Development and Characterization of Quercetin-Loaded Delivery Systems for Increasing Its Bioavailability in Cervical Cancer Cells
Abstract
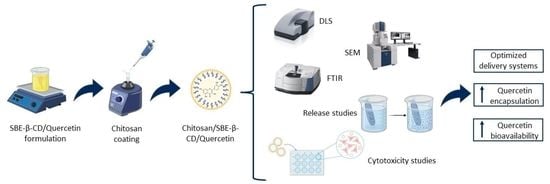
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
2.2.1. Preparation of SBE-β-CD/Quercetin Inclusion Complexes
2.2.2. Preparation of Chitosan/SBE-β-CD/Quercetin Delivery Systems
2.2.3. UV/vis Absorbance Spectrum
2.2.4. Physico-Chemical Characterization Studies
2.2.5. Encapsulation Efficiency
2.2.6. Scanning Electron Microscopy
2.2.7. Fourier Transform Infrared Spectroscopy
2.2.8. Release Studies
2.2.9. Cell Culture
2.2.10. Cell Internalization
2.2.11. Cell Viability Assays
2.2.12. Statistical Analysis
3. Results and Discussion
3.1. UV/vis Absorbance Spectrum
3.2. Characterization of SBE-β-CD/Quercetin Inclusion Complexes
3.3. Characterization of Chitosan/SBE-β-CD/Quercetin Delivery Systems
3.4. Scanning Electron Microscopy
3.5. Fourier Transform Infrared Spectroscopy
3.6. Release Studies
3.7. Cell Internlization Studies
3.8. Cell Viability Studies
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Dobrzynska, M.; Napierala, M.; Florek, E. Flavonoid Nanoparticles: A Promising Approach for Cancer Therapy. Biomolecules 2020, 10, 1268. [Google Scholar] [CrossRef] [PubMed]
- Madunić, J.; Madunić, I.V.; Gajski, G.; Popić, J.; Garaj-Vrhovac, V. Apigenin: A dietary flavonoid with diverse anticancer properties. Cancer Lett. 2018, 413, 11–22. [Google Scholar] [CrossRef]
- Imran, M.; Rauf, A.; Abu-Izneid, T.; Nadeem, M.; Shariati, M.A.; Khan, I.A.; Imran, A.; Orhan, I.E.; Rizwan, M.; Atif, M.; et al. Luteolin, a flavonoid, as an anticancer agent: A review. Biomed. Pharmacother. 2019, 112, 108612. [Google Scholar] [CrossRef]
- Franconi, R.; Massa, S.; Paolini, F.; Vici, P.; Venuti, A. Plant-Derived Natural Compounds in Genetic Vaccination and Therapy for HPV-Associated Cancers. Cancers 2020, 12, 3101. [Google Scholar] [CrossRef]
- Ferreira, M.; Costa, D.; Sousa, Â. Flavonoids-Based Delivery Systems towards Cancer Therapies. Bioengineering 2022, 9, 197. [Google Scholar] [CrossRef]
- Gomes, D.; Silvestre, S.; Duarte, A.P.; Venuti, A.; Soares, C.P.; Passarinha, L.; Sousa, Â. In Silico Approaches: A Way to Unveil Novel Therapeutic Drugs for Cervical Cancer Management. Pharmaceuticals 2021, 14, 741. [Google Scholar] [CrossRef]
- Song, Y.-K.; Yoon, J.-H.; Woo, J.K.; Kang, J.-H.; Lee, K.-R.; Oh, S.H.; Chung, S.-J.; Maeng, H.-J. Quercetin Is a Flavonoid Breast Cancer Resistance Protein Inhibitor with an Impact on the Oral Pharmacokinetics of Sulfasalazine in Rats. Pharmaceutics 2020, 12, 397. [Google Scholar] [CrossRef]
- Almeida, A.M.; Queiroz, J.A.; Sousa, F.; Sousa, Â. Cervical cancer and HPV infection: Ongoing therapeutic research to counteract the action of E6 and E7 oncoproteins. Drug Discov. Today 2019, 24, 2044–2057. [Google Scholar] [CrossRef]
- World Health Organization (WHO). GLOBOCAN 2020—The Global Cancer Observatory: Cancer Today—All Cancers; World Health Organization (WHO) International Agency for Research on Cancer: Lion, France, 2020; Available online: https://gco.iarc.fr/today/data/factsheets/cancers/39-All-cancers-fact-sheet.pdf (accessed on 16 December 2022).
- World Health Organization (WHO). GLOBOCAN 2020—The Global Cancer Observatory—Cancer Today Low Income; World Health Organization (WHO) International Agency for Research on Cancer: Lion, France, 2020; Available online: https://gco.iarc.fr/today/data/factsheets/populations/989-low-income-fact-sheets.pdf (accessed on 20 December 2022).
- Cordeiro, M.N.; Lima, R.D.C.P.D.; Paolini, F.; Melo, A.R.D.S.; Campos, A.P.F.; Venuti, A.; De Freitas, A.C. Current research into novel therapeutic vaccines against cervical cancer. Expert Rev. Anticancer. Ther. 2018, 18, 365–376. [Google Scholar] [CrossRef] [PubMed]
- Pal, A.; Kundu, R. Human Papillomavirus E6 and E7: The Cervical Cancer Hallmarks and Targets for Therapy. Front. Microbiol. 2020, 10, 3116. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Farthing, A.J.; Vousden, K.H. Functions of human papillomavirus E6 and E7 oncoproteins. Trends Microbiol. 1994, 2, 170–174. [Google Scholar] [CrossRef]
- Gomes, D.; Yaduvanshi, S.; Silvestre, S.; Duarte, A.P.; Santos, A.O.; Soares, C.P.; Kumar, V.; Passarinha, L.; Sousa, Â. Taxifolin and Lucidin as Potential E6 Protein Inhibitors: p53 Function Re-Establishment and Apoptosis Induction in Cervical Cancer Cells. Cancers 2022, 14, 2834. [Google Scholar] [CrossRef]
- Barra, F.; Lorusso, D.; Maggiore, U.L.R.; Ditto, A.; Bogani, G.; Raspagliesi, F.; Ferrero, S. Investigational drugs for the treatment of cervical cancer. Expert Opin. Investig. Drugs 2017, 26, 389–402. [Google Scholar] [CrossRef]
- Ordikhani, F.; Arslan, M.E.; Marcelo, R.; Sahin, I.; Grigsby, P.; Schwarz, J.K.; Azab, A.K. Drug Delivery Approaches for the Treatment of Cervical Cancer. Pharmaceutics 2016, 8, 23. [Google Scholar] [CrossRef]
- Yousefi, M.; Shadnoush, M.; Sohrabvandi, S.; Khorshidian, N.; Mortazavian, A.M. Encapsulation Systems for Delivery of Flavonoids: A Review. Biointerface Res. Appl. Chem. 2021, 11, 13934–13951. [Google Scholar] [CrossRef]
- Krauland, A.H.; Alonso, M.J. Chitosan/cyclodextrin nanoparticles as macromolecular drug delivery system. Int. J. Pharm. 2007, 340, 134–142. [Google Scholar] [CrossRef] [PubMed]
- Trapani, A.; Garcia-Fuentes, M.; Alonso, M.J. Novel drug nanocarriers combining hydrophilic cyclodextrins and chitosan. Nanotechnology 2008, 19, 185101. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nguyen, H.T.; Goycoolea, F.M. Chitosan/Cyclodextrin/TPP Nanoparticles Loaded with Quercetin as Novel Bacterial Quorum Sensing Inhibitors. Molecules 2017, 22, 1975. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fülöp, Z.; Saokham, P.; Loftsson, T. Sulfobutylether-β-cyclodextrin/chitosan nano- and microparticles and their physicochemical characteristics. Int. J. Pharm. 2014, 472, 282–287. [Google Scholar] [CrossRef] [PubMed]
- Sundararajan, M.; Thomas, P.A.; Venkadeswaran, K.; Jeganathan, K.; Geraldine, P. Synthesis and Characterization of Chrysin-Loaded β-Cyclodextrin-Based Nanosponges to Enhance In-Vitro Solubility, Photostability, Drug Release, Antioxidant Effects and Antitumorous Efficacy. J. Nanosci. Nanotechnol. 2017, 17, 8742–8751. [Google Scholar] [CrossRef]
- Konecsni, K.; Low, N.H.; Nickerson, M.T. Chitosan–tripolyphosphate submicron particles as the carrier of entrapped rutin. Food Chem. 2012, 134, 1775–1779. [Google Scholar] [CrossRef] [PubMed]
- Grabovac, V.; Guggi, D.; Bernkop-Schnürch, A. Comparison of the mucoadhesive properties of various polymers. Adv. Drug Deliv. Rev. 2005, 57, 1713–1723. [Google Scholar] [CrossRef] [PubMed]
- Bonferoni, M.C.; Sandri, G.; Rossi, S.; Ferrari, F.; Caramella, C. Chitosan and its salts for mucosal and transmucosal delivery. Expert Opin. Drug Deliv. 2009, 6, 923–939. [Google Scholar] [CrossRef]
- Rodolfo, C.; Eusébio, D.; Ventura, C.; Nunes, R.; Florindo, H.F.; Costa, D.; Sousa, Â. Design of Experiments to Achieve an Efficient Chitosan-Based DNA Vaccine Delivery System. Pharmaceutics 2021, 13, 1369. [Google Scholar] [CrossRef] [PubMed]
- Sun, M.; Nie, S.; Pan, X.; Zhang, R.; Fan, Z.; Wang, S. Quercetin-nanostructured lipid carriers: Characteristics and anti-breast cancer activities in vitro. Colloids Surfaces B: Biointerfaces 2014, 113, 15–24. [Google Scholar] [CrossRef]
- Ge, Y.; Zhang, Y.; He, S.; Nie, F.; Teng, G.; Gu, N. Fluorescence Modified Chitosan-Coated Magnetic Nanoparticles for High-Efficient Cellular Imaging. Nanoscale Res. Lett. 2009, 4, 287–295. [Google Scholar] [CrossRef] [Green Version]
- Rahimi, S.; Khoee, S.; Ghandi, M. Preparation and characterization of rod-like chitosan–quinoline nanoparticles as pH-responsive nanocarriers for quercetin delivery. Int. J. Biol. Macromol. 2019, 128, 279–289. [Google Scholar] [CrossRef]
- Simon, A.T.; Dutta, D.; Chattopadhyay, A.; Ghosh, S.S. Quercetin-Loaded Luminescent Hydroxyapatite Nanoparticles for Theranostic Application in Monolayer and Spheroid Cultures of Cervical Cancer Cell Line In Vitro. ACS Appl. Bio Mater. 2021, 4, 4495–4506. [Google Scholar] [CrossRef]
- D’Onofre Couto, B.; Novaes da Costa, R.; Castro Laurindo, W.; Moraes da Silva, H.; Rocha da Silva, C.; Sélia dos Reis Coimbra, J.; Barbosa Mageste, A.; de Cássia Dias, S.; José Boggione Santos, I. Characterization, Techno-Functional Properties, and Encapsulation Efficiency of Self-Assembled β-Lactoglobulin Nanostructures. Food Chem. 2021, 356, 129719. [Google Scholar] [CrossRef]
- Zhu, P.; Chen, L.; Zhao, Y.; Gao, C.; Yang, J.; Liao, X.; Liu, D.; Yang, B. A novel host-guest complex based on biotin functionalized polyamine-β-cyclodextrin for tumor targeted delivery of luteolin. J. Mol. Struct. 2021, 1237, 130339. [Google Scholar] [CrossRef]
- Trapani, A.; Lopedota, A.; Franco, M.; Cioffi, N.; Ieva, E.; Garcia-Fuentes, M.; Alonso, M.J. A comparative study of chitosan and chitosan/cyclodextrin nanoparticles as potential carriers for the oral delivery of small peptides. Eur. J. Pharm. Biopharm. 2010, 75, 26–32. [Google Scholar] [CrossRef]
- Prabha, S.; Arya, G.; Chandra, R.; Ahmed, B.; Nimesh, S. Effect of size on biological properties of nanoparticles employed in gene delivery. Artif. Cells Nanomed. Biotechnol. 2014, 44, 83–91. [Google Scholar] [CrossRef]
- Clogston, J.D.; Patri, A.K. Zeta potential measurement. Methods Mol. Biol. 2011, 697, 63–70. [Google Scholar] [CrossRef] [PubMed]
- Yue, Z.-G.; Wei, W.; Lv, P.-P.; Yue, H.; Wang, L.-Y.; Su, Z.-G.; Ma, G.-H. Surface Charge Affects Cellular Uptake and Intracellular Trafficking of Chitosan-Based Nanoparticles. Biomacromolecules 2011, 12, 2440–2446. [Google Scholar] [CrossRef]
- Marcucci, F.; Lefoulon, F. Active targeting with particulate drug carriers in tumor therapy: Fundamentals and recent progress. Drug Discov. Today 2004, 9, 219–228. [Google Scholar] [CrossRef] [PubMed]
- Zhao, F.; Zhao, Y.; Liu, Y.; Chang, X.; Chen, C.; Zhao, Y. Cellular Uptake, Intracellular Trafficking, and Cytotoxicity of Nanomaterials. Small 2011, 7, 1322–1337. [Google Scholar] [CrossRef]
- Harush-Frenkel, O.; Debotton, N.; Benita, S.; Altschuler, Y. Targeting of nanoparticles to the clathrin-mediated endocytic pathway. Biochem. Biophys. Res. Commun. 2007, 353, 26–32. [Google Scholar] [CrossRef] [PubMed]
- Zhang, K.; Fang, H.; Chen, Z.; Taylor, J.-S.A.; Wooley, K. Shape Effects of Nanoparticles Conjugated with Cell-Penetrating Peptides (HIV Tat PTD) on CHO Cell Uptake. Bioconjugate Chem. 2008, 19, 1880–1887. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chithrani, B.D.; Ghazani, A.A.; Chan, W.C.W. Determining the Size and Shape Dependence of Gold Nanoparticle Uptake into Mammalian Cells. Nano Lett. 2006, 6, 662–668. [Google Scholar] [CrossRef] [PubMed]
- Granja, A.; Lima-Sousa, R.; Alves, C.G.; de Melo-Diogo, D.; Pinheiro, M.; Sousa, C.T.; Correia, I.J.; Reis, S. Mitoxantrone-loaded lipid nanoparticles for breast cancer therapy–Quality-by-design approach and efficacy assessment in 2D and 3D in vitro cancer models. Int. J. Pharm. 2021, 607, 121044. [Google Scholar] [CrossRef]
- Amaro-Gahete, J.; Benítez, A.; Otero, R.; Esquivel, D.; Jiménez-Sanchidrián, C.; Morales, J.; Caballero, Á.; Romero-Salguero, F.J. A Comparative Study of Particle Size Distribution of Graphene Nanosheets Synthesized by an Ultrasound-Assisted Method. Nanomaterials 2019, 9, 152. [Google Scholar] [CrossRef] [Green Version]
- Yadav, N.; Tripathi, A.K.; Parveen, A. PLGA-Quercetin Nano-Formulation Inhibits Cancer Progression via Mitochondrial Dependent Caspase-3,7 and Independent FoxO1 Activation with Concomitant PI3K/AKT Suppression. Pharmaceutics 2022, 14, 1326. [Google Scholar] [CrossRef] [PubMed]
- Patel, A.R.; Heussen, P.C.M.; Hazekamp, J.; Drost, E.; Velikov, K.P. Quercetin loaded biopolymeric colloidal particles prepared by simultaneous precipitation of quercetin with hydrophobic protein in aqueous medium. Food Chem. 2012, 133, 423–429. [Google Scholar] [CrossRef]
- Ibrahim, Y.H.-E.; Regdon, G.; Kristó, K.; Kelemen, A.; Adam, M.E.; Hamedelniel, E.I.; Sovány, T. Design and characterization of chitosan/citrate films as carrier for oral macromolecule delivery. Eur. J. Pharm. Sci. 2020, 146, 105270. [Google Scholar] [CrossRef] [PubMed]
- Souza, M.P.; Vaz, A.F.M.; Correia, M.T.S.; Cerqueira, M.A.; Vicente, A.A.; Carneiro-da-Cunha, M.G. Quercetin-Loaded Lecithin/Chitosan Nanoparticles for Functional Food Applications. Food Bioprocess Technol. 2014, 7, 1149–1159. [Google Scholar] [CrossRef] [Green Version]
- Yang, Z.; Peng, H.; Wang, W.; Liu, T. Crystallization Behavior of Poly(ε-Caprolactone)/Layered Double Hydroxide Nanocomposites. J. Appl. Polym. Sci. 2010, 116, 2658–2667. [Google Scholar] [CrossRef]
- Deepika, M.S.; Thangam, R.; Sheena, T.S.; Sasirekha, R.; Sivasubramanian, S.; Babu, M.D.; Jeganathan, K.; Thirumurugan, R. A novel rutin-fucoidan complex based phytotherapy for cervical cancer through achieving enhanced bioavailability and cancer cell apoptosis. Biomed. Pharmacother. 2018, 109, 1181–1195. [Google Scholar] [CrossRef] [PubMed]
- Kumari, P.; Paul, M.; Bobde, Y.; Soniya, K.; Rompicharla, S.V.K.; Ghosh, B.; Biswas, S. Albumin-based lipoprotein nanoparticles for improved delivery and anticancer activity of curcumin for cancer treatment. Nanomedicine 2020, 15, 2851–2869. [Google Scholar] [CrossRef]
- Li, J.; Shi, M.; Ma, B.; Niu, R.; Zhang, H.; Kun, L. Antitumor activity and safety evaluation of nanaparticle-based delivery of quercetin through intravenous administration in mice. Mater. Sci. Eng. C 2017, 77, 803–810. [Google Scholar] [CrossRef] [PubMed]
- Saraswat, A.L.; Maher, T.J. Development and optimization of stealth liposomal system for enhanced in vitro cytotoxic effect of quercetin. J. Drug Deliv. Sci. Technol. 2020, 55, 101477. [Google Scholar] [CrossRef]
- Van Thoai, D.; Nguyen, D.T.; Dang, L.H.; Nguyen, N.H.; Nguyen, V.T.; Doan, P.; Nguyen, B.T.; Van Thu, L.; Tung, N.N.; Quyen, T.N. Lipophilic effect of various pluronic-grafted gelatin copolymers on the quercetin delivery efficiency in these self-assembly nanogels. J. Polym. Res. 2020, 27, 369. [Google Scholar] [CrossRef]
- Ni, S.; Hu, C.; Sun, R.; Zhao, G.; Xia, Q. Nanoemulsions-Based Delivery Systems for Encapsulation of Quercetin: Preparation, Characterization, and Cytotoxicity Studies. J. Food Process. Eng. 2017, 40, e12374. [Google Scholar] [CrossRef]
- Ghafelehbashi, R.; Yaraki, M.T.; Saremi, L.H.; Lajevardi, A.; Haratian, M.; Astinchap, B.; Rashidi, A.M.; Moradian, R. A pH-responsive citric-acid/α-cyclodextrin-functionalized Fe3O4 nanoparticles as a nanocarrier for quercetin: An experimental and DFT study. Mater. Sci. Eng. C 2020, 109, 110597. [Google Scholar] [CrossRef]
- Malekzadeh, A.M.; Ramazani, A.; Rezaei, S.J.T.; Niknejad, H. Design and construction of multifunctional hyperbranched polymers coated magnetite nanoparticles for both targeting magnetic resonance imaging and cancer therapy. J. Colloid Interface Sci. 2017, 490, 64–73. [Google Scholar] [CrossRef]
- Akal, Z.; Alpsoy, L.; Baykal, A. Biomedical applications of SPION@APTES@PEG-folic acid@carboxylated quercetin nanodrug on various cancer cells. Appl. Surf. Sci. 2016, 378, 572–581. [Google Scholar] [CrossRef]
- Alpsoy, L.; Baykal, A.; Kurtan, U.; Ülker, Z. Synthesis and Characterization of Carboxylated Luteolin (CL)-Functionalized SPION. J. Supercond. Nov. Magn. 2017, 30, 2797–2804. [Google Scholar] [CrossRef]
- Yu, C.; Gao, C.; Lü, S.; Chen, C.; Huang, Y.; Liu, M. Redox-responsive shell-sheddable micelles self-assembled from amphiphilic chondroitin sulfate-cholesterol conjugates for triggered intracellular drug release. Chem. Eng. J. 2013, 228, 290–299. [Google Scholar] [CrossRef]






| SBE-β-CD Concentration (mg/mL) | Size (nm) | PdI | Zeta Potential (mV) |
|---|---|---|---|
| 6 | 2782.5 ± 974.6 | 1 | ND |
| 3 | 2468.33 ± 207.4 | 0.123 ± 0.019 | −21.03 ± 0.723 |
| 1.5 | 1758 ± 410.6 | 1 | ND |
| Type of Chitosan | Chitosan Concentration (mg/mL) | Size (nm) | PdI | Zeta Potential (mV) |
|---|---|---|---|---|
| HMW | 2 | 2714.67 ± 927.87 | 1 | ND |
| 1.5 | 1379.5 ± 164.76 | 1 | ND | |
| 1 | 272.07 ± 2.87 | 0.287 ± 0.011 | +38 ± 1.34 | |
| 0.9 | 299.6 ± 10.6 | 0.302 ± 0.032 | +41.1 ± 1.85 | |
| 0.75 | 300.8 ± 4.291 | 0.249 ± 0.018 | +42.7 ± 0.62 | |
| 0.6 | 296.7 ± 14.03 | 0.248 ± 0.014 | +39.4 ± 3.99 | |
| 0.5 | 328.8 ± 19.37 | 0.218 ± 0.021 | +38.1 ± 5.35 | |
| LMW | 1 | 302.0 ± 17.80 | 0.285 ± 0.032 | +47.4 ± 1.88 |
| 0.9 | 275.9 ± 25.07 | 0.255 ± 0.044 | +46.3 ± 1.35 | |
| 0.75 | 269.0 ± 17.63 | 0.152 ± 0.092 | +42.3 ± 1.35 | |
| 0.6 | 1014 ± 776.8 | 0.732 ± 0.379 | ND | |
| 5 kDa | 1 | 282.6 ± 10.76 | 0.056 ± 0.030 | +39.5 ± 2.01 |
| 0.9 | 1002 ± 17.24 | 0.186 ± 0.022 | ND | |
| 0.75 | 18,920 ± 11,110 | 0.439 ± 0.487 | ND |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ferreira, M.; Gomes, D.; Neto, M.; Passarinha, L.A.; Costa, D.; Sousa, Â. Development and Characterization of Quercetin-Loaded Delivery Systems for Increasing Its Bioavailability in Cervical Cancer Cells. Pharmaceutics 2023, 15, 936. https://doi.org/10.3390/pharmaceutics15030936
Ferreira M, Gomes D, Neto M, Passarinha LA, Costa D, Sousa Â. Development and Characterization of Quercetin-Loaded Delivery Systems for Increasing Its Bioavailability in Cervical Cancer Cells. Pharmaceutics. 2023; 15(3):936. https://doi.org/10.3390/pharmaceutics15030936
Chicago/Turabian StyleFerreira, Miguel, Diana Gomes, Miguel Neto, Luís A. Passarinha, Diana Costa, and Ângela Sousa. 2023. "Development and Characterization of Quercetin-Loaded Delivery Systems for Increasing Its Bioavailability in Cervical Cancer Cells" Pharmaceutics 15, no. 3: 936. https://doi.org/10.3390/pharmaceutics15030936
APA StyleFerreira, M., Gomes, D., Neto, M., Passarinha, L. A., Costa, D., & Sousa, Â. (2023). Development and Characterization of Quercetin-Loaded Delivery Systems for Increasing Its Bioavailability in Cervical Cancer Cells. Pharmaceutics, 15(3), 936. https://doi.org/10.3390/pharmaceutics15030936