Influence of Folate-Targeted Gold Nanoparticles on Subcellular Localization and Distribution into Lysosomes
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Synthesis of Folate-PEG3.5kDa-SH (FA-PEG3.5kDa-SH)
2.3. Synthesis of Rhodamine-PEG2kDa-SH (Rho-PEG2kDa-SH)
2.4. Synthesis of Folate-Pentane-Rhodamine (FA-C5-Rho)
2.5. Gold Nanoparticle (AuNP) Preparation
2.6. AuNP Surface Decoration Efficiency
2.7. Preparation of Fluorescent Folate Decorated AuNPs (Rho-FAx-AuNPs)
2.8. Decorated AuNP Size and Morphology
2.9. Transmission Electron Microscopy (TEM) Imaging
2.10. Cell Culture
2.11. AuNP Association with KBFR-high and MCF-7FR-low Cells
2.11.1. Atomic Absorption Spectroscopy
2.11.2. Flow Cytometric Analysis
2.11.3. Confocal Microscopy
2.11.4. Transmission Electron Microscopy (TEM) of Cells
2.12. Intracellular Trafficking Studies
2.12.1. Kinetic Analysis of AuNP Trafficking to Lysosomes
2.12.2. Uptake Pathway Inhibition Assay
2.13. Statistical Analysis
3. Results and Discussion
3.1. Synthesis and Characterization of Thiolated Derivatives for AuNP Decoration and Fluorescent Folate
3.1.1. Folate-PEG3.5kDa-SH Synthesis
3.1.2. Rho-PEG2kDa-SH Synthesis
3.1.3. Folate-1,5-diaminopentane-rhodamine (FA-C5-Rho) Synthesis
3.2. Gold Nanoparticle Production
3.3. AuNP Surface Decoration
3.4. Cell Association and Uptake Studies
3.5. Intracellular Trafficking Study
Lysosomal Disposition
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Attia, M.F.; Anton, N.; Wallyn, J.; Omran, Z.; Vandamme, T.F. An overview of active and passive targeting strategies to improve the nanocarriers efficiency to tumour sites. J. Pharm. Pharmacol. 2019, 71, 1185–1198. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rosenblum, D.; Joshi, N.; Tao, W.; Karp, J.M.; Peer, D. Progress and challenges towards targeted delivery of cancer therapeutics. Nat. Commun. 2018, 9, 1410. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kirpotin, D.B.; Drummond, D.C.; Shao, Y.; Shalaby, M.R.; Hong, K.; Nielsen, U.B.; Marks, J.D.; Benz, C.C.; Park, J.W. Antibody targeting of long-circulating lipidic nanoparticles does not increase tumor localization but does increase internalization in animal models. Cancer Res. 2006, 66, 6732–6740. [Google Scholar] [CrossRef] [Green Version]
- Austin, C.D.; De Mazière, A.M.; Pisacane, P.I.; van Dijk, S.M.; Eigenbrot, C.; Sliwkowski, M.X.; Klumperman, J.; Scheller, R.H. Endocytosis and sorting of ErbB2 and the site of action of cancer therapeutics trastuzumab and geldanamycin. Mol. Biol. Cell 2004, 15, 5268–5282. [Google Scholar] [CrossRef] [PubMed]
- Ren, X.-R.; Wei, J.; Lei, G.; Wang, J.; Lu, J.; Xia, W.; Spector, N.; Barak, L.S.; Clay, T.M.; Osada, T. Polyclonal HER2-specific antibodies induced by vaccination mediate receptor internalization and degradation in tumor cells. Breast Cancer Res. 2012, 14, R89. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hommelgaard, A.M.; Lerdrup, M.; van Deurs, B. Association with membrane protrusions makes ErbB2 an internalization-resistant receptor. Mol. Biol. Cell 2004, 15, 1557–1567. [Google Scholar] [CrossRef] [Green Version]
- Longva, K.E.; Pedersen, N.M.; Haslekås, C.; Stang, E.; Madshus, I.H. Herceptin-induced inhibition of ErbB2 signaling involves reduced phosphorylation of Akt but not endocytic down-regulation of ErbB2. Int. J. Cancer 2005, 116, 359–367. [Google Scholar] [CrossRef]
- Moody, P.R.; Sayers, E.J.; Magnusson, J.P.; Alexander, C.; Borri, P.; Watson, P.; Jones, A.T. Receptor crosslinking: A general method to trigger internalization and lysosomal targeting of therapeutic receptor: Ligand complexes. Mol. Ther. 2015, 23, 1888–1898. [Google Scholar] [CrossRef] [Green Version]
- Wymant, J.M.; Sayers, E.J.; Muir, D.; Jones, A.T. Strategic Trastuzumab Mediated Crosslinking Driving Concomitant HER2 and HER3 Endocytosis and Degradation in Breast Cancer. J. Cancer 2020, 11, 3288–3302. [Google Scholar] [CrossRef]
- Zhu, W.; Okollie, B.; Artemov, D. Controlled internalization of Her-2/neu receptors by cross-linking for targeted delivery. Cancer Biol. Ther. 2007, 6, 1960–1966. [Google Scholar] [CrossRef]
- Mayor, S.; Rothberg, K.G.; Maxfield, F.R. Sequestration of GPI-anchored proteins in caveolae triggered by cross-linking. Science 1994, 264, 1948–1951. [Google Scholar] [CrossRef]
- Montet, X.; Funovics, M.; Montet-Abou, K.; Weissleder, R.; Josephson, L. Multivalent effects of RGD peptides obtained by nanoparticle display. J. Med. Chem. 2006, 49, 6087–6093. [Google Scholar] [CrossRef] [PubMed]
- Elias, D.R.; Poloukhtine, A.; Popik, V.; Tsourkas, A. Effect of ligand density, receptor density, and nanoparticle size on cell targeting. Nanomed. Nanotechnol. Biol. Med. 2013, 9, 194–201. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhao, M.; Kircher, M.F.; Josephson, L.; Weissleder, R. Differential conjugation of tat peptide to superparamagnetic nanoparticles and its effect on cellular uptake. Bioconj. Chem. 2002, 13, 840–844. [Google Scholar] [CrossRef] [PubMed]
- Giljohann, D.A.; Seferos, D.S.; Patel, P.C.; Millstone, J.E.; Rosi, N.L.; Mirkin, C.A. Oligonucleotide loading determines cellular uptake of DNA-modified gold nanoparticles. Nano Lett. 2007, 7, 3818–3821. [Google Scholar] [CrossRef] [PubMed]
- Moradi, E.; Vllasaliu, D.; Garnett, M.; Falcone, F.; Stolnik, S. Ligand density and clustering effects on endocytosis of folate modified nanoparticles. RSC Adv. 2012, 2, 3025–3033. [Google Scholar] [CrossRef]
- Saha, A.; Basiruddin, S.K.; Maity, A.R.; Jana, N.R. Synthesis of nanobioconjugates with a controlled average number of biomolecules between 1 and 100 per nanoparticle and observation of multivalency dependent interaction with proteins and cells. Langmuir 2013, 29, 13917–13924. [Google Scholar] [CrossRef]
- Woythe, L.; Tito, N.B.; Albertazzi, L. A quantitative view on multivalent nanomedicine targeting. Adv. Drug Deliv. Rev. 2021, 169, 1–21. [Google Scholar] [CrossRef]
- Sapsford, K.E.; Algar, W.R.; Berti, L.; Gemmill, K.B.; Casey, B.J.; Oh, E.; Stewart, M.H.; Medintz, I.L. Functionalizing nanoparticles with biological molecules: Developing chemistries that facilitate nanotechnology. Chem. Rev. 2013, 113, 1904–2074. [Google Scholar]
- Rothberg, K.G.; Ying, Y.S.; Kolhouse, J.F.; Kamen, B.A.; Anderson, R.G. The glycophospholipid-linked folate receptor internalizes folate without entering the clathrin-coated pit endocytic pathway. J. Cell Biol. 1990, 110, 637–649. [Google Scholar] [CrossRef] [Green Version]
- Sabharanjak, S.; Sharma, P.; Parton, R.G.; Mayor, S. GPI-anchored proteins are delivered to recycling endosomes via a distinct cdc42-regulated, clathrin-independent pinocytic pathway. Dev. Cell 2002, 2, 411–423. [Google Scholar] [CrossRef] [Green Version]
- Sabharanjak, S.; Mayor, S. Folate receptor endocytosis and trafficking. Adv. Drug Deliv. Rev. 2004, 56, 1099–1109. [Google Scholar] [CrossRef] [PubMed]
- Slowing, I.; Trewyn, B.G.; Lin, V.S.Y. Effect of surface functionalization of MCM-41-type mesoporous silica nanoparticles on the endocytosis by human cancer cells. J. Am. Chem. Soc. 2006, 128, 14792–14793. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, H.; Lou, C.; Xu, M.; Wu, C.; Miyoshi, H.; Liu, Y. Investigation of folate-conjugated fluorescent silica nanoparticles for targeting delivery to folate receptor-positive tumors and their internalization mechanism. Int. J. Nanomed. 2011, 6, 2023. [Google Scholar]
- Niu, L.; Meng, L.; Lu, Q. Folate-Conjugated PEG on single walled carbon nanotubes for targeting delivery of doxorubicin to cancer cells. Macromol. Biosci. 2013, 13, 735–744. [Google Scholar] [CrossRef]
- Suen, W.-L.L.; Chau, Y. Size-dependent internalisation of folate-decorated nanoparticles via the pathways of clathrin and caveolae-mediated endocytosis in ARPE-19 cells. J. Pharm. Pharmacol. 2014, 66, 564–573. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.-L.; Van Cuong, N.; Hsieh, M.-F. Endocytosis pathways of the folate tethered star-shaped PEG-PCL micelles in cancer cell lines. Polymers 2014, 6, 634–650. [Google Scholar] [CrossRef] [Green Version]
- Cao, D.; Tian, S.; Huang, H.; Chen, J.; Pan, S. Divalent folate modification on PEG: An effective strategy for improving the cellular uptake and targetability of PEGylated polyamidoamine–polyethylenimine copolymer. Mol. Pharm. 2015, 12, 240–252. [Google Scholar] [CrossRef]
- Dalal, C.; Saha, A.; Jana, N.R. Nanoparticle Multivalency Directed Shifting of Cellular Uptake Mechanism. J. Phys. Chem. C 2016, 120, 6778–6786. [Google Scholar] [CrossRef] [Green Version]
- Brazzale, C.; Mastrotto, F.; Moody, P.; Watson, P.D.; Balasso, A.; Malfanti, A.; Mantovani, G.; Caliceti, P.; Alexander, C.; Jones, A.T. Control of targeting ligand display by pH-responsive polymers on gold nanoparticles mediates selective entry into cancer cells. Nanoscale 2017, 9, 11137–11147. [Google Scholar] [CrossRef]
- Sims, G.E.C.; Snape, T.J. A method for the estimation of polyethylene glycol in plasma protein fractions. Anal. Biochem. 1980, 107, 60–63. [Google Scholar] [CrossRef] [PubMed]
- Ellman, G.L. Tissue sulfhvdrvl sroups. Arch. Biochem. Biophys. 1959, 82, 72–77. [Google Scholar] [CrossRef] [PubMed]
- Turkevich, J.; Stevenson, P.C.; Hillier, J. The formation of colloidal gold. J. Phys. Chem. 1953, 57, 670–673. [Google Scholar] [CrossRef]
- Mastrotto, F.; Caliceti, P.; Amendola, V.; Bersani, S.; Magnusson, J.P.; Meneghetti, M.; Mantovani, G.; Alexander, C.; Salmaso, S. Polymer control of ligand display on gold nanoparticles for multimodal switchable cell targeting. Chem. Commun. 2011, 47, 9846–9848. [Google Scholar] [CrossRef]
- Liu, X.; Atwater, M.; Wang, J.; Huo, Q. Extinction coefficient of gold nanoparticles with different sizes and different capping ligands. Colloids Surf. B Biointerfaces 2007, 58, 3–7. [Google Scholar] [CrossRef]
- Gallon, E.; Matini, T.; Sasso, L.; Mantovani, G.; Armiñan de Benito, A.; Sanchis, J.; Caliceti, P.; Alexander, C.; Vicent, M.J.; Salmaso, S. Triblock copolymer nanovesicles for pH-responsive targeted delivery and controlled release of siRNA to cancer cells. Biomacromolecules 2015, 16, 1924–1937. [Google Scholar] [CrossRef]
- Roberts-Dalton, H.D.; Cocks, A.; Falcon-Perez, J.M.; Sayers, E.J.; Webber, J.P.; Watson, P.; Clayton, A.; Jones, A.T. Fluorescence labelling of extracellular vesicles using a novel thiol-based strategy for quantitative analysis of cellular delivery and intracellular traffic. Nanoscale 2017, 9, 13693–13706. [Google Scholar] [CrossRef]
- Matini, T.; Francini, N.; Battocchio, A.; Spain, S.G.; Mantovani, G.; Vicent, M.J.; Sanchis, J.; Gallon, E.; Mastrotto, F.; Salmaso, S.; et al. Synthesis and characterization of variable conformation pH responsive block co-polymers for nucleic acid delivery and targeted cell entry. Polym. Chem. 2014, 5, 1626–1636. [Google Scholar] [CrossRef] [Green Version]
- Leamon, C.P.; Low, P.S. Receptor-mediated drug delivery. In Drug Delivery: Principles and Applications; John Wiley & Sons: Hoboken, NJ, USA, 2005; pp. 167–187. [Google Scholar]
- Gabizon, A.; Horowitz, A.T.; Goren, D.; Tzemach, D.; Mandelbaum-Shavit, F.; Qazen, M.M.; Zalipsky, S. Targeting folate receptor with folate linked to extremities of poly (ethylene glycol)-grafted liposomes: In vitro studies. Bioconj. Chem. 1999, 10, 289–298. [Google Scholar] [CrossRef]
- Jain, P.K.; Lee, K.S.; El-Sayed, I.H.; El-Sayed, M.A. Calculated absorption and scattering properties of gold nanoparticles of different size, shape, and composition: Applications in biological imaging and biomedicine. J. Phys. Chem. B 2006, 110, 7238–7248. [Google Scholar] [CrossRef] [Green Version]
- Link, S.; El-Sayed, M.A. Spectral Properties and Relaxation Dynamics of Surface Plasmon Electronic Oscillations in Gold and Silver Nanodots and Nanorods; ACS Publications: Washington, DC, USA, 1999; Volume 103, pp. 8410–8426. [Google Scholar]
- Park, J.-W.; Shumaker-Parry, J.S. Structural study of citrate layers on gold nanoparticles: Role of intermolecular interactions in stabilizing nanoparticles. J. Am. Chem. Soc. 2014, 136, 1907–1921. [Google Scholar] [CrossRef] [PubMed]
- Ulman, A. Formation and structure of self-assembled monolayers. Chem. Rev. 1996, 96, 1533–1554. [Google Scholar] [CrossRef]
- Bakshi, A.K.; Haider, T.; Tiwari, R.; Soni, V. Critical parameters for design and development of multivalent nanoconstructs: Recent trends. Drug Deliv. Transl. Res. 2022, 12, 2335–2358. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Tian, S.; Petros, R.A.; Napier, M.E.; DeSimone, J.M. The complex role of multivalency in nanoparticles targeting the transferrin receptor for cancer therapies. J. Am. Chem. Soc. 2010, 132, 11306–11313. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, J.; Min, J.; Eghtesadi, S.A.; Kane, R.S.; Chilkoti, A. Quantitative study of the interaction of multivalent ligand-modified nanoparticles with breast cancer cells with tunable receptor density. ACS Nano 2020, 14, 372–383. [Google Scholar] [CrossRef] [PubMed]
- Schulze, H.; Kolter, T.; Sandhoff, K. Principles of lysosomal membrane degradation: Cellular topology and biochemistry of lysosomal lipid degradation. Biochim. Biophys. Acta (BBA)-Mol. Cell Res. 2009, 1793, 674–683. [Google Scholar] [CrossRef] [Green Version]
- Ballabio, A.; Bonifacino, J.S. Lysosomes as dynamic regulators of cell and organismal homeostasis. Nat. Rev. Mol. Cell Biol. 2020, 21, 101–118. [Google Scholar] [CrossRef]
- Wang, C.; Wang, Q.; Wang, H.; Li, Z.; Chen, J.; Zhang, Z.; Zeng, H.; Yu, X.; Yang, X.; Yang, X. Hydroxyethyl starch-folic acid conjugates stabilized theranostic nanoparticles for cancer therapy. J. Control. Release 2023, 353, 391–410. [Google Scholar] [CrossRef]
- Rana, S.; Shetake, N.G.; Barick, K.C.; Pandey, B.N.; Salunke, H.G.; Hassan, P.A. Folic acid conjugated Fe3 O4 magnetic nanoparticles for targeted delivery of doxorubicin. Dalton Trans. 2016, 45, 17401–17408. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Villeneuve, G.; Wang, Z. Control of epidermal growth factor receptor endocytosis by receptor dimerization, rather than receptor kinase activation. EMBO Rep. 2005, 6, 942–948. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pierre, C.A.S.; Leonard, D.; Corvera, S.; Kurt-Jones, E.A.; Finberg, R.W. Antibodies to cell surface proteins redirect intracellular trafficking pathways. Exp. Mol. Pathol. 2011, 91, 723–732. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, A.P.; Aguet, F.; Danuser, G.; Schmid, S.L. Local clustering of transferrin receptors promotes clathrin-coated pit initiation. J. Cell Biol. 2010, 191, 1381–1393. [Google Scholar] [CrossRef] [Green Version]
- Lee, R.J.; Low, P.S. Delivery of liposomes into cultured KB cells via folate receptor-mediated endocytosis. J. Biol. Chem. 1994, 269, 3198–3204. [Google Scholar] [CrossRef]
- Turek, J.J.; Leamon, C.P.; Low, P.S. Endocytosis of folate-protein conjugates: Ultrastructural localization in KB cells. J. Cell Sci. 1993, 106, 423–430. [Google Scholar] [CrossRef] [PubMed]
- Humphries Iv, W.H.; Szymanski, C.J.; Payne, C.K. Endo-lysosomal vesicles positive for Rab7 and LAMP1 are terminal vesicles for the transport of dextran. PLoS ONE 2011, 6, e26626. [Google Scholar] [CrossRef] [Green Version]
- Paulos, C.M.; Reddy, J.A.; Leamon, C.P.; Turk, M.J.; Low, P.S. Ligand binding and kinetics of folate receptor recycling in vivo: Impact on receptor-mediated drug delivery. Mol. Pharmacol. 2004, 66, 1406–1414. [Google Scholar] [CrossRef] [Green Version]
- Kirchhausen, T.; Macia, E.; Pelish, H.E. Use of dynasore, the small molecule inhibitor of dynamin, in the regulation of endocytosis. Methods Enzymol. 2008, 438, 77–93. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhai, G.; Wu, J.; Yu, B.; Guo, C.; Yang, X.; Lee, R.J. A transferrin receptor-targeted liposomal formulation for docetaxel. J. Nanosci. Nanotechnol. 2010, 10, 5129–5136. [Google Scholar] [CrossRef]
- Preta, G.; Cronin, J.G.; Sheldon, I.M. Dynasore-not just a dynamin inhibitor. Cell Commun. Signal. 2015, 13, 24. [Google Scholar] [CrossRef] [Green Version]
- Griffiths, G.; Gruenberg, J.; Marsh, M.; Wohlmann, J.; Jones, A.T.; Parton, R.G. Nanoparticle entry into cells; the cell biology weak link. Adv. Drug Deliv. Rev. 2022, 188, 114403. [Google Scholar] [CrossRef]
- Anderson, R.G.W. Potocytosis of small molecules and ions by caveolae. Trends Cell Biol. 1993, 3, 69–72. [Google Scholar] [CrossRef]
- Conner, S.D.; Schmid, S.L. Regulated portals of entry into the cell. Nature 2003, 422, 37–44. [Google Scholar] [CrossRef] [PubMed]
- Parton, R.G.; Del Pozo, M.A.; Vassilopoulos, S.; Nabi, I.R.; Le Lay, S.; Lundmark, R.; Kenworthy, A.K.; Camus, A.; Blouin, C.M.; Sessa, W.C. Caveolae: The FAQs. Traffic 2020, 21, 181–185. [Google Scholar] [CrossRef] [PubMed]
- Tao-Cheng, J.-H. Stimulation-induced differential redistributions of clathrin and clathrin-coated vesicles in axons compared to soma/dendrites. Mol. Brain 2020, 13, 141. [Google Scholar] [CrossRef] [PubMed]
- Suk, J.S.; Xu, Q.; Kim, N.; Hanes, J.; Ensign, L.M. PEGylation as a strategy for improving nanoparticle-based drug and gene delivery. Adv. Drug Deliv. Rev. 2016, 99, 28–51. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vermeulen, L.M.P.; Brans, T.; Samal, S.K.; Dubruel, P.; Demeester, J.; De Smedt, S.C.; Remaut, K.; Braeckmans, K. Endosomal size and membrane leakiness influence proton sponge-based rupture of endosomal vesicles. ACS Nano 2018, 12, 2332–2345. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sayers, E.J.; Peel, S.E.; Schantz, A.; England, R.M.; Beano, M.; Bates, S.M.; Desai, A.S.; Puri, S.; Ashford, M.B.; Jones, A.T. Endocytic profiling of cancer cell models reveals critical factors influencing LNP-mediated mRNA delivery and protein expression. Mol. Ther. 2019, 27, 1950–1962. [Google Scholar] [CrossRef] [PubMed]





 ) or not (
) or not ( ) of free folic acid. The data were normalized to the Mean Fluorescence Intensity value of cells incubated with particles without folic acid. Data are reported as the mean ± SD of three independent experiments performed in triplicate. Statistical significance: *** p < 0.001, **** p < 0.0001.
) of free folic acid. The data were normalized to the Mean Fluorescence Intensity value of cells incubated with particles without folic acid. Data are reported as the mean ± SD of three independent experiments performed in triplicate. Statistical significance: *** p < 0.001, **** p < 0.0001.
 ) or not (
) or not ( ) of free folic acid. The data were normalized to the Mean Fluorescence Intensity value of cells incubated with particles without folic acid. Data are reported as the mean ± SD of three independent experiments performed in triplicate. Statistical significance: *** p < 0.001, **** p < 0.0001.
) of free folic acid. The data were normalized to the Mean Fluorescence Intensity value of cells incubated with particles without folic acid. Data are reported as the mean ± SD of three independent experiments performed in triplicate. Statistical significance: *** p < 0.001, **** p < 0.0001.

 ) and Rho-FA50-AuNPs (
) and Rho-FA50-AuNPs ( ) within lysosomal compartments of KBFR-high cells over time. The data were normalized to the Mean Fluorescence intensity value of Rho-FA50-AuNPs at 4 h post-incubation. Error bars represent SD between mean normalized values of three independent experiments. Statistical analysis was performed for Rho-FA10-AuNPs vs Rho-FA50-AuNPs at each timepoint. Statistical significance: ns: p > 0.05, ** p < 0.01, *** p < 0.001.
) within lysosomal compartments of KBFR-high cells over time. The data were normalized to the Mean Fluorescence intensity value of Rho-FA50-AuNPs at 4 h post-incubation. Error bars represent SD between mean normalized values of three independent experiments. Statistical analysis was performed for Rho-FA10-AuNPs vs Rho-FA50-AuNPs at each timepoint. Statistical significance: ns: p > 0.05, ** p < 0.01, *** p < 0.001.
 ) and Rho-FA50-AuNPs (
) and Rho-FA50-AuNPs ( ) within lysosomal compartments of KBFR-high cells over time. The data were normalized to the Mean Fluorescence intensity value of Rho-FA50-AuNPs at 4 h post-incubation. Error bars represent SD between mean normalized values of three independent experiments. Statistical analysis was performed for Rho-FA10-AuNPs vs Rho-FA50-AuNPs at each timepoint. Statistical significance: ns: p > 0.05, ** p < 0.01, *** p < 0.001.
) within lysosomal compartments of KBFR-high cells over time. The data were normalized to the Mean Fluorescence intensity value of Rho-FA50-AuNPs at 4 h post-incubation. Error bars represent SD between mean normalized values of three independent experiments. Statistical analysis was performed for Rho-FA10-AuNPs vs Rho-FA50-AuNPs at each timepoint. Statistical significance: ns: p > 0.05, ** p < 0.01, *** p < 0.001.


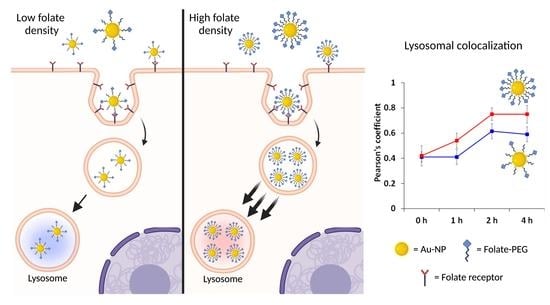
 ), Rho-FA50-AuNPs (
), Rho-FA50-AuNPs ( ) and the monovalent conjugate FA-C5-Rho (
) and the monovalent conjugate FA-C5-Rho ( ) with Dex-647 used as probe for lysosomes. Statistical analysis is reported in Table S2.
) with Dex-647 used as probe for lysosomes. Statistical analysis is reported in Table S2.
 ), Rho-FA50-AuNPs (
), Rho-FA50-AuNPs ( ) and the monovalent conjugate FA-C5-Rho (
) and the monovalent conjugate FA-C5-Rho ( ) with Dex-647 used as probe for lysosomes. Statistical analysis is reported in Table S2.
) with Dex-647 used as probe for lysosomes. Statistical analysis is reported in Table S2.
 ) or not (
) or not ( ) of Dynasore. The data were normalized to the Mean Fluorescence intensity value of cells non treated with Dynasore. Error bars represent SD between mean normalized values of three independent experiments. Statistical significance: ns: p > 0.05, **** p < 0.0001. (C–D’) TEM images of KBFR-high cells incubated with Rho-FA50-AuNPs. Red arrows point to AuNPs. Panel (C’,D’) are magnifications of the white boxes of panel (C,D), respectively. Black dots are AuNP.
) of Dynasore. The data were normalized to the Mean Fluorescence intensity value of cells non treated with Dynasore. Error bars represent SD between mean normalized values of three independent experiments. Statistical significance: ns: p > 0.05, **** p < 0.0001. (C–D’) TEM images of KBFR-high cells incubated with Rho-FA50-AuNPs. Red arrows point to AuNPs. Panel (C’,D’) are magnifications of the white boxes of panel (C,D), respectively. Black dots are AuNP.
 ) or not (
) or not ( ) of Dynasore. The data were normalized to the Mean Fluorescence intensity value of cells non treated with Dynasore. Error bars represent SD between mean normalized values of three independent experiments. Statistical significance: ns: p > 0.05, **** p < 0.0001. (C–D’) TEM images of KBFR-high cells incubated with Rho-FA50-AuNPs. Red arrows point to AuNPs. Panel (C’,D’) are magnifications of the white boxes of panel (C,D), respectively. Black dots are AuNP.
) of Dynasore. The data were normalized to the Mean Fluorescence intensity value of cells non treated with Dynasore. Error bars represent SD between mean normalized values of three independent experiments. Statistical significance: ns: p > 0.05, **** p < 0.0001. (C–D’) TEM images of KBFR-high cells incubated with Rho-FA50-AuNPs. Red arrows point to AuNPs. Panel (C’,D’) are magnifications of the white boxes of panel (C,D), respectively. Black dots are AuNP.
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Daniele, R.; Brazzale, C.; Arpac, B.; Tognetti, F.; Pesce, C.; Malfanti, A.; Sayers, E.; Mastrotto, F.; Jones, A.T.; Salmaso, S.; et al. Influence of Folate-Targeted Gold Nanoparticles on Subcellular Localization and Distribution into Lysosomes. Pharmaceutics 2023, 15, 864. https://doi.org/10.3390/pharmaceutics15030864
Daniele R, Brazzale C, Arpac B, Tognetti F, Pesce C, Malfanti A, Sayers E, Mastrotto F, Jones AT, Salmaso S, et al. Influence of Folate-Targeted Gold Nanoparticles on Subcellular Localization and Distribution into Lysosomes. Pharmaceutics. 2023; 15(3):864. https://doi.org/10.3390/pharmaceutics15030864
Chicago/Turabian StyleDaniele, Raffaella, Chiara Brazzale, Busra Arpac, Francesco Tognetti, Cristiano Pesce, Alessio Malfanti, Edward Sayers, Francesca Mastrotto, Arwyn T. Jones, Stefano Salmaso, and et al. 2023. "Influence of Folate-Targeted Gold Nanoparticles on Subcellular Localization and Distribution into Lysosomes" Pharmaceutics 15, no. 3: 864. https://doi.org/10.3390/pharmaceutics15030864
APA StyleDaniele, R., Brazzale, C., Arpac, B., Tognetti, F., Pesce, C., Malfanti, A., Sayers, E., Mastrotto, F., Jones, A. T., Salmaso, S., & Caliceti, P. (2023). Influence of Folate-Targeted Gold Nanoparticles on Subcellular Localization and Distribution into Lysosomes. Pharmaceutics, 15(3), 864. https://doi.org/10.3390/pharmaceutics15030864