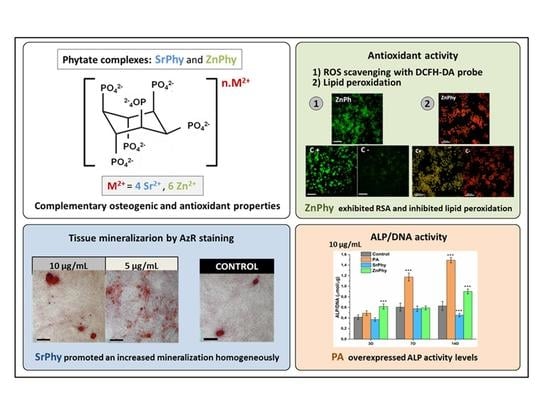
New Insights into the In Vitro Antioxidant Routes and Osteogenic Properties of Sr/Zn Phytate Compounds
Abstract
:1. Introduction
2. Materials and Methods
2.1. Synthesis of Metallic Phytate Derivatives
2.2. Radical Scavenger Activity Using a DPPH Assay
2.3. Formation of Ferrous Chelate Complexes
2.4. In Vitro Biological Tests
2.4.1. Cell Cultures
2.4.2. Cytotoxicity
2.4.3. Antioxidant Activity Using Reactive Oxygen Species Quantification
2.4.4. Qualitative and Quantitative Assessment of Lipid Peroxidation
2.4.5. Cell Viability
2.4.6. Alkaline Phosphatase Activity
2.4.7. Qualitative and Quantitative Determination of Matrix Mineralization Degree
3. Results and Discussion
3.1. Antioxidant Capacity Assessed Using a DPPH Assay
3.2. Ferrous Ions’ Chelating Ability
3.3. In Vitro Biological Performance
3.3.1. Cytotoxicity
3.3.2. Quantification of ROS Production
3.3.3. Lipid Peroxidation Inhibition
3.3.4. Cell Viability
3.3.5. ALP Activity Quantification
3.3.6. Matrix Mineralization Degree
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ALP | Alkaline phosphatase |
| AzR | Alizarin red |
| basal-DMEM | Basal medium Dulbecco’s Modified Eagle’s Medium |
| BMD | Bone mineral density |
| CPC | Cetylpyridinium chloride |
| DCF | Dichlorofluorescein |
| DCFH-DA | 2,7′-Dichlorodihydrofluorescein diacetate |
| DMEM-MHG | Dulbecco’s Modified Eagle’s Medium-high glucose |
| DPPH | 2,2-diphenyl-1-picrylhydrazyl |
| hMSC | Human mesenchymal stem cells |
| EDTA | Ethylenediaminetetraacetic acid |
| FBS | Fetal bovine serum |
| hUC-MSCs | Human umbilical cord mesenchymal stem cells |
| MDA | Malondialdehyde |
| MSCM | Mesenchymal Stem Cell Medium Kit |
| OPG | Osteoprotegerin, osteogenic-DMEM-LG, osteogenic medium DMEM Medium-low glucose |
| PA | Phytic acid |
| PBS | Phosphate-buffered saline |
| ROS | Reactive oxygen species |
| RANK | Receptor activator of nuclear factor kappa Β |
| RANKL | Receptor activator of nuclear factor kappa Β ligand |
| RAW264.7 | Murine macrophage cell line |
| RSA | Radical scavenger activity |
| SrPhy | Strontium phytate |
| SD | Standard deviation |
| TNF | Tumour necrosis factor |
| ZnPhy | Zinc phytate |
References
- Liu, Z.-Q. Bridging free radical chemistry with drug discovery: A promising way for finding novel drugs efficiently. Eur. J. Med. Chem. 2020, 189, 112020. [Google Scholar] [CrossRef] [PubMed]
- Choukroun, E.; Surmenian, J.; Simonpieri, A.; Choukroun, J. Oxidative Stress and Osteoimmunology: The two Missing Pieces of the Oral Osseointegration Puzzle. Immunol. Res. Ther. J. 2011, 3, 119. [Google Scholar]
- Domazetovic, V.; Marcucci, G.; Iantomasi, T.; Brandi, M.L.; Vincenzini, M.T. Oxidative stress in bone remodeling: Role of antioxidants. Clin. Cases Miner. Bone Metab. 2017, 14, 209–216. [Google Scholar] [CrossRef] [PubMed]
- Baek, K.H.; Oh, K.W.; Lee, W.Y.; Lee, S.S.; Kim, M.K.; Kwon, H.S.; Rhee, E.J.; Han, J.H.; Song, K.H.; Cha, B.Y.; et al. Association of oxidative stress with postmenopausal osteoporosis and the effects of hydrogen peroxide on osteoclast formation in human bone marrow cell cultures. Calcif. Tissue Int. 2010, 87, 226–235. [Google Scholar] [CrossRef] [PubMed]
- Tian, Y.; Ma, X.; Yang, C.; Su, P.; Yin, C.; Qian, A.-R. The impact of oxidative stress on the bone system in response to the space special environment. Int. J. Mol. Sci. 2017, 18, 2321. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yasuda, H. Discovery of the RANKL/RANK/OPG system. J. Bone Miner. Metab. 2021, 39, 2–11. [Google Scholar] [CrossRef]
- Castaneda, O.A.; Lee, S.-C.; Ho, C.-T.; Huang, T.-C. Macrophages in oxidative stress and models to evaluate the antioxidant function of dietary natural compounds. J. Food Drug Anal. 2017, 25, 111–118. [Google Scholar] [CrossRef] [Green Version]
- Boyce, B.F.; Xing, L. Functions of RANKL/RANK/OPG in bone modeling and remodeling. Arch. Biochem. Biophys. 2008, 473, 139–146. [Google Scholar] [CrossRef] [Green Version]
- Li, H.; Qi, Z.; Zheng, S.; Chang, Y.; Kong, W.; Fu, C.; Yu, Z.; Yang, X.; Pan, S. The Application of Hyaluronic Acid-Based Hydrogels in Bone and Cartilage Tissue Engineering. Adv. Mater. Sci. Eng. 2019, 2019, 3027303. [Google Scholar] [CrossRef] [Green Version]
- Catalá, A. Lipid peroxidation of membrane phospholipids generates hydroxy-alkenals and oxidized phospholipids active in physiological and/or pathological conditions. Chem. Phys. Lipids. 2009, 157, 1–11. [Google Scholar] [CrossRef]
- Ayala, A.; Muñoz, M.; Argüelles, S. Lipid peroxidation: Production, metabolism, and signaling mechanisms of malondialdehyde and 4-hydroxy-2-nonenal. Oxidative Med. Cell. Longev. 2014, 2014, 360438. [Google Scholar] [CrossRef]
- Sasaki, C.J.; Cottam, G.L. Lipid peroxidation following thermal injury. J. Burn Care Rehabil. 1983, 4, 251–254. [Google Scholar] [CrossRef]
- Gaschler, M.M.; Stockwell, B.R. Lipid peroxidation in cell death. Biochem. Biophys. Res. Commun. 2017, 482, 419–425. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Liang, X.-F.; Li, J.; Yuan, X.; Fang, J. Effects of supplemental phytic acid on the apparent digestibility and utilization of dietary amino acids and minerals in juvenile grass carp ( Ctenopharyngodon idellus ). Aquac. Nutr. 2017, 24, 850–857. [Google Scholar] [CrossRef]
- Qin, J.; Shi, X.; Li, H.; Zhao, R.; Li, G.; Zhang, S.; Ding, L.; Cui, X.; Zhao, Y.; Zhang, R. Performance and failure process of green recycling solutions for preparing high degradation resistance coating on biomedical magnesium alloys. Green Chem. 2022, 24, 8113–8130. [Google Scholar] [CrossRef]
- Erdman, J.W.; Poneros-Schneier, A. Phytic acid interactions with divalent cations in foods and in the gastrointestinal tract. Adv. Exp. Med. Biol. 1989, 249, 161–171. [Google Scholar] [CrossRef]
- Serraino, M.R.; Thompson, L.U. Removal of Phytic Acid and Protein-Phytic Acid Interactions in Rapeseed. J. Agric. Food Chem. 1984, 32, 38–40. [Google Scholar] [CrossRef]
- Zajdel, A.; Wilczok, A.; Wȩglarz, L.; Dzierżewicz, Z. Phytic acid inhibits lipid peroxidation in vitro. Biomed Res. Int. 2013, 2013, 147307. [Google Scholar] [CrossRef] [Green Version]
- Graf, E.; Empson, K.L.; Eaton, J.W. Phytic acid. A natural antioxidant. J. Biol. Chem. 1987, 262, 11647–11650. [Google Scholar] [CrossRef]
- Ko, K.M.; Godin, D.V. Ferric ion-induced lipid peroxidation in erythrocyte membranes: Effects of phytic acid and butylated hydroxytoluene. Mol. Cell. Biochem. 1990, 95, 125–131. [Google Scholar] [CrossRef]
- Lee, B.J.; Hendricks, D.G. Phytic Acid Protective Effect Against Beef Round Muscle Lipid Peroxidation. J. Food Sci. 1995, 60, 241–244. [Google Scholar] [CrossRef]
- del Mar Arriero, M.; Ramis, J.M.; Perelló, J.; Monjo, M. Inositol hexakisphosphate inhibits osteoclastogenesis on RAW 264.7 cells and human primary osteoclasts. PLoS ONE 2012, 7, e43187. [Google Scholar] [CrossRef] [Green Version]
- Del Mar Arriero, M.; Ramis, J.M.; Perelló, J.; Monjo, M. Differential response of MC3T3-E1 and human mesenchymal stem cells to inositol hexakisphosphate. Cell. Physiol. Biochem. 2012, 30, 974–986. [Google Scholar] [CrossRef]
- Addison, W.N.; McKee, M.D. Inositol hexakisphosphate inhibits mineralization of MC3T3-E1 osteoblast cultures. Bone 2010, 46, 1100–1107. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Liu, K.; Lu, M.; Liu, L.; Yan, Y.; Chu, Z.; Ge, Y.; Wang, T.; Qiu, J.; Bu, S.; et al. Micro/nanostructured calcium phytate coating on titanium fabricated by chemical conversion deposition for biomedical application. Mater. Sci. Eng. C 2021, 118, 111402. [Google Scholar] [CrossRef] [PubMed]
- Mora-Boza, A.; López-Donaire, M.L.; Saldaña, L.; Vilaboa, N.; Vázquez-Lasa, B.; Román, J.S. Glycerylphytate compounds with tunable ion affinity and osteogenic properties. Sci. Rep. 2019, 9, 11491. [Google Scholar] [CrossRef] [Green Version]
- Mora-Boza, A.; García-Fernández, L.; Barbosa, F.A.; Oliveira, A.L.; Vázquez-Lasa, B.; Román, J.S. Glycerylphytate crosslinker as a potential osteoinductor of chitosan-based systems for guided bone regeneration. Carbohydr. Polym. 2020, 241, 116269. [Google Scholar] [CrossRef]
- Lopez-Gonzalez, A.A.; Grases, F.; Perello, J.; Tur, F.; Costa-Bauza, A.; Monroy, N.; Mari, B.; Vicente-Herrero, T. Phytate levels and bone parameters: A retrospective pilot clinical trial. Front. Biosci. 2010, 2, 1093–1098. [Google Scholar] [CrossRef] [Green Version]
- Grases, F.; Sanchis, P.; Prieto, R.M.; Perelló, J.; López-González, Á.A. Effect of Tetracalcium Dimagnesium Phytate on Bone Characteristics in Ovariectomized Rats. J. Med. Food. 2010, 13, 1301–1306. [Google Scholar] [CrossRef]
- Yang, F.; Yang, D.; Tu, J.; Zheng, Q.; Cai, L.; Wang, L. Strontium enhances osteogenic differentiation of mesenchymal stem cells and in vivo bone formation by activating Wnt/catenin signaling. Stem Cells 2011, 29, 981–991. [Google Scholar] [CrossRef]
- Peng, S.; Zhou, G.; Luk, K.D.K.; Cheung, K.; Li, Z.; Lam, W.M.; Zhou, Z.; Lu, W.W. Strontium promotes osteogenic differentiation of mesenchymal stem cells through the Ras/MAPK signaling pathway. Cell Physiol. Biochem. 2009, 23, 165–174. [Google Scholar] [CrossRef] [PubMed]
- Jiménez, M.; Abradelo, C.; Román, J.S.; Rojo, L. Bibliographic review on the state of the art of strontium and zinc based regenerative therapies. Recent developments and clinical applications. J. Mater. Chem. B 2019, 7, 1974–1985. [Google Scholar] [CrossRef] [PubMed]
- Baltaci, A.K.; Yuce, K.; Mogulkoc, R. Zinc Metabolism and Metallothioneins. Biol. Trace Elem. Res. 2018, 183, 22–31. [Google Scholar] [CrossRef] [PubMed]
- Cabrera, W.E.; Schrooten, I.; De Broe, M.E.; D’Haese, P.C. Strontium and Bone. J. Bone Miner. Res. 1999, 14, 661–668. [Google Scholar] [CrossRef]
- Marie, P.J. Strontium ranelate: A physiological approach for optimizing bone formation and resorption. Bone 2006, 38, 10–14. [Google Scholar] [CrossRef]
- Rojo, L.; Radley-Searle, S.; Fernandez-Gutierrez, M.; Rodriguez-Lorenzo, L.M.; Abradelo, C.; Deb, S.; Roman, J.S. The synthesis and characterisation of strontium and calcium folates with potential osteogenic activity. J. Mater. Chem. B 2015, 3, 2708–2713. [Google Scholar] [CrossRef]
- Powell, S.R. The Antioxidant Properties of Zinc. J. Nutr. 2000, 130, 1447S–1454S. [Google Scholar] [CrossRef] [Green Version]
- Seo, H.-J.; Cho, Y.-E.; Kim, T.; Shin, H.-I.; Kwun, I.-S. Zinc may increase bone formation through stimulating cell proliferation, alkaline phosphatase activity and collagen synthesis in osteoblastic MC3T3-E1 cells. Nutr. Res. Pract. 2010, 4, 356–361. [Google Scholar] [CrossRef] [Green Version]
- Fernández-Villa, D.; Asensio, G.; Silva, M.; Ramírez-Jiménez, R.A.; Saldaña, L.; Vilaboa, N.; Leite-Oliveira, A.; San Román, J.; Vázquez-Lasa, B.; Rojo, L. Vitamin B9 derivatives as carriers of bioactive cations for musculoskeletal regeneration applications: Synthesis, characterization and biological evaluation. Eur. J. Med. Chem. 2021, 212, 113152. [Google Scholar] [CrossRef]
- Martín-Del-Campo, M.; Sampedro, J.G.; Flores-Cedillo, M.L.; Rosales-Ibañez, R.; Rojo, L. Bone regeneration induced by strontium folate loaded biohybrid scaffolds. Molecules 2019, 24, 1660. [Google Scholar] [CrossRef] [Green Version]
- Martin-del-campo, M.; Rosales-ibañez, R.; Alvarado, K.; Sampedro, J.G.; Garcia-Sepulveda, C.A.; Deb, S.; San Román, J.; Rojo, L. Strontium folate loaded biohybrid scaffolds seeded with dental pulp stem cells induce in vivo bone regeneration in critical sized defects. Biomater. Sci. 2016, 4, 1596–1604. [Google Scholar] [CrossRef] [PubMed]
- Asensio, G.; Benito-Garzón, L.; Ramírez-Jiménez, R.A.; Guadilla, Y.; Gonzalez-Rubio, J.; Abradelo, C.; Parra, J.; Martín-López, M.R.; Aguilar, M.R.; Vázquez-Lasa, B.; et al. Biomimetic Gradient Scaffolds Containing Hyaluronic Acid and Sr/Zn Folates for Osteochondral Tissue Engineering. Polymers 2022, 14, 12. [Google Scholar] [CrossRef] [PubMed]
- Asensio, G.; Hernández-Arriaga, A.M.; Martín-Del-Campo, M.; Prieto, M.A.; Rojo, L.; Vázquez-Lasa, B. A study on Sr/Zn phytate complexes: Structural properties and antimicrobial synergistic effects against Streptococcus mutans. Sci. Rep. 2022, 12, 1–11. [Google Scholar] [CrossRef]
- Dinis, T.C.; Maderia, V.M.; Almeida, L.M. Action of Phenolic Derivatives (Acetaminophen). Arch. Biochem. Biophys. 1994, 315, 161–169. [Google Scholar] [CrossRef]
- Carragee, E.J.; Hurwitz, E.L.; Weiner, B.K. A critical review of recombinant human bone morphogenetic protein-2 trials in spinal surgery: Emerging safety concerns and lessons learned. Spine J. 2011, 11, 471–491. [Google Scholar] [CrossRef]
- Cooper, C.; Fox, K.M.; Borer, J.S. Ischaemic cardiac events and use of strontium ranelate in postmenopausal osteoporosis: A nested case-control study in the CPRD. Osteoporos. Int. 2014, 25, 737–745. [Google Scholar] [CrossRef] [Green Version]
- Abrahamsen, B.; Grove, E.; Vestergaard, P. Nationwide registry-based analysis of cardiovascular risk factors and adverse outcomes in patients treated with strontium ranelate. Osteoporos. Int. 2014, 25, 757–762. [Google Scholar] [CrossRef]
- Milkovic, L.; Gasparovic, A.C.; Cindric, M.; Mouthuy, P.-A.; Zarkovic, N. Short Overview of ROS as Cell Function Regulators and Their Implications in Therapy Concepts. Cells 2019, 8, 793. [Google Scholar] [CrossRef] [Green Version]
- Bagchi, D.; Bagchi, M.; Stohs, S. Comparative in vitro oxygen radical scavenging ability of zinc methionine and selected zinc salts and antioxidants. Gen. Pharmacol. 1997, 28, 85–91. [Google Scholar] [CrossRef]
- Prasad, A.S. Zinc is an Antioxidant and Anti-Inflammatory Agent: Its Role in Human Health. Front. Nutr. 2014, 1, 14. [Google Scholar] [CrossRef] [Green Version]
- Halliwell, B.; Chirico, S. Lipid peroxidation: Significance and its mechanism. Am. J. Clin. Nutr. 1993, 57, 715–725. [Google Scholar] [CrossRef] [PubMed]
- Toyokuni, S. Iron and carcinogenesis: From Fenton reaction to target genes. Redox Rep. 2002, 7, 189–197. [Google Scholar] [CrossRef] [PubMed]
- Torres, J.; Domínguez, S.; Cerdá, M.F.; Obal, G.; Mederos, A.; Irvine, R.F.; Díaz, A.; Kremer, C. Solution behaviour of myo -inositol hexakisphosphate in the presence of multivalent cations. Prediction of a neutral pentamagnesium species under cytosolic/nuclear conditions. J. Inorg. Biochem. 2005, 99, 828–840. [Google Scholar] [CrossRef] [PubMed]
- Marolt, G.; Gričar, E.; Pihlar, B.; Kolar, M. Complex Formation of Phytic Acid With Selected Monovalent and Divalent Metals. Front. Chem. 2020, 8, 82746. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.; Lee, Y.D.; Kim, H.J.; Lee, Z.H.; Kim, H.-H. SOD2 and Sirt3 Control Osteoclastogenesis by Regulating Mitochondrial ROS. J. Bone Miner. Res. 2017, 32, 397–406. [Google Scholar] [CrossRef]
- Callaway, D.A.; Jiang, J.X. Reactive oxygen species and oxidative stress in osteoclastogenesis, skeletal aging and bone diseases. J. Bone Miner. Metab. 2015, 33, 359–370. [Google Scholar] [CrossRef]
- El-Refai, A.A.; Ghoniem, G.A.; El-Khateeb, A.Y.; Hassaan, M.M. Eco-friendly synthesis of metal nanoparticles using ginger and garlic extracts as biocompatible novel antioxidant and antimicrobial agents. J. Nanostructure Chem. 2018, 8, 71–81. [Google Scholar] [CrossRef] [Green Version]
- Elkodous, M.A.; El-Sayyad, G.S.; Maksoud, M.I.A.A.; Abdelrahman, I.Y.; Mosallam, F.M.; Gobara, M.; El-Batal, A.I. Fabrication of Ultra-Pure Anisotropic Zinc Oxide Nanoparticles via Simple and Cost-Effective Route: Implications for UTI and EAC Medications. Biol. Trace Elem. Res. 2020, 196, 297–317. [Google Scholar] [CrossRef]
- Rajeshkumar, S.; Kumar, S.V.; Ramaiah, A.; Agarwal, H.; Lakshmi, T.; Roopan, S.M. Biosynthesis of zinc oxide nanoparticles usingMangifera indica leaves and evaluation of their antioxidant and cytotoxic properties in lung cancer (A549) cells. Enzym. Microb. Technol. 2018, 117, 91–95. [Google Scholar] [CrossRef]
- Press, D. Novel conductive polypyrrole / zinc oxide/chitosan bionanocomposite: Synthesis, characterization, antioxidant, and antibacterial activities. Int. J. Nanomedicine. 2015, 10, 217–227. [Google Scholar]
- Wu, S.; Du, Y.; Hu, Y.; Shi, X.; Zhang, L. Antioxidant and antimicrobial activity of xylan-chitooligomer-zinc complex. Food Chem. 2013, 138, 1312–1319. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.; Zheng, X.P.; Chen, L.L. Study on antioxidant activity of dihydromyricetin-zinc(II) complex. Adv. Mater. Res. 2011, 183–185, 863–867. [Google Scholar] [CrossRef]
- Baran, A.; Karakılıç, E.; Faiz, Ö.; Özen, F. Synthesis of chalcone-containing zinc and cobalt metallophthalocyanines; investigation of their photochemical, DPPH radical scavenging and metal chelating characters. Org. Commun. 2020, 13, 65–78. [Google Scholar] [CrossRef]
- Fuhrman, B.; Oiknine, J.; Aviram, M. Iron induces lipid peroxidation in cultured macrophages, increases their ability to oxidatively modify LDL, and affects their secretory properties. Atherosclerosis 1994, 111, 65–78. [Google Scholar] [CrossRef] [PubMed]
- Tsikas, D. Assessment of lipid peroxidation by measuring malondialdehyde (MDA) and relatives in biological samples: Analytical and biological challenges. Anal. Biochem. 2017, 524, 13–30. [Google Scholar] [CrossRef] [PubMed]
- Moore, K.; Roberts, L.J. Measurement of lipid peroxidation. Free Radic. Res. 1998, 28, 659–671. [Google Scholar] [CrossRef]
- Huntosova, V.; Horvath, D.; Seliga, R.; Wagnieres, G. Influence of oxidative stress on time-resolved oxygen detection by [ru(Phen)3 ]2+ in vivo and in vitro. Molecules 2021, 26, 485. [Google Scholar] [CrossRef]
- Bose, C.; Hindle, A.; Lee, J.; Kopel, J.; Tonk, S.; Palade, P.T.; Singhal, S.S.; Awasthi, S.; Singh, S.P. Anticancer activity of Ω-6 fatty acids through increased 4-hne in breast cancer cells. Cancers 2021, 13, 6377. [Google Scholar] [CrossRef]
- Del Favero, G.; Hohenbichler, J.; Mayer, R.M.; Rychlik, M.; Marko, D. Mycotoxin Altertoxin II Induces Lipid Peroxidation Connecting Mitochondrial Stress Response to NF-κB Inhibition in THP-1 Macrophages. Chem. Res. Toxicol. 2020, 33, 492–504. [Google Scholar] [CrossRef] [Green Version]
- Sullivan, J.F.; Jetton, M.M.; Hahn, H.K.; Burch, R.E. Enhanced lipid peroxidation in liver microsomes of zinc-deficient rats. Am J Clin Nutr. 1980, 33, 51–56. [Google Scholar] [CrossRef]
- Tunçdemir, M.; Ertürküner, S.P.; Özçelik, D. Investigation of lipid peroxidation and antiapoptotic effects of zinc aganist liver damage in diabetic rats. Hum. Exp. Toxicol. 2017, 36, 813–822. [Google Scholar] [CrossRef] [PubMed]
- Zago, M.P.; Verstraeten, S.V.; Oteiza, P.I. Zinc in the prevention of Fe2+-initiated lipid and protein oxidation. Biol. Res. 2000, 33, 143–150. [Google Scholar] [CrossRef] [PubMed]
- Irvine, R.F.; Schell, M.J. Back in the water: The return of the inositol phosphate. Nat. Rev. Mol. Cell Biol. 2001, 2, 327–338. [Google Scholar] [CrossRef]
- Liu, Y.; Wu, J.; Zhang, H.; Wu, Y.; Tang, C. Covalent immobilization of the phytic acid-magnesium layer on titanium improves the osteogenic and antibacterial properties. Colloids Surfaces B Biointerfaces. 2021, 203, 111768. [Google Scholar] [CrossRef]
- Wang, Y.; Lou, J.; Zeng, L.; Xiang, J.; Zhang, S.; Wang, J.; Xiong, F.; Li, C.; Zhao, Y.; Zhang, R. Osteogenic potential of a novel microarc oxidized coating formed on Ti6Al4V alloys. Appl. Surf. Sci. 2017, 412, 29–36. [Google Scholar] [CrossRef]
- Wang, Q.; Ding, C.; Zhou, Y.; Luo, J.; Li, J. Universal and biocompatible hydroxyapatite coating induced by phytic acid-metal complex multilayer. Colloids Surf. B Biointerfaces 2018, 169, 478–485. [Google Scholar] [CrossRef]
- Li, J.; Wang, S.; Dong, Y. Regeneration of pulp-dentine complex-like tissue in a rat experimental model under an inflammatory microenvironment using high phosphorous-containing bioactive glasses. Int. Endod. J. 2021, 54, 1129–1141. [Google Scholar] [CrossRef] [PubMed]
- Bonnelye, E.; Chabadel, A.; Saltel, F.; Jurdic, P. Dual effect of strontium ranelate: Stimulation of osteoblast differentiation and inhibition of osteoclast formation and resorption in vitro. Bone 2008, 42, 129–138. [Google Scholar] [CrossRef]
- Capuccini, C.; Torricelli, P.; Sima, F.; Boanini, E.; Ristoscu, C.; Bracci, B.; Socol, G.; Fini, M.; Mihailescu, I.; Bigi, A. Strontium-substituted hydroxyapatite coatings synthesized by pulsed-laser deposition: In vitro osteoblast and osteoclast response. Acta Biomater. 2008, 4, 1885–1893. [Google Scholar] [CrossRef]
- Park, J.-W.; Kim, H.-K.; Kim, Y.-J.; Jang, J.-H.; Song, H.; Hanawa, T. Osteoblast response and osseointegration of a Ti-6Al-4V alloy implant incorporating strontium. Acta Biomater. 2010, 6, 2843–2851. [Google Scholar] [CrossRef]








Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Asensio, G.; Martín-del-Campo, M.; Ramírez, R.A.; Rojo, L.; Vázquez-Lasa, B. New Insights into the In Vitro Antioxidant Routes and Osteogenic Properties of Sr/Zn Phytate Compounds. Pharmaceutics 2023, 15, 339. https://doi.org/10.3390/pharmaceutics15020339
Asensio G, Martín-del-Campo M, Ramírez RA, Rojo L, Vázquez-Lasa B. New Insights into the In Vitro Antioxidant Routes and Osteogenic Properties of Sr/Zn Phytate Compounds. Pharmaceutics. 2023; 15(2):339. https://doi.org/10.3390/pharmaceutics15020339
Chicago/Turabian StyleAsensio, Gerardo, Marcela Martín-del-Campo, Rosa Ana Ramírez, Luis Rojo, and Blanca Vázquez-Lasa. 2023. "New Insights into the In Vitro Antioxidant Routes and Osteogenic Properties of Sr/Zn Phytate Compounds" Pharmaceutics 15, no. 2: 339. https://doi.org/10.3390/pharmaceutics15020339
APA StyleAsensio, G., Martín-del-Campo, M., Ramírez, R. A., Rojo, L., & Vázquez-Lasa, B. (2023). New Insights into the In Vitro Antioxidant Routes and Osteogenic Properties of Sr/Zn Phytate Compounds. Pharmaceutics, 15(2), 339. https://doi.org/10.3390/pharmaceutics15020339