Efficient Delivery of Gemcitabine by Estrogen Receptor-Targeted PEGylated Liposome and Its Anti-Lung Cancer Activity In Vivo and In Vitro
Abstract
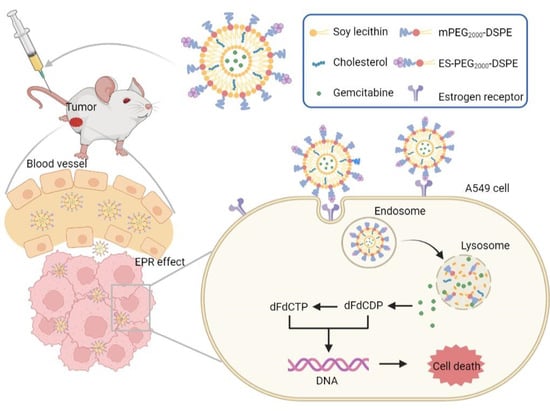
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Cell Lines and Animals
2.3. Preparation of GEM Liposomes
2.4. Characterization of Liposomes
2.5. In Vitro Stability of Liposomes
2.6. Drug Release Behavior
2.7. Cytotoxicity Assay
2.8. Cellular Uptake
2.9. Endocytosis Mechanism
2.10. In Vivo Tumor-Targeting Study
2.11. In Vivo Antitumor Efficacy
2.12. Statistical Analysis
3. Results and Discussion
3.1. Characterization of Liposomes
3.2. In Vitro Stability of Liposomes
3.3. Drug Release Behavior
3.4. Cytotoxicity Assay
3.5. Cellular Uptake
3.6. Endocytosis Mechanism
3.7. In Vivo Tumor-Targeting Study
3.8. In Vivo Antitumor Efficacy
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ruiz-Cordero, R.; Devine, W.P. Targeted Therapy and Checkpoint Immunotherapy in Lung Cancer. Surg. Pathol. Clin. 2020, 13, 17–33. [Google Scholar] [CrossRef] [PubMed]
- Bray, F.; Ferlay, J.; Soerjomataram, I.; Siegel, R.L.; Torre, L.A.; Jemal, A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2018, 68, 394–424. [Google Scholar] [CrossRef] [Green Version]
- Chakraborty, S.; Nath, D.; Barbhuiya, P.A.; Choudhury, Y.; Uddin, A. Silencing lung cancer genes using miRNAs identified by 7mer-seed matching. Comput. Biol. Chem. 2021, 92, 107483. [Google Scholar] [CrossRef] [PubMed]
- Nagasaka, M.; Gadgeel, S.M. Role of chemotherapy and targeted therapy in early-stage non-small cell lung cancer. Expert Rev. Anticancer. Ther. 2018, 18, 63–70. [Google Scholar] [CrossRef]
- An, Q.; Shi, C.-X.; Guo, H.; Xie, S.-M.; Yang, Y.-Y.; Liu, Y.-N.; Liu, Z.-H.; Zhou, C.-Z.; Niu, F.-J. Development and characterization of octreotide-modified curcumin plus docetaxel micelles for potential treatment of non-small-cell lung cancer. Pharm. Dev. Technol. 2019, 24, 1164–1174. [Google Scholar] [CrossRef] [PubMed]
- Du, B.; Wen, X.; Wang, Y.; Lin, M.; Lai, J. Gemcitabine and checkpoint blockade exhibit synergistic anti-tumor effects in a model of murine lung carcinoma. Int. Immunopharmacol. 2020, 86, 106694. [Google Scholar] [CrossRef]
- Duma, N.; Santana-Davila, R.; Molina, J.R. Non-Small Cell Lung Cancer: Epidemiology, Screening, Diagnosis, and Treatment. Mayo Clin. Proc. 2019, 94, 1623–1640. [Google Scholar] [CrossRef] [PubMed]
- Schiller, J.H.; Harrington, D.; Belani, C.P.; Langer, C.; Sandler, A.; Krook, J.; Zhu, J.; Johnson, D.H. Comparison of Four Chemotherapy Regimens for Advanced Non–Small-Cell Lung Cancer. N. Engl. J. Med. 2002, 346, 92–98. [Google Scholar] [CrossRef]
- Hatami, E.; Nagesh, P.K.; Jaggi, M.; Chauhan, S.C.; Yallapu, M.M. Gambogic acid potentiates gemcitabine induced anticancer activity in non-small cell lung cancer. Eur. J. Pharmacol. 2020, 888, 173486. [Google Scholar] [CrossRef] [PubMed]
- Hayashi, H.; Kurata, T.; Nakagawa, K. Gemcitabine: Efficacy in the Treatment of Advanced Stage Nonsquamous Non-Small Cell Lung Cancer. Clin. Med. Insights Oncol. 2011, 5, 177–184. [Google Scholar] [CrossRef] [PubMed]
- Mini, E.; Nobili, S.; Caciagli, B.; Landini, I.; Mazzei, T. Cellular pharmacology of gemcitabine. Ann. Oncol. 2006, 17 (Suppl. S5), v7–v12. [Google Scholar] [CrossRef]
- Sun, X.; Xu, X.; Chen, Y.; Guan, R.; Cheng, T.; Wang, Y.; Jin, R.; Song, M.; Hang, T. Danggui Buxue Decoction Sensitizes the Response of Non-Small-Cell Lung Cancer to Gemcitabine via Regulating Deoxycytidine Kinase and P-glycoprotein. Molecules 2019, 24, 2011. [Google Scholar] [CrossRef] [Green Version]
- Delaney, L.J.; Eisenbrey, J.R.; Brown, D.; Brody, J.R.; Jimbo, M.; Oeffinger, B.E.; Stanczak, M.; Forsberg, F.; Liu, J.-B.; Wheatley, M.A. Gemcitabine-loaded microbubble system for ultrasound imaging and therapy. Acta Biomater. 2021, 130, 385–394. [Google Scholar] [CrossRef]
- Garrido-Laguna, I.; Hidalgo, M. Pancreatic cancer: From state-of-the-art treatments to promising novel therapies. Nat. Rev. Clin. Oncol. 2015, 12, 319–334. [Google Scholar] [CrossRef]
- Reddy, L.H.; Couvreur, P. Novel Approaches to Deliver Gemcitabine to Cancers. Curr. Pharm. Des. 2008, 14, 1124–1137. [Google Scholar] [CrossRef] [PubMed]
- Youngren-Ortiz, S.R.; Hill, D.B.; Hoffmann, P.R.; Morris, K.R.; Barrett, E.G.; Forest, M.G.; Chougule, M.B. Development of Optimized, Inhalable, Gemcitabine-Loaded Gelatin Nanocarriers for Lung Cancer. J. Aerosol Med. Pulm. Drug Deliv. 2017, 30, 299–321. [Google Scholar] [CrossRef]
- Yu, Q.; Qiu, Y.; Li, J.; Tang, X.; Wang, X.; Cun, X.; Xu, S.; Liu, Y.; Li, M.; Zhang, Z.; et al. Targeting cancer-associated fibroblasts by dual-responsive lipid-albumin nanoparticles to enhance drug perfusion for pancreatic tumor therapy. J. Control. Release 2020, 321, 564–575. [Google Scholar] [CrossRef]
- Duan, H.; Liu, C.; Hou, Y.; Liu, Y.; Zhang, Z.; Zhao, H.; Xin, X.; Liu, W.; Zhang, X.; Chen, L.; et al. Sequential Delivery of Quercetin and Paclitaxel for the Fibrotic Tumor Microenvironment Remodeling and Chemotherapy Potentiation via a Dual-Targeting Hybrid Micelle-in-Liposome System. ACS Appl. Mater. Interfaces 2022, 14, 10102–10116. [Google Scholar] [CrossRef] [PubMed]
- Lai, P.-S.; Lou, P.-J.; Peng, C.-L.; Pai, C.-L.; Yen, W.-N.; Huang, M.-Y.; Young, T.-H.; Shieh, M.-J. Doxorubicin delivery by polyamidoamine dendrimer conjugation and photochemical internalization for cancer therapy. J. Control. Release 2007, 122, 39–46. [Google Scholar] [CrossRef] [PubMed]
- Din, F.; Aman, W.; Ullah, I.; Qureshi, O.S.; Mustapha, O.; Shafique, S.; Zeb, A. Effective use of nanocarriers as drug delivery systems for the treatment of selected tumors. Int. J. Nanomed. 2017, 12, 7291–7309. [Google Scholar] [CrossRef] [Green Version]
- Zhang, L.; Kong, L.; He, S.-Y.; Liu, X.-Z.; Liu, Y.; Zang, J.; Ju, R.-J.; Li, X.-T. The anti-ovarian cancer effect of RPV modified paclitaxel plus schisandra B liposomes in SK-OV-3 cells and tumor-bearing mice. Life Sci. 2021, 285, 120013. [Google Scholar] [CrossRef] [PubMed]
- Yu, J.; Wang, Y.; Zhou, S.; Li, J.; Wang, J.; Chi, D.; Wang, X.; Lin, G.; He, Z.; Wang, Y. Remote loading paclitaxel–doxorubicin prodrug into liposomes for cancer combination therapy. Acta Pharm. Sin. B 2020, 10, 1730–1740. [Google Scholar] [CrossRef] [PubMed]
- Nunes, S.S.; Fernandes, R.S.; Cavalcante, C.H.; César, I.D.C.; Leite, E.A.; Lopes, S.C.A.; Ferretti, A.; Rubello, D.; Townsend, D.M.; de Oliveira, M.C.; et al. Influence of PEG coating on the biodistribution and tumor accumulation of pH-sensitive liposomes. Drug Deliv. Transl. Res. 2019, 9, 123–130. [Google Scholar] [CrossRef]
- Mohamed, M.; Abu Lila, A.S.; Shimizu, T.; Alaaeldin, E.; Hussein, A.; Sarhan, H.A.; Szebeni, J.; Ishida, T. PEGylated liposomes: Immunological responses. Sci. Technol. Adv. Mater. 2019, 20, 710–724. [Google Scholar] [CrossRef] [Green Version]
- Mo, L.; Song, J.G.; Lee, H.; Zhao, M.; Kim, H.Y.; Lee, Y.J.; Ko, H.W.; Han, H.-K. PEGylated hyaluronic acid-coated liposome for enhanced in vivo efficacy of sorafenib via active tumor cell targeting and prolonged systemic exposure. Nanomedicine 2018, 14, 557–567. [Google Scholar] [CrossRef]
- Choi, J.H.; Lee, J.S.; Park, K.M.; Bae, J.W.; Lee, Y.; Park, K.D. Multi-layered nanogels with MMP-sheddable PEG masks: Preparation and promotion of tumor cell uptake by controlling surface characteristics. Colloids Surf. B Biointerfaces 2017, 156, 71–78. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Chen, W.; Li, H.; Huang, G.; Zhou, Y.; Wang, Y.; Wan, W.; You, B.; Liu, Y.; Zhang, X. Folate-receptor mediated pH/reduction-responsive biomimetic nanoparticles for dually activated multi-stage anticancer drug delivery. Int. J. Pharm. 2020, 585, 119456. [Google Scholar] [CrossRef] [PubMed]
- Li, N.; Xie, X.; Hu, Y.; He, H.; Fu, X.; Fang, T.; Li, C. Herceptin-conjugated liposomes co-loaded with doxorubicin and simvastatin in targeted prostate cancer therapy. Am. J. Transl. Res. 2019, 11, 1255–1269. [Google Scholar]
- Chen, T.; Gong, T.; Zhao, T.; Fu, Y.; Zhang, Z.; Gong, T. A comparison study between lycobetaine-loaded nanoemulsion and liposome using nRGD as therapeutic adjuvant for lung cancer therapy. Eur. J. Pharm. Sci. 2018, 111, 293–302. [Google Scholar] [CrossRef]
- Brzozowski, A.M.; Pike, A.C.; Dauter, Z.; Hubbard, R.E.; Bonn, T.; Engström, O.; Öhman, L.; Greene, G.L.; Gustafsson, J.A.; Carlquist, M. Molecular basis of agonism and antagonism in the oestrogen receptor. Nature 1997, 389, 753–758. [Google Scholar] [CrossRef]
- Yang, X.; Xue, L.Y.; Liu, Y.; Guo, L.; Wen, P.; Lin, D. Clinicopathological and prognostic significance of ERα and ERβ expression in lung carcinomas: A tissue microarray study. Chin. J. Oncol. 2013, 35, 678–683. [Google Scholar]
- Marquez-Garban, D.C.; Mah, V.; Alavi, M.; Maresh, E.L.; Chen, H.-W.; Bagryanova, L.; Horvath, S.; Chia, D.; Garon, E.; Goodglick, L.; et al. Progesterone and estrogen receptor expression and activity in human non-small cell lung cancer. Steroids 2011, 76, 910–920. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Baik, C.S.; Eaton, K.D. Estrogen Signaling in Lung Cancer: An Opportunity for Novel Therapy. Cancers 2012, 4, 969–988. [Google Scholar] [CrossRef] [Green Version]
- Hao, Q.; Xu, G.; Yang, Y.; Sun, Y.; Cong, D.; Li, H.; Liu, X.; Wang, Z.; Zhang, Z.; Chen, J.; et al. Oestrone-targeted liposomes for mitoxantrone delivery via oestrogen receptor—Synthesis, physicochemical characterization and in-vitro evaluation. J. Pharm. Pharmacol. 2017, 69, 991–1001. [Google Scholar] [CrossRef] [PubMed]
- Karuppaiah, A.; Babu, D.; Selvaraj, D.; Natrajan, T.; Rajan, R.; Gautam, M.; Ranganathan, H.; Siram, K.; Nesamony, J.; Sankar, V. Building and behavior of a pH-stimuli responsive chitosan nanoparticles loaded with folic acid conjugated gemcitabine silver colloids in MDA-MB-453 metastatic breast cancer cell line and pharmacokinetics in rats. Eur. J. Pharm. Sci. 2021, 165, 105938. [Google Scholar] [CrossRef]
- Yao, X.-M.; Niu, F.-J.; Kong, L.; Cai, F.-Y.; Jing, M.; Fu, M.; Liu, J.-J.; He, S.-Y.; Zhang, L.; Liu, X.-Z.; et al. GGP modified daunorubicin plus dioscin liposomes inhibit breast cancer by suppressing epithelial–mesenchymal transition. Drug Dev. Ind. Pharm. 2020, 46, 916–930. [Google Scholar] [CrossRef] [PubMed]
- Choudhary, R.; Khurana, D.; Kumar, A.; Subudhi, S. Stability analysis of Al2O3/water nanofluids. J. Exp. Nanosci. 2017, 12, 140–151. [Google Scholar] [CrossRef] [Green Version]
- Tunsirikongkon, A.; Pyo, Y.-C.; Kim, D.-H.; Lee, S.-E.; Park, J.-S. Optimization of Polyarginine-Conjugated PEG Lipid Grafted Proliposome Formulation for Enhanced Cellular Association of a Protein Drug. Pharmaceutics 2019, 11, 272. [Google Scholar] [CrossRef] [Green Version]
- Nguyen, H.T.; Tran, T.H.; Thapa, R.K.; Phung, C.D.; Shin, B.S.; Jeong, J.-H.; Choi, H.-G.; Yong, C.S.; Kim, J.O. Targeted co-delivery of polypyrrole and rapamycin by trastuzumab-conjugated liposomes for combined chemo-photothermal therapy. Int. J. Pharm. 2017, 527, 61–71. [Google Scholar] [CrossRef]
- Hashizaki, K.; Taguchi, H.; Itoh, C.; Sakai, H.; Abe, M.; Saito, Y.; Ogawa, N. Effects of Poly(ethylene glycol) (PEG) Concentration on the Permeability of PEG-Grafted Liposomes. Chem. Pharm. Bull. 2005, 53, 27–31. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tai, K.; Rappolt, M.; Mao, L.; Gao, Y.; Yuan, F. Stability and release performance of curcumin-loaded liposomes with varying content of hydrogenated phospholipids. Food Chem. 2020, 326, 126973. [Google Scholar] [CrossRef]
- Yi, S.; Zhang, C.; Hu, J.; Meng, Y.; Chen, L.; Yu, H.; Li, S.; Wang, G.; Zheng, G.; Qiu, Z. Preparation, Characterization, and In Vitro Pharmacodynamics and Pharmacokinetics Evaluation of PEGylated Urolithin A Liposomes. AAPS PharmSciTech 2021, 22, 26. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Lan, J.; Li, Z.; Zeng, R.; Wang, Y.; Zhen, L.; Jin, H.; Ding, Y.; Zhang, T. A Novel Diosgenin-Based Liposome Delivery System Combined with Doxorubicin for Liver Cancer Therapy. Pharmaceutics 2022, 14, 1685. [Google Scholar] [CrossRef] [PubMed]
- Zheng, T.; Feng, H.; Liu, L.; Peng, J.; Xiao, H.; Yu, T.; Zhou, Z.; Li, Y.; Zhang, Y.; Bai, X.; et al. Enhanced antiproliferative effect of resveratrol in head and neck squamous cell carcinoma using GE11 peptide conjugated liposome. Int. J. Mol. Med. 2019, 43, 1635–1642. [Google Scholar] [CrossRef] [Green Version]
- Abu Lila, A.S.; Kizuki, S.; Doi, Y.; Suzuki, T.; Ishida, T.; Kiwada, H. Oxaliplatin encapsulated in PEG-coated cationic liposomes induces significant tumor growth suppression via a dual-targeting approach in a murine solid tumor model. J. Control. Release 2009, 137, 8–14. [Google Scholar] [CrossRef]
- Chang, J.; Jallouli, Y.; Kroubi, M.; Yuan, X.-B.; Feng, W.; Kang, C.-S.; Pu, P.-Y.; Betbeder, D. Characterization of endocytosis of transferrin-coated PLGA nanoparticles by the blood–brain barrier. Int. J. Pharm. 2009, 379, 285–292. [Google Scholar] [CrossRef] [PubMed]
- Nam, H.Y.; Kwon, S.M.; Chung, H.; Lee, S.-Y.; Kwon, S.-H.; Jeon, H.; Kim, Y.; Park, J.H.; Kim, J.; Her, S.; et al. Cellular uptake mechanism and intracellular fate of hydrophobically modified glycol chitosan nanoparticles. J. Control. Release 2009, 135, 259–267. [Google Scholar] [CrossRef] [PubMed]
- Tang, H.; Rui, M.; Mai, J.; Guo, W.; Xu, Y. Reimaging biological barriers affecting distribution and extravasation of PEG/peptide- modified liposomes in xenograft SMMC7721 tumor. Acta Pharm. Sin. B 2020, 10, 546–556. [Google Scholar] [CrossRef]
- Maruyama, M.; Tojo, H.; Toi, K.; Ienaka, Y.; Hyodo, K.; Kikuchi, H.; Ogawara, K.-I.; Higaki, K. Effect of Doxorubicin Release Rate from Polyethylene Glycol-Modified Liposome on Anti-tumor Activity in B16-BL6 Tumor-Bearing Mice. J. Pharm. Sci. 2022, 111, 293–297. [Google Scholar] [CrossRef]
- Jahan, S.T.; Sadat, S.M.A.; Walliser, M.; Haddadi, A. Targeted Therapeutic Nanoparticles: An Immense Promise to Fight against Cancer. J. Drug Deliv. 2017, 2017, 9090325. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, M.; Hagan, C.T.; Foley, H.; Tian, X.; Yang, F.; Au, K.M.; Mi, Y.; Medik, Y.; Roche, K.; Wagner, K.; et al. Co-delivery of etoposide and cisplatin in dual-drug loaded nanoparticles synergistically improves chemoradiotherapy in non-small cell lung cancer models. Acta Biomater. 2021, 124, 327–335. [Google Scholar] [CrossRef] [PubMed]
- Yang, W.; Hu, Q.; Xu, Y.; Liu, H.; Zhong, L. Antibody fragment-conjugated gemcitabine and paclitaxel-based liposome for effective therapeutic efficacy in pancreatic cancer. Mater. Sci. Eng. C 2018, 89, 328–335. [Google Scholar] [CrossRef]
- Tang, Z.; Feng, W.; Yang, Y.; Wang, Q. Gemcitabine-loaded RGD modified liposome for ovarian cancer: Preparation, characterization and pharmacodynamic studies. Drug Des. Dev. Ther. 2019, 13, 3281–3290. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, Z.; Chu, W.; Sun, Q.; Zhao, L.; Tan, X.; Zhang, Y.; Yin, T.; He, H.; Gou, J.; Tang, X. Micelle-contained and PEGylated hybrid liposomes of combined gemcitabine and cisplatin delivery for enhancing antitumor activity. Int. J. Pharm. 2021, 602, 120619. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Wang, Q.; Liu, Z.; Zheng, X. Preparation, pharmacokinetics and tumour-suppressive activity of berberine liposomes. J. Pharm. Pharmacol. 2017, 69, 625–632. [Google Scholar] [CrossRef] [PubMed]
- Ezhilarasan, D.; Lakshmi, T.; Mallineni, S.K. Nano-based targeted drug delivery for lung cancer: Therapeutic avenues and challenges. Nanomedicine 2022, 17, 1855–1869. [Google Scholar] [CrossRef]
- Park, Y.I.; Kwon, S.-H.; Lee, G.; Motoyama, K.; Kim, M.W.; Lin, M.; Niidome, T.; Choi, J.H.; Lee, R. pH-sensitive multi-drug liposomes targeting folate receptor β for efficient treatment of non-small cell lung cancer. J. Control. Release 2021, 330, 1–14. [Google Scholar] [CrossRef]
- Jha, A.; Viswanadh, M.K.; Burande, A.S.; Mehata, A.K.; Poddar, S.; Yadav, K.; Mahto, S.K.; Parmar, A.S.; Muthu, M.S. DNA biodots based targeted theranostic nanomedicine for the imaging and treatment of non-small cell lung cancer. Int. J. Biol. Macromol. 2020, 150, 413–425. [Google Scholar] [CrossRef]
- Nan, Y. Lung carcinoma therapy using epidermal growth factor receptor-targeted lipid polymeric nanoparticles co-loaded with cisplatin and doxorubicin. Oncol. Rep. 2019, 42, 2087–2096. [Google Scholar] [CrossRef]









| Formulation | Particle Size (nm) | PDI | Zeta Potential (mV) | EE (%) |
|---|---|---|---|---|
| L-GEM | 118.80 ± 0.05 | 0.14 ± 0.01 | −25.67 ± 0.61 | 48.27 ± 3.61 |
| ES-L-GEM | 120.10 ± 0.16 | 0.14 ± 0.01 | −24.97 ± 0.34 | 48.90 ± 1.73 |
| SSL-GEM | 129.60 ± 0.68 | 0.19 ± 0.01 | −10.04 ± 0.56 | 55.93 ± 2.98 |
| ES-SSL-GEM | 131.20 ± 0.62 | 0.20 ± 0.01 | −9.73 ± 0.41 | 56.35 ± 1.38 |
| Formulation | 24 h IC50 | 48 h IC50 | 72 h IC50 |
|---|---|---|---|
| GEM | 25.98 ± 0.69 * | 22.79 ± 0.61 * | 19.52 ± 0.52 * |
| L-GEM | 23.32 ± 0.89 * | 20.41 ± 0.51 * | 6.89 ± 0.43 * |
| ES-L-GEM | 21.49 ± 1.49 * | 8.87 ± 1.52 * | 1.05 ± 0.85 |
| SSL-GEM | 10.39 ± 2.45 | 5.02 ± 1.45 | 0.22 ± 0.07 |
| ES-SSL-GEM | 8.24 ± 0.31 | 3.56 ± 0.48 | 0.09 ± 0.02 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tang, H.; Zhang, Z.; Zhu, M.; Xie, Y.; Lv, Z.; Liu, R.; Shen, Y.; Pei, J. Efficient Delivery of Gemcitabine by Estrogen Receptor-Targeted PEGylated Liposome and Its Anti-Lung Cancer Activity In Vivo and In Vitro. Pharmaceutics 2023, 15, 988. https://doi.org/10.3390/pharmaceutics15030988
Tang H, Zhang Z, Zhu M, Xie Y, Lv Z, Liu R, Shen Y, Pei J. Efficient Delivery of Gemcitabine by Estrogen Receptor-Targeted PEGylated Liposome and Its Anti-Lung Cancer Activity In Vivo and In Vitro. Pharmaceutics. 2023; 15(3):988. https://doi.org/10.3390/pharmaceutics15030988
Chicago/Turabian StyleTang, Huan, Zheng Zhang, Ming Zhu, Yizhuo Xie, Zhe Lv, Rui Liu, Yujia Shen, and Jin Pei. 2023. "Efficient Delivery of Gemcitabine by Estrogen Receptor-Targeted PEGylated Liposome and Its Anti-Lung Cancer Activity In Vivo and In Vitro" Pharmaceutics 15, no. 3: 988. https://doi.org/10.3390/pharmaceutics15030988
APA StyleTang, H., Zhang, Z., Zhu, M., Xie, Y., Lv, Z., Liu, R., Shen, Y., & Pei, J. (2023). Efficient Delivery of Gemcitabine by Estrogen Receptor-Targeted PEGylated Liposome and Its Anti-Lung Cancer Activity In Vivo and In Vitro. Pharmaceutics, 15(3), 988. https://doi.org/10.3390/pharmaceutics15030988