Preclinical Evaluation of a Lead Specific Chelator (PSC) Conjugated to Radiopeptides for 203Pb and 212Pb-Based Theranostics
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials and Reagents
2.2. Synthesis of Lead Specific Chelator (PSC) and Chelator-Conjugated Peptides
2.3. Measurement of 212Pb Radioactivity
2.4. Radiolabeling
2.5. Stability of Radiometal Complex and Radiopeptides
2.6. Radio-iTLC and Radio-HPLC
2.7. In Vivo Biodistribution and SPECT Imaging of [212Pb]Pb-PSC-PEG-T
2.8. Statistics
3. Results
3.1. Calibration of HPGe and Dose Calibrator for 212Pb
3.2. Radiolabeling
3.3. Stability of Radiocomplex and Radiopeptides in Saline and Serum
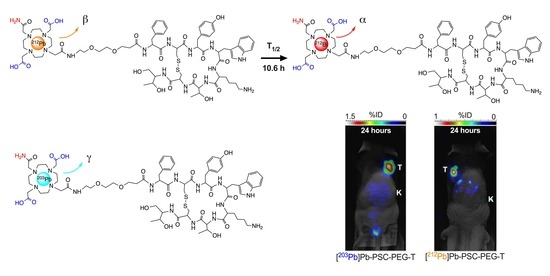
3.4. Biodistribution and Micro-SPECT Imaging
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Feijtel, D.; de Jong, M.; Nonnekens, J. Peptide receptor radionuclide therapy: Looking back, looking forward. Curr. Top. Med. Chem. 2020, 20, 2959–2969. [Google Scholar] [CrossRef] [PubMed]
- Sgouros, G.; Bodei, L.; McDevitt, M.R.; Nedrow, J.R. Radiopharmaceutical therapy in cancer: Clinical advances and challenges. Nat. Rev. Drug Discov. 2020, 19, 589–608. [Google Scholar] [CrossRef] [PubMed]
- Kwekkeboom, D.J.; Krenning, E.P. Peptide Receptor Radionuclide Therapy in the Treatment of Neuroendocrine Tumors. Hematol. Oncol. Clin. N. Am. 2016, 30, 179–191. [Google Scholar] [CrossRef] [PubMed]
- Otte, A. Neuroendocrine tumors: Peptide receptors radionuclide therapy (PRRT). Hell. J. Nucl. Med. 2016, 19, 182. [Google Scholar] [PubMed]
- Ladner, R.C.; Sato, A.K.; Gorzelany, J.; de Souza, M. Phage display-derived peptides as therapeutic alternatives to antibodies. Drug Discov. Today 2004, 9, 525–529. [Google Scholar] [CrossRef]
- Westerlund-Wikström, B. Peptide display on bacterial flagella: Principles and applications. Int. J. Med. Microbiol. 2000, 290, 223–230. [Google Scholar] [CrossRef]
- Lowman, H. Bacteriophage display and discovery of peptide leads for drug development. Annu. Rev. Biophys. Biomol. Struct. 1997, 26, 401–424. [Google Scholar] [CrossRef] [PubMed]
- Zalutsky, M.R. Targeted alpha-particle therapy of microscopic disease: Providing a further rationale for clinical investigation. J. Nucl. Med. 2006, 47, 1238–1240. [Google Scholar] [PubMed]
- Sgouros, G. Alpha-particles for targeted therapy. Adv. Drug Deliv. Rev. 2008, 60, 1402–1406. [Google Scholar] [CrossRef] [PubMed]
- Kratochwil, C.; Bruchertseifer, F.; Giesel, F.L.; Weis, M.; Verburg, F.A.; Mottaghy, F.; Kopka, K.; Apostolidis, C.; Haberkorn, U.; Morgenstern, A. 225Ac-PSMA-617 for PSMA targeting alpha-radiation therapy of patients with metastatic castration-resistant prostate cancer. J. Nucl. Med. 2016, 57, 1941–1944. [Google Scholar] [CrossRef] [Green Version]
- Sgouros, G.; Roeske, J.C.; McDevitt, M.R.; Palm, S.; Allen, B.J.; Fisher, D.R.; Brill, A.B.; Song, H.; Howell, R.W.; Akabani, G.; et al. MIRD Pamphlet No. 22 (abridged): Radiobiology and dosimetry of alpha-particle emitters for targeted radionuclide therapy. J. Nucl. Med. 2010, 51, 311–328. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Akabani, G.; Kennel, S.J.; Zalutsky, M.R. Microdosimetric analysis of alpha-particle-emitting targeted radiotherapeutics using histological images. J. Nucl. Med. 2003, 44, 792–805. [Google Scholar] [PubMed]
- Baidoo, K.E.; Yong, K.; Brechbiel, M.W. Molecular pathways: Targeted alpha-particle radiation therapy. Clin. Cancer Res. 2013, 19, 530–537. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zucchini, G.L.; Friedman, A.M. Isotopic generator for 212Pb and 212Bi. Int. J. Nucl. Med. Biol. 1982, 9, 83–84. [Google Scholar] [CrossRef] [PubMed]
- Hassfjell, S.P.; Hoff, P. A generator for production of 212Pb and 212Bi. Appl. Radiat. Isot. 1994, 45, 1021–1025. [Google Scholar] [CrossRef]
- Boldyrev, P.P.; Bortash, A.I.; Zagryadskii, V.A.; Zakharov, A.S.; Nikolaev, V.I.; Proshin, M.A.; Chuvilin, D.Y.; Shatrov, A.V.; Vesnovskii, S.P. Pb-212/Bi-212 GENERATOR FOR NUCLEAR MEDICINE. At. Energy+ 2012, 111, 422–427. [Google Scholar] [CrossRef]
- Booth, B.J.; Ramakrishnan, B.; Narayan, K.; Wollacott, A.M.; Babcock, G.J.; Shriver, Z.; Viswanathan, K. Extending human IgG half-life using structure-guided design. In MAbs; Taylor & Francis: Oxfordshire, UK, 2018; pp. 1098–1110. [Google Scholar] [CrossRef]
- Li, M.; Zhang, X.; Quinn, T.P.; Lee, D.; Liu, D.; Kunkel, F.; Zimmerman, B.E.; McAlister, D.; Olewein, K.; Menda, Y. Automated cassette-based production of high specific activity [203/212Pb] peptide-based theranostic radiopharmaceuticals for image-guided radionuclide therapy for cancer. Appl. Radiat. Isot. 2017, 127, 52–60. [Google Scholar] [CrossRef]
- Li, M.; Sagastume, E.E.; Lee, D.; McAlister, D.; DeGraffenreid, A.J.; Olewine, K.R.; Graves, S.; Copping, R.; Mirzadeh, S.; Zimmerman, B.E. 203/212Pb Theranostic Radiopharmaceuticals for Image-guided Radionuclide Therapy for Cancer. Curr. Med. Chem. 2020, 27, 7003–7031. [Google Scholar] [CrossRef]
- Bartoś, B.; Lyczko, K.; Kasperek, A.; Krajewski, S.; Bilewicz, A. Search of ligands suitable for 212 Pb/212 Bi in vivo generators. J. Radioanal. Nucl. Chem. 2013, 295, 205–209. [Google Scholar] [CrossRef] [Green Version]
- Henriksen, G.; Schoultz, B.W.; Hoff, P.; Larsen, R.H. Potential in vivo generator for alpha-particle therapy with 212Bi: Presentation of a system to minimize escape of daughter nuclide after decay of 212Pb to 212Bi. Radiochim. Acta 2003, 91, 109–114. [Google Scholar] [CrossRef]
- Mirzadeh, S.; Kumar, K.; Gansow, O.A. The chemical fate of 212 Bi-DOTA formed by β-decay of 212 Pb (DOTA) 2. Radiochim. Acta 1993, 60, 1–10. [Google Scholar] [CrossRef]
- Su, F.-M.; Beaumier, P.; Axworthy, D.; Atcher, R.; Fritzberg, A. Pretargeted radioimmunotherapy in tumored mice using an in vivo 212Pb/212Bi generator. Nucl. Med. Biol. 2005, 32, 741–747. [Google Scholar] [CrossRef] [PubMed]
- Zaid, N.R.; Kletting, P.; Winter, G.; Prasad, V.; Beer, A.J.; Glatting, G. A Physiologically Based Pharmacokinetic Model for In Vivo Alpha Particle Generators Targeting Neuroendocrine Tumors in Mice. Pharmaceutics 2021, 13, 2132. [Google Scholar] [CrossRef] [PubMed]
- Lee, D.; Li, M.; Bednarz, B.; Schultz, M.K. Modeling Cell and Tumor-Metastasis Dosimetry with the Particle and Heavy Ion Transport Code System (PHITS) Software for Targeted Alpha-Particle Radionuclide Therapy. Radiat. Res. 2018, 190, 236–247. [Google Scholar] [CrossRef]
- Ingham, A.; Kostelnik, T.I.; McNeil, B.L.; Patrick, B.O.; Choudhary, N.; de Guadalupe Jaraquemada-Peláez, M.; Orvig, C. Getting a lead on Pb 2+-amide chelators for 203/212 Pb radiopharmaceuticals. Dalton Trans. 2021, 50, 11579–11595. [Google Scholar] [CrossRef] [PubMed]
- Lange, J.L.; Davey, P.R.; Ma, M.T.; White, J.M.; Morgenstern, A.; Bruchertseifer, F.; Blower, P.J.; Paterson, B.M. An octadentate bis (semicarbazone) macrocycle: A potential chelator for lead and bismuth radiopharmaceuticals. Dalton Trans. 2020, 49, 14962–14974. [Google Scholar] [CrossRef] [PubMed]
- Miao, Y.; Hylarides, M.; Fisher, D.R.; Shelton, T.; Moore, H.; Wester, D.W.; Fritzberg, A.R.; Winkelmann, C.T.; Hoffman, T.; Quinn, T.P. Melanoma therapy via peptide-targeted α-radiation. Clin. Cancer Res. 2005, 11, 5616–5621. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- dos Santos, J.C.; Schäfer, M.; Bauder-Wüst, U.; Lehnert, W.; Leotta, K.; Morgenstern, A.; Kopka, K.; Haberkorn, U.; Mier, W.; Kratochwil, C. Development and dosimetry of 203 Pb/212 Pb-labelled PSMA ligands: Bringing “the lead” into PSMA-targeted alpha therapy? Eur. J. Nucl. Med. Mol. Imaging 2019, 46, 1081–1091. [Google Scholar] [CrossRef] [Green Version]
- Delpassand, E.S.; Tworowska, I.; Esfandiari, R.; Torgue, J.; Hurt, J.; Shafie, A.; Núñez, R. Targeted Alpha-Emitter Therapy With 212Pb-DOTAMTATE for the Treatment of Metastatic SSTR-Expressing Neuroendocrine Tumors: First-in-Human, Dose-Escalation Clinical Trial. J. Nucl. Med. 2022, 63 (Suppl. S9), 1326–1333. [Google Scholar] [CrossRef]
- Zaid, N.R.; Kletting, P.; Beer, A.J.; Rozgaja Stallons, T.A.; Torgue, J.J.; Glatting, G. Mathematical Modeling of In Vivo Alpha Particle Generators and Chelator Stability. Cancer Biother. Radiopharm. 2021. online ahead of print. [Google Scholar] [CrossRef]
- Maaland, A.F.; Saidi, A.; Torgue, J.; Heyerdahl, H.; Stallons, T.A.R.; Kolstad, A.; Dahle, J. Targeted alpha therapy for chronic lymphocytic leukaemia and non-Hodgkin’s lymphoma with the anti-CD37 radioimmunoconjugate 212Pb-NNV003. PLoS ONE 2020, 15, e0230526. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huff, E. TRU. Spec and RE. Spec chromatography: Basic studies and applications. In Proceedings of the 34th ORNL/DOE Conference on Analytical Chemistry in Energy Technology, Gatlinburg, TN, USA, 5–7 October 1993. [Google Scholar]
- Jussing, E.; Milton, S.; Samén, E.; Moein, M.M.; Bylund, L.; Axelsson, R.; Siikanen, J.; Tran, T.A. Clinically Applicable Cyclotron-Produced Gallium-68 Gives High-Yield Radiolabeling of DOTA-Based Tracers. Biomolecules 2021, 11, 1118. [Google Scholar] [CrossRef]
- Mueller, D.; Klette, I.; Baum, R.P.; Gottschaldt, M.; Schultz, M.K.; Breeman, W.A. Simplified NaCl based 68Ga concentration and labeling procedure for rapid synthesis of 68Ga radiopharmaceuticals in high radiochemical purity. Bioconjug. Chem. 2012, 23, 1712–1717. [Google Scholar] [CrossRef] [PubMed]
- Auranen, K.; McCutchan, E.A. Nuclear Data Sheets for A= 212. Nucl. Data Sheets 2020, 168, 117–267. [Google Scholar] [CrossRef]
- Martin, M. Nuclear data sheets for A = 208. Nucl. Data Sheets 2007, 108, 1583–1806. [Google Scholar] [CrossRef]
- Kailey, B.; van de Bunt, M.; Cheley, S.; Johnson, P.R.; MacDonald, P.E.; Gloyn, A.L.; Rorsman, P.; Braun, M. SSTR2 is the functionally dominant somatostatin receptor in human pancreatic β-and α-cells. Am. J. Physiol.-Endocrinol. Metab. 2012, 303, E1107–E1116. [Google Scholar] [CrossRef] [Green Version]
- Horwitz, E.P.; Dietz, M.L.; Rhoads, S.; Felinto, C.; Gale, N.H.; Houghton, J. A lead-selective extraction chromatographic resin and its application to the isolation of lead from geological samples. Anal. Chim. Acta 1994, 292, 263–273. [Google Scholar] [CrossRef]
- Li, M.; Liu, D.; Lee, D.; Cheng, Y.; Baumhover, N.J.; Marks, B.M.; Sagastume, E.A.; Ballas, Z.K.; Johnson, F.L.; Morris, Z.S. Targeted Alpha-Particle Radiotherapy and Immune Checkpoint Inhibitors Induces Cooperative Inhibition on Tumor Growth of Malignant Melanoma. Cancers 2021, 13, 3676. [Google Scholar] [CrossRef] [PubMed]
- Miao, Y.; Figueroa, S.D.; Fisher, D.R.; Moore, H.A.; Testa, R.F.; Hoffman, T.J.; Quinn, T.P. 203Pb-labeled alpha-melanocyte-stimulating hormone peptide as an imaging probe for melanoma detection. J. Nucl. Med. 2008, 49, 823–829. [Google Scholar] [CrossRef] [Green Version]
- Yang, J.; Xu, J.; Cheuy, L.; Gonzalez, R.; Fisher, D.R.; Miao, Y. Evaluation of a Novel Pb-203-Labeled Lactam-Cyclized Alpha-Melanocyte-Stimulating Hormone Peptide for Melanoma Targeting. Mol. Pharm. 2019, 16, 1694–1702. [Google Scholar] [CrossRef] [PubMed]
- Kang, C.S.; Song, H.A.; Milenic, D.E.; Baidoo, K.E.; Brechbiel, M.W.; Chong, H.-S. Preclinical evaluation of NETA-based bifunctional ligand for radioimmunotherapy applications using 212Bi and 213Bi: Radiolabeling, serum stability, and biodistribution and tumor uptake studies. Nucl. Med. Biol. 2013, 40, 600–605. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pearson, R.G. Hard and soft acids and bases, HSAB, part 1: Fundamental principles. J. Chem. Educ. 1968, 45, 581. [Google Scholar] [CrossRef]
- Ramler, J.; Lichtenberg, P.D.D.C. Molecular Bismuth Cations: Assessment of Soft Lewis Acidity. Chemistry 2020, 26, 10250. [Google Scholar] [CrossRef] [PubMed]
- Sadler, P.J.; Li, H.; Sun, H. Coordination chemistry of metals in medicine: Target sites for bismuth. Coord. Chem. Rev. 1999, 185, 689–709. [Google Scholar] [CrossRef]
- Cuenot, F.; Meyer, M.; Espinosa, E.; Bucaille, A.; Burgat, R.; Guilard, R.; Marichal-Westrich, C. New Insights into the Complexation of Lead (II) by 1, 4, 7, 10-Tetrakis (carbamoylmethyl)-1, 4, 7, 10-tetraazacyclododecane (DOTAM): Structural, Thermodynamic, and Kinetic Studies. Eur. J. Inorg. Chem. 2008, 2008, 267–283. [Google Scholar] [CrossRef]
- Nugent, J.W.; Lee, H.-S.; Reibenspies, J.H.; Hancock, R.D. Spectroscopic, structural, and thermodynamic aspects of the stereochemically active lone pair on lead (II): Structure of the lead (II) dota complex. Polyhedron 2015, 91, 120–127. [Google Scholar] [CrossRef]
- Filss, C.; Heinzel, A.; Miiller, B.; Vogg, A.T.; Langen, K.-J.; Mottaghy, F.M. Relevant tumor sink effect in prostate cancer patients receiving 177Lu-PSMA-617 radioligand therapy. Nuklearmedizin 2018, 57, 19–25. [Google Scholar] [CrossRef] [PubMed]
- Tuncel, M.; Telli, T.; Tuncalı, M.Ç.; Karabulut, E. Predictive factors of tumor sink effect: Insights from 177 Lu-Prostate-specific membrane antigen therapy. Ann. Nucl. Med. 2021, 35, 529–539. [Google Scholar] [CrossRef]
- Russ, G.A.; Bigler, R.E.; Tilbury, R.S.; Woodard, H.Q.; Laughlin, J.S. Metabolic Studies with Radiobismuth: I. Retention and Distribution of in the Normal Rat. Radiat. Res. 1975, 63, 443–454. [Google Scholar] [CrossRef]
- Stallons, T.A.R.; Saidi, A.; Tworowska, I.; Delpassand, E.S.; Torgue, J.J. Preclinical Investigation of (212)Pb-DOTAMTATE for Peptide Receptor Radionuclide Therapy in a Neuroendocrine Tumor Model. Mol. Cancer Ther. 2019, 18, 1012–1021. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nicolas, G.P.; Mansi, R.; McDougall, L.; Kaufmann, J.; Bouterfa, H.; Wild, D.; Fani, M. Biodistribution, pharmacokinetics, and dosimetry of 177Lu-, 90Y-, and 111In-labeled somatostatin receptor antagonist OPS201 in comparison to the agonist 177Lu-DOTATATE: The mass effect. J. Nucl. Med. 2017, 58, 1435–1441. [Google Scholar] [CrossRef] [Green Version]
- Koumarianou, E.; Pawlak, D.; Korsak, A.; Mikolajczak, R. Comparison of receptor affinity of nat Sc-DOTA-TATE versus nat Ga-DOTA-TATE. Nucl. Med. Rev. 2011, 14, 85–89. [Google Scholar] [CrossRef] [Green Version]
- Orcutt, K.D.; Henry, K.E.; Habjan, C.; Palmer, K.; Heimann, J.; Cupido, J.M.; Gottumukkala, V.; Cissell, D.D.; Lyon, M.C.; Hussein, A.I. Dosimetry of [212Pb] VMT01, a MC1R-Targeted Alpha Therapeutic Compound, and Effect of Free 208Tl on Tissue Absorbed Doses. Molecules 2022, 27, 5831. [Google Scholar] [CrossRef] [PubMed]






| Radionuclide | Half-Life | Decay Mode | Energy (Intensity) |
|---|---|---|---|
| Pb-203 | 51.9 h | ℇ (100%) | γ: 279 keV (81%) |
| Pb-212 | 10.6 h | β− (100%) | β−: 40.9 keV (5%); 93.3 keV (81.5%); 171.4 keV (13.7%) γ: 238 keV (46.3%) |
| Bi-212 | 60.6 min | β− (64.06%) α (35.94%) | β−: 833.9 keV (55.4%) α: 6050.8 keV (25.1%) |
| Po-212 | 0.3 µs | α (100%) | α: 8784.9 keV (25.1%) |
| Tl-208 | 3 min | β− (100%) | β−: 441.5 keV (24.2%); 535.4 keV (22.2%); 649.5 keV (13.7%) γ: 510.8 (22.6%); 583.2 keV (85%); 2614.5 keV (99.8%) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, M.; Baumhover, N.J.; Liu, D.; Cagle, B.S.; Boschetti, F.; Paulin, G.; Lee, D.; Dai, Z.; Obot, E.R.; Marks, B.M.; et al. Preclinical Evaluation of a Lead Specific Chelator (PSC) Conjugated to Radiopeptides for 203Pb and 212Pb-Based Theranostics. Pharmaceutics 2023, 15, 414. https://doi.org/10.3390/pharmaceutics15020414
Li M, Baumhover NJ, Liu D, Cagle BS, Boschetti F, Paulin G, Lee D, Dai Z, Obot ER, Marks BM, et al. Preclinical Evaluation of a Lead Specific Chelator (PSC) Conjugated to Radiopeptides for 203Pb and 212Pb-Based Theranostics. Pharmaceutics. 2023; 15(2):414. https://doi.org/10.3390/pharmaceutics15020414
Chicago/Turabian StyleLi, Mengshi, Nicholas J. Baumhover, Dijie Liu, Brianna S. Cagle, Frédéric Boschetti, Guillaume Paulin, Dongyoul Lee, Zhiming Dai, Ephraim R. Obot, Brenna M. Marks, and et al. 2023. "Preclinical Evaluation of a Lead Specific Chelator (PSC) Conjugated to Radiopeptides for 203Pb and 212Pb-Based Theranostics" Pharmaceutics 15, no. 2: 414. https://doi.org/10.3390/pharmaceutics15020414
APA StyleLi, M., Baumhover, N. J., Liu, D., Cagle, B. S., Boschetti, F., Paulin, G., Lee, D., Dai, Z., Obot, E. R., Marks, B. M., Okeil, I., Sagastume, E. A., Gabr, M., Pigge, F. C., Johnson, F. L., & Schultz, M. K. (2023). Preclinical Evaluation of a Lead Specific Chelator (PSC) Conjugated to Radiopeptides for 203Pb and 212Pb-Based Theranostics. Pharmaceutics, 15(2), 414. https://doi.org/10.3390/pharmaceutics15020414