Sericin/Human Placenta-Derived Extracellular Matrix Scaffolds for Cutaneous Wound Treatment—Preparation, Characterization, In Vitro and In Vivo Analyses
Abstract
:1. Introduction
2. Materials and Methods
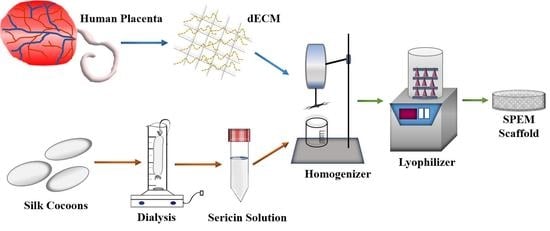
2.1. Isolation of Sericin
2.2. Decellularized Extracellular Matrix (dECM) from Human Placenta
2.3. Preparation of Sericin/Placenta-Derived Extracellular Matrix (SPEM) Scaffolds
2.4. Characterization
2.5. Hemolytic Assay
2.6. Swelling Study
2.7. Moisture Content
2.8. Antibacterial Activity
2.9. In Vitro Experiments
2.9.1. Cell Viability Assay
2.9.2. Wound Healing Assay
2.10. In Vivo Experiments
2.10.1. Chick Embryo Chorioallantoic Membrane Assay
2.10.2. Open Excision Wound Animal Model
2.11. Statistical Analysis
3. Results and Discussion
3.1. Characterization
3.2. Hemolytic Assay
3.3. Swelling Assay
3.4. Moisture Content
3.5. Antibacterial Activity
3.6. In Vitro Experiments
3.6.1. Cell Viability Assay
3.6.2. Wound Healing Assay
3.7. In-Vivo Experiments
3.7.1. Chick Embryo Chorioallantoic Membrane Assay
3.7.2. Open Excision Wound Animal Model
3.7.3. Histological Observation
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sun, G.; Qin, J.; Chen, F.; Wu, P. Recent Advances in Bioengineered Scaffolds for Cutaneous Wound Healing. Front. Bioeng. Biotechnol. 2022, 10, 244. [Google Scholar]
- Nosrati, H.; Aramideh Khouy, R.; Nosrati, A.; Khodaei, M.; Banitalebi-Dehkordi, M.; Ashrafi-Dehkordi, K.; Sanami, S.; Alizadeh, Z. Nanocomposite Scaffolds for Accelerating Chronic Wound Healing by Enhancing Angiogenesis. J. Nanobiotechnology 2021, 19, 1. [Google Scholar] [CrossRef]
- Sivaraj, D.; Chen, K.; Chattopadhyay, A.; Henn, D.; Wu, W.; Noishiki, C.; Magbual, N.J.; Mittal, S.; Mermin-Bunnell, A.M.; Bonham, C.A.; et al. Hydrogel Scaffolds to Deliver Cell Therapies for Wound Healing. Front. Bioeng. Biotechnol. 2021, 9, 660145. [Google Scholar] [CrossRef] [PubMed]
- Soubhagya, A.S.; Moorthi, A.; Prabaharan, M. Preparation and Characterization of Chitosan/Pectin/ZnO Porous Films for Wound Healing. Int. J. Biol. Macromol. 2020, 157, 135–145. [Google Scholar] [CrossRef] [PubMed]
- Garg, V.K.; Paliwal, S.K. Wound-Healing Activity of Ethanolic and Aqueous Extracts of Ficus Benghalensis. J. Adv. Pharm. Technol. Res. 2011, 2, 110. [Google Scholar] [CrossRef]
- Rasouli, R.; Barhoum, A.; Bechelany, M.; Dufresne, A. Nanofibers for Biomedical and Healthcare Applications. Macromol. Biosci. 2019, 19, 1800256. [Google Scholar] [CrossRef]
- Dhandayuthapani, B.; Yoshida, Y.; Maekawa, T.; Kumar, D.S. Polymeric Scaffolds in Tissue Engineering Application: A Review. Int. J. Polym. Sci. 2011, 2011, 290602. [Google Scholar] [CrossRef]
- Jayakumar, R.; Prabaharan, M. Chitosan for Biomaterials III: Structure-Property Relationships; Springer Nature: Cham, Switzerland, 2021; Volume 287. [Google Scholar]
- Fahimirad, S.; Abtahi, H.; Satei, P.; Ghaznavi-Rad, E.; Moslehi, M.; Ganji, A. Wound Healing Performance of PCL/Chitosan Based Electrospun Nanofiber Electrosprayed with Curcumin Loaded Chitosan Nanoparticles. Carbohydr. Polym. 2021, 259, 117640. [Google Scholar] [CrossRef]
- Lin, Z.-I.; Tsai, H.-L.; Liu, G.-L.; Lu, X.-H.; Cheng, P.-W.; Chi, P.-L.; Wang, C.-K.; Tsai, T.-H.; Wang, C.-C.; Yang, J.H.C.; et al. Preparation of CO2-Based Cationic Polycarbonate/Polyacrylonitrile Nanofibers with an Optimal Fibrous Microstructure for Antibacterial Applications. Macromol. Biosci. 2022, 22, 2200178. [Google Scholar] [CrossRef]
- Deepika, B.; Gopikrishna, A.; Girigoswami, A.; Banu, M.N.; Girigoswami, K. Applications of Nanoscaffolds in Tissue Engineering. Curr. Pharmacol. Rep. 2022, 8, 171–187. [Google Scholar] [CrossRef]
- Jo, Y.-Y.; Kweon, H.; Oh, J.-H. Sericin for Tissue Engineering. Appl. Sci. 2020, 10, 8457. [Google Scholar] [CrossRef]
- Padamwar, M.N.; Pawar, A.P.; Daithankar, A.V.; Mahadik, K.R. Silk Sericin as a Moisturizer: An in Vivo Study. J. Cosmet. Dermatol. 2005, 4, 250–257. [Google Scholar] [CrossRef]
- Lamboni, L.; Li, Y.; Liu, J.; Yang, G. Silk Sericin-Functionalized Bacterial Cellulose as a Potential Wound-Healing Biomaterial. Biomacromolecules 2016, 17, 3076–3084. [Google Scholar] [CrossRef]
- Martinez-Mora, C.; Mrowiec, A.; Garcia-Vizcaino, E.M.; Alcaraz, A.; Cenis, J.L.; Nicolás, F.J. Fibroin and Sericin from Bombyx Mori Silk Stimulate Cell Migration through Upregulation and Phosphorylation of C-Jun. PLoS ONE 2012, 7, e42271. [Google Scholar] [CrossRef]
- Aramwit, P.; Kanokpanont, S.; De-Eknamkul, W.; Srichana, T. Monitoring of Inflammatory Mediators Induced by Silk Sericin. J. Biosci. Bioeng. 2009, 107, 556–561. [Google Scholar] [CrossRef]
- Aramwit, P.; Kanokpanont, S.; De-Eknamkul, W.; Kamei, K.; Srichana, T. The Effect of Sericin with Variable Amino-Acid Content from Different Silk Strains on the Production of Collagen and Nitric Oxide. J. Biomater. Sci. Polym. Ed. 2009, 20, 1295–1306. [Google Scholar] [CrossRef]
- Aramwit, P.; Siritientong, T.; Srichana, T. Potential Applications of Silk Sericin, a Natural Protein from Textile Industry by-Products. Waste Manag. Res. 2012, 30, 217–224. [Google Scholar] [CrossRef]
- Kato, N.; Sato, S.; Yamanaka, A.; Yamada, H.; Fuwa, N.; Nomura, M. Silk Protein, Sericin, Inhibits Lipid Peroxidation and Tyrosinase Activity. Biosci. Biotechnol. Biochem. 1998, 62, 145–147. [Google Scholar] [CrossRef] [Green Version]
- Teramoto, H.; Kameda, T.; Tamada, Y. Preparation of Gel Film from Bombyx mori Silk Sericin and Its Characterization as a Wound Dressing. Biosci. Biotechnol. Biochem. 2008, 72, 3189–3196. [Google Scholar] [CrossRef] [Green Version]
- Aamodt, J.M.; Grainger, D.W. Extracellular Matrix-Based Biomaterial Scaffolds and the Host Response. Biomaterials 2016, 86, 68–82. [Google Scholar] [CrossRef] [Green Version]
- Rameshbabu, A.P.; Bankoti, K.; Datta, S.; Subramani, E.; Apoorva, A.; Ghosh, P.; Maity, P.P.; Manchikanti, P.; Chaudhury, K.; Dhara, S. Silk Sponges Ornamented with a Placenta-Derived Extracellular Matrix Augment Full-Thickness Cutaneous Wound Healing by Stimulating Neovascularization and Cellular Migration. ACS Appl. Mater. Interfaces 2018, 10, 16977–16991. [Google Scholar] [CrossRef] [PubMed]
- Hong, J.W.; Lee, W.J.; Hahn, S.B.; Kim, B.J.; Lew, D.H. The Effect of Human Placenta Extract in a Wound Healing Model. Ann. Plast. Surg. 2010, 65, 96–100. [Google Scholar] [CrossRef] [PubMed]
- De, D.; Chakraborty, P.D.; Bhattacharyya, D. Regulation of Trypsin Activity by Peptide Fraction of an Aqueous Extract of Human Placenta Used as Wound Healer. J. Cell. Physiol. 2011, 226, 2033–2040. [Google Scholar] [CrossRef] [PubMed]
- Benders, K.E.M.; van Weeren, P.R.; Badylak, S.F.; Saris, D.B.F.; Dhert, W.J.A.; Malda, J. Extracellular Matrix Scaffolds for Cartilage and Bone Regeneration. Trends Biotechnol. 2013, 31, 169–176. [Google Scholar] [CrossRef] [PubMed]
- Lobo, S.E.; Leonel, L.C.P.C.; Miranda, C.M.F.C.; Coelho, T.M.; Ferreira, G.A.S.; Mess, A.; Abrão, M.S.; Miglino, M.A. The Placenta as an Organ and a Source of Stem Cells and Extracellular Matrix: A Review. Cells Tissues Organs 2016, 201, 239–252. [Google Scholar] [CrossRef]
- Choi, J.S.; Kim, J.D.; Yoon, H.S.; Cho, Y.W. Full-Thickness Skin Wound Healing Using Human Placenta-Derived Extracellular Matrix Containing Bioactive Molecules. Tissue Eng. Part A 2013, 19, 329–339. [Google Scholar] [CrossRef] [Green Version]
- Vedakumari, W.S.; Prabu, P.; Babu, S.C.; Sastry, T.P. Fibrin Nanoparticles as Possible Vehicles for Drug Delivery. Biochim. Biophys. Acta (BBA) Gen. Subj. 2013, 1830, 4244–4253. [Google Scholar] [CrossRef]
- Vedakumari, S.W.; Jayalakshmi, R.; Sanjayan, C.G.; Jayavardhini, B.; Arya, K.; Murugesan, R. Fabrication of Microcomposites Based on Silk Sericin and Monetite for Bone Tissue Engineering. Polym. Bull. 2020, 77, 475–481. [Google Scholar] [CrossRef]
- Garrido, T.; Etxabide, A.; de la Caba, K.; Guerrero, P. Versatile Soy Protein Films and Hydrogels by the Incorporation of β-Chitin from Squid Pens (Loligo sp.). Green Chem. 2017, 19, 5923–5931. [Google Scholar] [CrossRef]
- Choudhary, P.; Ramalingam, B.; Das, S.K. Fabrication of Chitosan-Reinforced Multifunctional Graphene Nanocomposite as Antibacterial Scaffolds for Hemorrhage Control and Wound-Healing Application. ACS Biomater. Sci. Eng. 2020, 6, 5911–5929. [Google Scholar] [CrossRef]
- Shuai, C.; Xu, Y.; Feng, P.; Wang, G.; Xiong, S.; Peng, S. Antibacterial Polymer Scaffold Based on Mesoporous Bioactive Glass Loaded with in Situ Grown Silver. Chem. Eng. J. 2019, 374, 304–315. [Google Scholar] [CrossRef]
- Vedakumari, S.W.; Jancy, S.J.V.; Pravin, Y.R.; Bhoopathy, J.; Iyswariya, K.; Thomas, S.; Rubiya, R.; Prabakaran, L.; Kumar, C.; Prabu, P.; et al. Facile Synthesis of Sericin Modified Graphene Oxide Nanocomposites for Treating Ischemic Diseases. Environ. Res. 2022, 209, 112925. [Google Scholar] [CrossRef]
- Tao, L.; Zhonglong, L.; Ming, X.; Zezheng, Y.; Zhiyuan, L.; Xiaojun, Z.; Jinwu, W. In Vitro and In Vivo Studies of a Gelatin/Carboxymethyl Chitosan/LAPONITE® composite Scaffold for Bone Tissue Engineering. RSC Adv. 2017, 7, 54100–54110. [Google Scholar] [CrossRef] [Green Version]
- Chen, C.-K.; Lee, M.-C.; Lin, Z.-I.; Lee, C.-A.; Tung, Y.-C.; Lou, C.-W.; Law, W.-C.; Chen, N.-T.; Lin, K.-Y.A.; Lin, J.-H. Intensifying the Antimicrobial Activity of Poly (2-(Tert-Butylamino) Ethyl Methacrylate)/Polylactide Composites by Tailoring Their Chemical and Physical Structures. Mol. Pharm. 2018, 16, 709–723. [Google Scholar] [CrossRef]
- Jo, Y.-Y.; Kim, D.-W.; Choi, J.-Y.; Kim, S.-G. 4-Hexylresorcinol and Silk Sericin Increase the Expression of Vascular Endothelial Growth Factor via Different Pathways. Sci. Rep. 2019, 9, 3448. [Google Scholar] [CrossRef] [Green Version]
- Li, J.; Zhang, Y.-P.; Kirsner, R.S. Angiogenesis in Wound Repair: Angiogenic Growth Factors and the Extracellular Matrix. Microsc. Res. Tech. 2003, 60, 107–114. [Google Scholar] [CrossRef]













Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bhoopathy, J.; Dharmalingam, S.; Sathyaraj, W.V.; Rajendran, S.; Rymbai, S.; Senthil, R.; Atchudan, R. Sericin/Human Placenta-Derived Extracellular Matrix Scaffolds for Cutaneous Wound Treatment—Preparation, Characterization, In Vitro and In Vivo Analyses. Pharmaceutics 2023, 15, 362. https://doi.org/10.3390/pharmaceutics15020362
Bhoopathy J, Dharmalingam S, Sathyaraj WV, Rajendran S, Rymbai S, Senthil R, Atchudan R. Sericin/Human Placenta-Derived Extracellular Matrix Scaffolds for Cutaneous Wound Treatment—Preparation, Characterization, In Vitro and In Vivo Analyses. Pharmaceutics. 2023; 15(2):362. https://doi.org/10.3390/pharmaceutics15020362
Chicago/Turabian StyleBhoopathy, Jayavardhini, Sankari Dharmalingam, Weslen Vedakumari Sathyaraj, Selvarajan Rajendran, Shibormi Rymbai, Rethinam Senthil, and Raji Atchudan. 2023. "Sericin/Human Placenta-Derived Extracellular Matrix Scaffolds for Cutaneous Wound Treatment—Preparation, Characterization, In Vitro and In Vivo Analyses" Pharmaceutics 15, no. 2: 362. https://doi.org/10.3390/pharmaceutics15020362
APA StyleBhoopathy, J., Dharmalingam, S., Sathyaraj, W. V., Rajendran, S., Rymbai, S., Senthil, R., & Atchudan, R. (2023). Sericin/Human Placenta-Derived Extracellular Matrix Scaffolds for Cutaneous Wound Treatment—Preparation, Characterization, In Vitro and In Vivo Analyses. Pharmaceutics, 15(2), 362. https://doi.org/10.3390/pharmaceutics15020362