocompatibility Studies
2.12. Statistical Analysis
3. Results and Discussion
3.1. Morphology of the Nanofibers
3.2. Photothermal Activity
3.3. Drug Loading and In Vitro Drug Release
3.4. Antimicrobial Photodynamic Activity
3.4.1. Planktonic Bacteria
3.4.2. Adhered Bacteria
3.5. Biocompatibility Studies
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Renwick, M.J.; Brogan, D.M.; Mossialos, E. A systematic review and critical assessment of incentive strategies for discovery and development of novel antibiotics. J. Antibiot. 2016, 69, 73–88. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bowler, P.G.; Duerden, B.I.; Armstrong, D.G. Wound microbiology and associated approaches to wound management. Clin. Microbiol. Rev. 2001, 14, 244–269. [Google Scholar] [CrossRef] [Green Version]
- Broughton, G.; Janis, J.E.; Attinger, C.E. The basic science of wound healing. Plast. Reconstr. Surg. 2006, 117, 12S–34S. [Google Scholar] [CrossRef]
- Frykberg, R.G.; Banks, J. Challenges in the Treatment of Chronic Wounds. Adv. Wound Care (New Rochelle) 2015, 4, 560–582. [Google Scholar] [CrossRef] [Green Version]
- Edwards, R.; Harding, K.G. Bacteria and wound healing. Curr. Opin. Infect. Dis. 2004, 17, 91. [Google Scholar] [CrossRef]
- Percival, S.L.; Thomas, J.G.; Williams, D.W. Biofilms and bacterial imbalances in chronic wounds: Anti-Koch. Int. Wound J. 2010, 7, 169–175. [Google Scholar] [CrossRef]
- Hu, Y.; Ruan, X.; Lv, X.; Xu, Y.; Wang, W.; Cai, Y.; Ding, M.; Dong, H.; Shao, J.; Yang, D.; et al. Biofilm microenvironment-responsive nanoparticles for the treatment of bacterial infection. Nano Today 2022, 46, 101602. [Google Scholar] [CrossRef]
- Yuwen, L.; Xiao, H.; Lu, P.; Chen, X.; Li, J.; Xiu, W.; Gan, S.; Yang, D.; Wang, L. Amylase degradation enhanced NIR photothermal therapy and fluorescence imaging of bacterial biofilm infections. Biomater. Sci. 2022. [Google Scholar] [CrossRef]
- Wainwright, M.; Maisch, T.; Nonell, S.; Plaetzer, K.; Almeida, A.; Tegos, G.P.; Hamblin, M.R. Photoantimicrobials—Are we afraid of the light? Lancet Infect. Dis. 2017, 17, e49–e55. [Google Scholar] [CrossRef]
- Boluki, E.; Kazemian, H.; Peeridogaheh, H.; Alikhani, M.Y.; Shahabi, S.; Beytollahi, L.; Ghorbanzadeh, R. Antimicrobial activity of photodynamic therapy in combination with colistin against a pan-drug resistant Acinetobacter baumannii isolated from burn patient. Photodiagnosis Photodyn. Ther. 2017, 18, 1–5. [Google Scholar] [CrossRef]
- Fekrazad, R.; Zare, H.; Mohammadi Sepahvand, S.; Morsali, P. The effect of antimicrobial photodynamic therapy with radachlorin® on Staphylococcus aureus and Escherichia coli: An in vitro study. J. Lasers Med. Sci. 2014, 5, 82–85. [Google Scholar] [PubMed]
- García, I.; Ballesta, S.; Gilaberte, Y.; Rezusta, A.; Pascual, Á. Antimicrobial photodynamic activity of hypericin against methicillin-susceptible and resistant Staphylococcus aureus biofilms. Future Microbiol. 2015, 10, 347–356. [Google Scholar] [CrossRef]
- Grinholc, M.; Szramka, B.; Kurlenda, J.; Graczyk, A.; Bielawski, K.P. Bactericidal effect of photodynamic inactivation against methicillin-resistant and methicillin-susceptible Staphylococcus aureus is strain-dependent. J. Photochem. Photobiol. B 2008, 90, 57–63. [Google Scholar] [CrossRef] [PubMed]
- Dayyih, A.A.; Gutberlet, B.; Preis, E.; Engelhardt, K.H.; Amin, M.U.; Abdelsalam, A.M.; Bonsu, M.; Bakowsky, U. Thermoresponsive Liposomes for Photo-Triggered Release of Hypericin Cyclodextrin Inclusion Complex for Efficient Antimicrobial Photodynamic Therapy. ACS Appl. Mater. Interfaces 2022, 14, 31525–31540. [Google Scholar] [CrossRef]
- Ayoub, A.M.; Gutberlet, B.; Preis, E.; Abdelsalam, A.M.; Abu Dayyih, A.; Abdelkader, A.; Balash, A.; Schäfer, J.; Bakowsky, U. Parietin Cyclodextrin-Inclusion Complex as an Effective Formulation for Bacterial Photoinactivation. Pharmaceutics 2022, 14, 357. [Google Scholar] [CrossRef]
- Almeida, A.; Faustino, M.A.F.; Neves, M.G. Antimicrobial Photodynamic Therapy in the Control of COVID-19. Antibiotics 2020, 9, 320. [Google Scholar] [CrossRef] [PubMed]
- Kipshidze, N.; Yeo, N.; Kipshidze, N. Photodynamic therapy for COVID-19. Nat. Photonics 2020, 14, 651–652. [Google Scholar] [CrossRef]
- Svyatchenko, V.A.; Nikonov, S.D.; Mayorov, A.P.; Gelfond, M.L.; Loktev, V.B. Antiviral photodynamic therapy: Inactivation and inhibition of SARS-CoV-2 in vitro using methylene blue and Radachlorin. Photodiagnosis Photodyn. Ther. 2021, 33, 102112. [Google Scholar] [CrossRef]
- Polat, E.; Kang, K. Natural Photosensitizers in Antimicrobial Photodynamic Therapy. Biomedicines 2021, 9, 584. [Google Scholar] [CrossRef]
- Cieplik, F.; Deng, D.; Crielaard, W.; Buchalla, W.; Hellwig, E.; Al-Ahmad, A.; Maisch, T. Antimicrobial photodynamic therapy-what we know and what we don’t. Crit. Rev. Microbiol. 2018, 44, 571–589. [Google Scholar] [CrossRef]
- Costa, S.M.; Fangueiro, R.; Ferreira, D.P. Drug Delivery Systems for Photodynamic Therapy: The Potentiality and Versatility of Electrospun Nanofibers. Macromol. Biosci. 2022, 22, e2100512. [Google Scholar] [CrossRef] [PubMed]
- Arenbergerova, M.; Arenberger, P.; Bednar, M.; Kubat, P.; Mosinger, J. Light-activated nanofibre textiles exert antibacterial effects in the setting of chronic wound healing. Exp. Dermatol. 2012, 21, 619–624. [Google Scholar] [CrossRef]
- Guo, F.-C.; Chen, C.-Y. Zwitterionic Core-Sheath Nanofibers in Antibacterial Photodynamic Therapy. ACS Appl. Polym. Mater. 2022, 4, 4576–4587. [Google Scholar] [CrossRef]
- Contreras, A.; Raxworthy, M.J.; Wood, S.; Schiffman, J.D.; Tronci, G. Photodynamically Active Electrospun Fibers for Antibiotic-Free Infection Control. ACS Appl. Bio Mater. 2019, 2, 4258–4270. [Google Scholar] [CrossRef] [PubMed]
- El-Khordagui, L.; El-Sayed, N.; Galal, S.; El-Gowelli, H.; Omar, H.; Mohamed, M. Photosensitizer-eluting nanofibers for enhanced photodynamic therapy of wounds: A preclinical study in immunocompromized rats. Int. J. Pharm. 2017, 520, 139–148. [Google Scholar] [CrossRef]
- Tan, X.; Wang, J.; Pang, X.; Liu, L.; Sun, Q.; You, Q.; Tan, F.; Li, N. Indocyanine Green-Loaded Silver Nanoparticle@Polyaniline Core/Shell Theranostic Nanocomposites for Photoacoustic/Near-Infrared Fluorescence Imaging-Guided and Single-Light-Triggered Photothermal and Photodynamic Therapy. ACS Appl. Mater. Interfaces 2016, 8, 34991–35003. [Google Scholar] [CrossRef]
- Preis, E.; Anders, T.; Širc, J.; Hobzova, R.; Cocarta, A.-I.; Bakowsky, U.; Jedelská, J. Biocompatible indocyanine green loaded PLA nanofibers for in situ antimicrobial photodynamic therapy. Mater. Sci. Eng. C Mater. Biol. Appl. 2020, 115, 111068. [Google Scholar] [CrossRef]
- Qiu, H.; Zhu, S.; Pang, L.; Ma, J.; Liu, Y.; Du, L.; Wu, Y.; Jin, Y. ICG-loaded photodynamic chitosan/polyvinyl alcohol composite nanofibers: Anti-resistant bacterial effect and improved healing of infected wounds. Int. J. Pharm. 2020, 588, 119797. [Google Scholar] [CrossRef]
- Ege, Z.R.; Akan, A.; Oktar, F.N.; Lin, C.-C.; Karademir, B.; Gunduz, O. Encapsulation of indocyanine green in poly(lactic acid) nanofibers for using as a nanoprobe in biomedical diagnostics. Mater. Lett. 2018, 228, 148–151. [Google Scholar] [CrossRef]
- Ege, Z.R.; Akan, A.; Oktar, F.N.; Lin, C.C.; Kuruca, D.S.; Karademir, B.; Sahin, Y.M.; Erdemir, G.; Gunduz, O. Indocyanine green based fluorescent polymeric nanoprobes for in vitro imaging. J. Biomed. Mater. Res. Part B Appl. Biomater. 2020, 108, 538–554. [Google Scholar] [CrossRef]
- Epstein, J.; Sanderson, I.R.; Macdonald, T.T. Curcumin as a therapeutic agent: The evidence from in vitro, animal and human studies. Br. J. Nutr. 2010, 103, 1545–1557. [Google Scholar] [CrossRef] [Green Version]
- Wilken, R.; Veena, M.S.; Wang, M.B.; Srivatsan, E.S. Curcumin: A review of anti-cancer properties and therapeutic activity in head and neck squamous cell carcinoma. Mol. Cancer 2011, 10, 12. [Google Scholar] [CrossRef] [Green Version]
- Naksuriya, O.; Okonogi, S.; Schiffelers, R.M.; Hennink, W.E. Curcumin nanoformulations: A review of pharmaceutical properties and preclinical studies and clinical data related to cancer treatment. Biomaterials 2014, 35, 3365–3383. [Google Scholar] [CrossRef] [PubMed]
- Moghadamtousi, S.Z.; Kadir, H.A.; Hassandarvish, P.; Tajik, H.; Abubakar, S.; Zandi, K. A review on antibacterial, antiviral, and antifungal activity of curcumin. BioMed Res. Int. 2014, 2014, 186864. [Google Scholar] [CrossRef] [Green Version]
- Lehmann, J.; Agel, M.R.; Engelhardt, K.H.; Pinnapireddy, S.R.; Agel, S.; Duse, L.; Preis, E.; Wojcik, M.; Bakowsky, U. Improvement of Pulmonary Photodynamic Therapy: Nebulisation of Curcumin-Loaded Tetraether Liposomes. Pharmaceutics 2021, 13, 1243. [Google Scholar] [CrossRef]
- Agel, M.R.; Baghdan, E.; Pinnapireddy, S.R.; Lehmann, J.; Schäfer, J.; Bakowsky, U. Curcumin loaded nanoparticles as efficient photoactive formulations against gram-positive and gram-negative bacteria. Colloids Surf. B Biointerfaces 2019, 178, 460–468. [Google Scholar] [CrossRef] [PubMed]
- Duse, L.; Agel, M.R.; Pinnapireddy, S.R.; Schäfer, J.; Selo, M.A.; Ehrhardt, C.; Bakowsky, U. Photodynamic Therapy of Ovarian Carcinoma Cells with Curcumin-Loaded Biodegradable Polymeric Nanoparticles. Pharmaceutics 2019, 11, 282. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Preis, E.; Baghdan, E.; Agel, M.R.; Anders, T.; Pourasghar, M.; Schneider, M.; Bakowsky, U. Spray dried curcumin loaded nanoparticles for antimicrobial photodynamic therapy. Eur. J. Pharm. Biopharm. 2019, 142, 531–539. [Google Scholar] [CrossRef]
- Fereydouni, N.; Darroudi, M.; Movaffagh, J.; Shahroodi, A.; Butler, A.E.; Ganjali, S.; Sahebkar, A. Curcumin nanofibers for the purpose of wound healing. J. Cell. Physiol. 2019, 234, 5537–5554. [Google Scholar] [CrossRef]
- Fallah, M.; Bahrami, S.H.; Ranjbar-Mohammadi, M. Fabrication and characterization of PCL/gelatin/curcumin nanofibers and their antibacterial properties. J. Ind. Text. 2016, 46, 562–577. [Google Scholar] [CrossRef]
- Nguyen, T.T.T.; Ghosh, C.; Hwang, S.-G.; Tran, L.D.; Park, J.S. Characteristics of curcumin-loaded poly (lactic acid) nanofibers for wound healing. J. Mater. Sci. 2013, 48, 7125–7133. [Google Scholar] [CrossRef]
- Agarwal, S.; Wendorff, J.H.; Greiner, A. Use of electrospinning technique for biomedical applications. Polymer 2008, 49, 5603–5621. [Google Scholar] [CrossRef] [Green Version]
- Fu, Y.; Li, X.; Ren, Z.; Mao, C.; Han, G. Multifunctional Electrospun Nanofibers for Enhancing Localized Cancer Treatment. Small 2018, 14, e1801183. [Google Scholar] [CrossRef]
- Dubský, M.; Kubinová, S.; Sirc, J.; Voska, L.; Zajíček, R.; Zajícová, A.; Lesný, P.; Jirkovská, A.; Michálek, J.; Munzarová, M.; et al. Nanofibers prepared by needleless electrospinning technology as scaffolds for wound healing. J. Mater. Sci. Mater. Med. 2012, 23, 931–941. [Google Scholar] [CrossRef]
- Xiong, X.; Xu, Z.; Huang, H.; Wang, Y.; Zhao, J.; Guo, X.; Zhou, S. A NIR light triggered disintegratable nanoplatform for enhanced penetration and chemotherapy in deep tumor tissues. Biomaterials 2020, 245, 119840. [Google Scholar] [CrossRef]
- Chen, Y.; Gao, Y.; Chen, Y.; Liu, L.; Mo, A.; Peng, Q. Nanomaterials-based photothermal therapy and its potentials in antibacterial treatment. J. Control. Release 2020, 328, 251–262. [Google Scholar] [CrossRef]
- Qian, G.; Wen, T.; Shuai, Y.; Wu, X.; Zeng, Z.; Peng, S.; Shuai, C. Photothermal and Photodynamic Effects of g-C 3 N 4 Nanosheet/Bi 2 S 3 Nanorod Composites with Antibacterial Activity for Tracheal Injury Repair. ACS Appl. Nano Mater. 2022, 5, 16528–16543. [Google Scholar] [CrossRef]
- Carvalho, D.D.M.; Takeuchi, K.P.; Geraldine, R.M.; Moura, C.J.D.; Torres, M.C.L. Production, solubility and antioxidant activity of curcumin nanosuspension. Food Sci. Technol. 2015, 35, 115–119. [Google Scholar] [CrossRef] [Green Version]
- Leach, M.K.; Feng, Z.-Q.; Tuck, S.J.; Corey, J.M. Electrospinning fundamentals: Optimizing solution and apparatus parameters. J. Vis. Exp. 2011, 47, e2494. [Google Scholar] [CrossRef]
- Beachley, V.; Wen, X. Effect of electrospinning parameters on the nanofiber diameter and length. Mater. Sci. Eng. C Mater. Biol. Appl. 2009, 29, 663–668. [Google Scholar] [CrossRef]
- Reinhart, M.B.; Huntington, C.R.; Blair, L.J.; Heniford, B.T.; Augenstein, V.A. Indocyanine Green: Historical Context, Current Applications, and Future Considerations. Surg. Innov. 2016, 23, 166–175. [Google Scholar] [CrossRef]
- Li, M.; Liu, X.; Tan, L.; Cui, Z.; Yang, X.; Li, Z.; Zheng, Y.; Yeung, K.W.K.; Chu, P.K.; Wu, S. Noninvasive rapid bacteria-killing and acceleration of wound healing through photothermal/photodynamic/copper ion synergistic action of a hybrid hydrogel. Biomater. Sci. 2018, 6, 2110–2121. [Google Scholar] [CrossRef]
- Liu, M.; He, D.; Yang, T.; Liu, W.; Mao, L.; Zhu, Y.; Wu, J.; Luo, G.; Deng, J. An efficient antimicrobial depot for infectious site-targeted chemo-photothermal therapy. J. Nanobiotechnol. 2018, 16, 23. [Google Scholar] [CrossRef] [Green Version]
- Korupalli, C.; Huang, C.-C.; Lin, W.-C.; Pan, W.-Y.; Lin, P.-Y.; Wan, W.-L.; Li, M.-J.; Chang, Y.; Sung, H.-W. Acidity-triggered charge-convertible nanoparticles that can cause bacterium-specific aggregation in situ to enhance photothermal ablation of focal infection. Biomaterials 2017, 116, 1–9. [Google Scholar] [CrossRef]
- Mazinani, S.; Ajji, A.; Dubois, C. Morphology, structure and properties of conductive PS/CNT nanocomposite electrospun mat. Polymer 2009, 50, 3329–3342. [Google Scholar] [CrossRef]
- Ranjbar-Mohammadi, M.; Bahrami, S.H. Electrospun curcumin loaded poly(ε-caprolactone)/gum tragacanth nanofibers for biomedical application. Int. J. Biol. Macromol. 2016, 84, 448–456. [Google Scholar] [CrossRef]
- Akrami-Hasan-Kohal, M.; Tayebi, L.; Ghorbani, M. Curcumin-loaded naturally-based nanofibers as active wound dressing mats: Morphology, drug release, cell proliferation, and cell adhesion studies. New J. Chem. 2020, 44, 10343–10351. [Google Scholar] [CrossRef]
- Liu, Y.; Zhi, X.; Yang, M.; Zhang, J.; Lin, L.; Zhao, X.; Hou, W.; Zhang, C.; Zhang, Q.; Pan, F.; et al. Tumor-triggered drug release from calcium carbonate-encapsulated gold nanostars for near-infrared photodynamic/photothermal combination antitumor therapy. Theranostics 2017, 7, 1650–1662. [Google Scholar] [CrossRef]
- Xi, Y.; Ge, J.; Wang, M.; Chen, M.; Niu, W.; Cheng, W.; Xue, Y.; Lin, C.; Lei, B. Bioactive Anti-inflammatory, Antibacterial, Antioxidative Silicon-Based Nanofibrous Dressing Enables Cutaneous Tumor Photothermo-Chemo Therapy and Infection-Induced Wound Healing. ACS Nano 2020, 14, 2904–2916. [Google Scholar] [CrossRef]
- Zhao, C.; Chen, R.; Chen, Z.; Lu, Q.; Zhu, H.; Bu, Q.; Yin, J.; He, H. Bioinspired Multifunctional Cellulose Nanofibril-Based In Situ Liquid Wound Dressing for Multiple Synergistic Therapy of the Postoperative Infected Wound. ACS Appl. Mater. Interfaces 2021, 13, 51578–51591. [Google Scholar] [CrossRef]
- Allison, R.R.; Downie, G.H.; Cuenca, R.; Hu, X.-H.; Childs, C.J.H.; Sibata, C.H. Photosensitizers in clinical PDT. Photodiagnosis Photodyn. Ther. 2004, 1, 27–42. [Google Scholar] [CrossRef]
- Preis, E.; Wojcik, M.; Litscher, G.; Bakowsky, U. Editorial on the “Special Issue in Honor of Dr. Michael Weber’s 70th Birthday: Photodynamic Therapy: Rising Star in Pharmaceutical Applications”. Pharmaceutics 2022, 14, 1786. [Google Scholar] [CrossRef] [PubMed]
- Henderson, B.W.; Busch, T.M.; Snyder, J.W. Fluence rate as a modulator of PDT mechanisms. Lasers Surg. Med. 2006, 38, 489–493. [Google Scholar] [CrossRef] [PubMed]
- Okada, N.; Muraoka, E.; Fujisawa, S.; Machino, M. Effects of curcumin and capsaicin irradiated with visible light on murine oral mucosa. Vivo 2012, 26, 759–764. [Google Scholar]
- Raschpichler, M.; Preis, E.; Pinnapireddy, S.R.; Baghdan, E.; Pourasghar, M.; Schneider, M.; Bakowsky, U. Photodynamic inactivation of circulating tumor cells: An innovative approach against metastatic cancer. Eur. J. Pharm. Biopharm. 2020, 157, 38–46. [Google Scholar] [CrossRef] [PubMed]
- Stewart, P.S.; William Costerton, J. Antibiotic resistance of bacteria in biofilms. Lancet 2001, 358, 135–138. [Google Scholar] [CrossRef]
- Cheraghipour, K.; Ezatpour, B.; Masoori, L.; Marzban, A.; Sepahvand, A.; Rouzbahani, A.K.; Moridnia, A.; Khanizadeh, S.; Mahmoudvand, H. Anti-Candida Activity of Curcumin: A Systematic Review. Curr. Drug Discov. Technol. 2021, 18, 379–390. [Google Scholar] [CrossRef] [PubMed]
- Jennings, M.R.; Parks, R.J. Curcumin as an Antiviral Agent. Viruses 2020, 12, 1242. [Google Scholar] [CrossRef] [PubMed]
- Vahedian-Azimi, A.; Abbasifard, M.; Rahimi-Bashar, F.; Guest, P.C.; Majeed, M.; Mohammadi, A.; Banach, M.; Jamialahmadi, T.; Sahebkar, A. Effectiveness of Curcumin on Outcomes of Hospitalized COVID-19 Patients: A Systematic Review of Clinical Trials. Nutrients 2022, 14, 256. [Google Scholar] [CrossRef]
- Baghdan, E.; Raschpichler, M.; Lutfi, W.; Pinnapireddy, S.R.; Pourasghar, M.; Schäfer, J.; Schneider, M.; Bakowsky, U. Nano spray dried antibacterial coatings for dental implants. Eur. J. Pharm. Biopharm. 2019, 139, 59–67. [Google Scholar] [CrossRef]
- Athanasiou, K.A.; Niederauer, G.G.; Agrawal, C. Sterilization, toxicity, biocompatibility and clinical applications of polylactic acid/ polyglycolic acid copolymers. Biomaterials 1996, 17, 93–102. [Google Scholar] [CrossRef] [PubMed]





| Sample Name | PLA Content | ICG Content | CUR Content |
|---|---|---|---|
| PLA.NF | 6 wt% | 0 wt% | 0 wt% |
| PLA.NF.ICG | 6 wt% | 3 wt% | 0 wt% |
| PLA.NF.CUR | 6 wt% | 0 wt% | 10 wt% |
| PLA.NF.ICG.CUR | 6 wt% | 3 wt% | 10 wt% |
| Sample Name | ICG Concentration ± SD [µg/cm2] | CUR Concentration ± SD [µg/cm2] |
|---|---|---|
| PLA.NF | 0 | 0 |
| PLA.NF.ICG | 6.30 ± 0.50 | 0 |
| PLA.NF.CUR | 0 | 30.46 ± 0.83 |
| PLA.NF.ICG.CUR | 6.36 ± 0.16 | 23.23 ± 0.50 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gutberlet, B.; Preis, E.; Roschenko, V.; Bakowsky, U. Photothermally Controlled Drug Release of Poly(d,l-lactide) Nanofibers Loaded with Indocyanine Green and Curcumin for Efficient Antimicrobial Photodynamic Therapy. Pharmaceutics 2023, 15, 327. https://doi.org/10.3390/pharmaceutics15020327
Gutberlet B, Preis E, Roschenko V, Bakowsky U. Photothermally Controlled Drug Release of Poly(d,l-lactide) Nanofibers Loaded with Indocyanine Green and Curcumin for Efficient Antimicrobial Photodynamic Therapy. Pharmaceutics. 2023; 15(2):327. https://doi.org/10.3390/pharmaceutics15020327
Chicago/Turabian StyleGutberlet, Bernd, Eduard Preis, Valeri Roschenko, and Udo Bakowsky. 2023. "Photothermally Controlled Drug Release of Poly(d,l-lactide) Nanofibers Loaded with Indocyanine Green and Curcumin for Efficient Antimicrobial Photodynamic Therapy" Pharmaceutics 15, no. 2: 327. https://doi.org/10.3390/pharmaceutics15020327
APA StyleGutberlet, B., Preis, E., Roschenko, V., & Bakowsky, U. (2023). Photothermally Controlled Drug Release of Poly(d,l-lactide) Nanofibers Loaded with Indocyanine Green and Curcumin for Efficient Antimicrobial Photodynamic Therapy. Pharmaceutics, 15(2), 327. https://doi.org/10.3390/pharmaceutics15020327