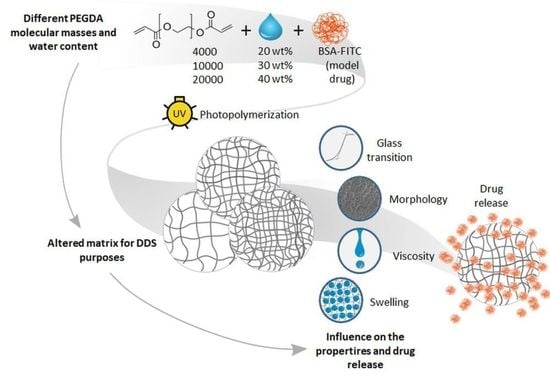
Influence of PEGDA Molecular Weight and Concentration on the In Vitro Release of the Model Protein BSA–FITC from Photo Crosslinked Systems
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. TES Buffer Preparation
2.3. PEGDA Polymerization
2.4. Morphology Analysis
2.5. Differential Scanning Calorimetry
2.6. Swelling Behavior Evaluation
2.7. Rheological Measurements
2.8. In Vitro Release of BSA–FITC
2.9. Statistical Analysis
3. Results
3.1. Rheological Behavior
3.2. Surface Morphology
3.3. Thermal Properties
3.4. Swelling Behavior
3.5. In Vitro Drug Release
4. Discussion
4.1. Swelling Behavior
4.2. Viscosity of PEGDA Solutions
4.3. Surface Morphology
4.4. Thermal Properties
4.5. In Vitro Drug Release
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Seyfoddin, A.; Dezfooli, S.M.; Greene, C.A. Engineering Drug Delivery Systems; Woodhead Publishing: Duxford, UK, 2020; ISBN 0081025483. [Google Scholar]
- Indurkhya, A. Influence of Drug Properties and Routes of Drug Administration on the Design of Controlled Release System. In Dosage Form Design Considerations; Tekade, R.K., Ed.; Academic Press: London, UK; San Diego, CA, USA; Cambridge, UK; Oxford, UK, 2018; Chapter 6; ISBN 9780128144237. [Google Scholar]
- Li, C.; Wang, J.; Wang, Y.; Gao, H.; Wei, G.; Huang, Y.; Yu, H.; Gan, Y.; Wang, Y.; Mei, L.; et al. Recent progress in drug delivery. Acta Pharm. Sin. B 2019, 9, 1145–1162. [Google Scholar] [CrossRef] [PubMed]
- Sandler, N.; Preis, M. Printed Drug-Delivery Systems for Improved Patient Treatment. Trends Pharmacol. Sci. 2016, 37, 1070–1080. [Google Scholar] [CrossRef]
- Domsta, V.; Seidlitz, A. 3D-Printing of Drug-Eluting Implants: An Overview of the Current Developments Described in the Literature. Molecules 2021, 26, 4066. [Google Scholar] [CrossRef]
- Elkasabgy, N.A.; Mahmoud, A.A.; Maged, A. 3D printing: An appealing route for customized drug delivery systems. Int. J. Pharm. 2020, 588, 119732. [Google Scholar] [CrossRef]
- Auriemma, G.; Tommasino, C.; Falcone, G.; Esposito, T.; Sardo, C.; Aquino, R.P. Additive Manufacturing Strategies for Personalized Drug Delivery Systems and Medical Devices: Fused Filament Fabrication and Semi Solid Extrusion. Molecules 2022, 27, 2784. [Google Scholar] [CrossRef] [PubMed]
- Khaled, S.A.; Burley, J.C.; Alexander, M.R.; Yang, J.; Roberts, C.J. 3D printing of five-in-one dose combination polypill with defined immediate and sustained release profiles. J. Control. Release 2015, 217, 308–314. [Google Scholar] [CrossRef]
- Robles-Martinez, P.; Xu, X.; Trenfield, S.J.; Awad, A.; Goyanes, A.; Telford, R.; Basit, A.W.; Gaisford, S. 3D Printing of a Multi-Layered Polypill Containing Six Drugs Using a Novel Stereolithographic Method. Pharmaceutics 2019, 11, 274. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Januskaite, P.; Xu, X.; Ranmal, S.R.; Gaisford, S.; Basit, A.W.; Tuleu, C.; Goyanes, A. I Spy with My Little Eye: A Paediatric Visual Preferences Survey of 3D Printed Tablets. Pharmaceutics 2020, 12, 1100. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Sun, L.; Mei, Z.; Zhang, F.; He, M.; Fletcher, C.; Wang, F.; Yang, J.; Bi, D.; Jiang, Y.; et al. 3D printed biodegradable implants as an individualized drug delivery system for local chemotherapy of osteosarcoma. Mater. Des. 2020, 186, 108336. [Google Scholar] [CrossRef]
- Palo, M.; Holländer, J.; Suominen, J.; Yliruusi, J.; Sandler, N. 3D printed drug delivery devices: Perspectives and technical challenges. Expert Rev. Med. Devices 2017, 14, 685–696. [Google Scholar] [CrossRef]
- Pandey, M.; Choudhury, H.; Fern, J.L.C.; Kee, A.T.K.; Kou, J.; Jing, J.L.J.; Her, H.C.; Yong, H.S.; Ming, H.C.; Bhattamisra, S.K.; et al. 3D printing for oral drug delivery: A new tool to customize drug delivery. Drug Deliv. Transl. Res. 2020, 10, 986–1001. [Google Scholar] [CrossRef] [PubMed]
- Fazel-Rezai, R. Biomedical Engineering—Frontiers and Challenges; IntechOpen: Rijeka, Croatia, 2011; ISBN 978-953-307-309-5. [Google Scholar]
- Konasch, J.; Riess, A.; Mau, R.; Teske, M.; Rekowska, N.; Eickner, T.; Grabow, N.; Seitz, H. A Novel Hybrid Additive Manufacturing Process for Drug Delivery Systems with Locally Incorporated Drug Depots. Pharmaceutics 2019, 11, 661. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Choi, J.R.; Yong, K.W.; Choi, J.Y.; Cowie, A.C. Recent advances in photo-crosslinkable hydrogels for biomedical applications. Biotechniques 2019, 66, 40–53. [Google Scholar] [CrossRef] [Green Version]
- Ito, Y. Photochemistry for Biomedical Applications: From Device Fabrication to Diagnosis and Therapy; Springer: Singapore, 2018; ISBN 978-981-13-0151-3. [Google Scholar]
- Jain, A.; Jain, A.; Gulbake, A.; Shilpi, S.; Hurkat, P.; Jain, S.K. Peptide and protein delivery using new drug delivery systems. Crit. Rev. Ther. Drug Carr. Syst. 2013, 30, 293–329. [Google Scholar] [CrossRef] [PubMed]
- Tekade, R.K. Basic Fundamentals of Drug Delivery; Academic Press: London, UK; San Diego, CA, USA, 2019; ISBN 0128179090. [Google Scholar]
- Morishita, M.; Goto, T.; Nakamura, K.; Lowman, A.M.; Takayama, K.; Peppas, N.A. Novel oral insulin delivery systems based on complexation polymer hydrogels: Single and multiple administration studies in type 1 and 2 diabetic rats. J. Control. Release 2006, 110, 587–594. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Abdella, S.; Youssef, S.H.; Afinjuomo, F.; Song, Y.; Fouladian, P.; Upton, R.; Garg, S. 3D Printing of Thermo-Sensitive Drugs. Pharmaceutics 2021, 13, 1524. [Google Scholar] [CrossRef]
- Martinez, P.R.; Goyanes, A.; Basit, A.W.; Gaisford, S. Fabrication of drug-loaded hydrogels with stereolithographic 3D printing. Int. J. Pharm. 2017, 532, 313–317. [Google Scholar] [CrossRef] [Green Version]
- Madzarevic, M.; Medarevic, D.; Vulovic, A.; Sustersic, T.; Djuris, J.; Filipovic, N.; Ibric, S. Optimization and Prediction of Ibuprofen Release from 3D DLP Printlets Using Artificial Neural Networks. Pharmaceutics 2019, 11, 544. [Google Scholar] [CrossRef] [Green Version]
- Healy, A.V.; Fuenmayor, E.; Doran, P.; Geever, L.M.; Higginbotham, C.L.; Lyons, J.G. Additive Manufacturing of Personalized Pharmaceutical Dosage Forms via Stereolithography. Pharmaceutics 2019, 11, 645. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hanna, K.; Yasar-Inceoglu, O.; Yasar, O. Drug Delivered Poly(ethylene glycol) Diacrylate (PEGDA) Hydrogels and Their Mechanical Characterization Tests for Tissue Engineering Applications. MRS Adv. 2018, 3, 1697–1702. [Google Scholar] [CrossRef]
- Warr, C.; Valdoz, J.C.; Bickham, B.P.; Knight, C.J.; Franks, N.A.; Chartrand, N.; van Ry, P.M.; Christensen, K.A.; Nordin, G.P.; Cook, A.D. Biocompatible PEGDA Resin for 3D Printing. ACS Appl. Bio Mater. 2020, 3, 2239–2244. [Google Scholar] [CrossRef] [PubMed]
- O’Donnell, K.; Boyd, A.; Meenan, B.J. Controlling Fluid Diffusion and Release through Mixed-Molecular-Weight Poly(ethylene) Glycol Diacrylate (PEGDA) Hydrogels. Materials 2019, 12, 3381. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rekowska, N.; Huling, J.; Brietzke, A.; Arbeiter, D.; Eickner, T.; Konasch, J.; Riess, A.; Mau, R.; Seitz, H.; Grabow, N.; et al. Thermal, Mechanical and Biocompatibility Analyses of Photochemically Polymerized PEGDA250 for Photopolymerization-Based Manufacturing Processes. Pharmaceutics 2022, 14, 628. [Google Scholar] [CrossRef]
- Liu, S.; Yeo, D.C.; Wiraja, C.; Tey, H.L.; Mrksich, M.; Xu, C. Peptide delivery with poly(ethylene glycol) diacrylate microneedles through swelling effect. Bioeng. Transl. Med. 2017, 2, 258–267. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.; Hou, M.; Yang, R.; Zhang, L.; Xu, Z.; Kang, Y.; Xue, P. PEGDA/PVP Microneedles with Tailorable Matrix Constitutions for Controllable Transdermal Drug Delivery. Macromol. Mater. Eng. 2018, 303, 1800233. [Google Scholar] [CrossRef]
- Shadish, J.A.; DeForest, C.A. Site-Selective Protein Modification: From Functionalized Proteins to Functional Biomaterials. Matter 2020, 2, 50–77. [Google Scholar] [CrossRef]
- Davidovich-Pinhas, M.; Bianco-Peled, H. Novel mucoadhesive system based on sulfhydryl-acrylate interactions. J. Mater. Sci. Mater. Med. 2010, 21, 2027–2034. [Google Scholar] [CrossRef]
- Mazzoccoli, J.P.; Feke, D.L.; Baskaran, H.; Pintauro, P.N. Mechanical and cell viability properties of crosslinked low- and high-molecular weight poly(ethylene glycol) diacrylate blends. J. Biomed. Mater. Res. A 2010, 93, 558–566. [Google Scholar] [CrossRef] [Green Version]
- Lee, S.; Tong, X.; Yang, F. The effects of varying poly(ethylene glycol) hydrogel crosslinking density and the crosslinking mechanism on protein accumulation in three-dimensional hydrogels. Acta Biomater. 2014, 10, 4167–4174. [Google Scholar] [CrossRef]
- Qiao, X.; Wang, L.; Ma, J.; Deng, Q.; Liang, Z.; Zhang, L.; Peng, X.; Zhang, Y. High sensitivity analysis of water-soluble, cyanine dye labeled proteins by high-performance liquid chromatography with fluorescence detection. Anal. Chim. Acta 2009, 640, 114–120. [Google Scholar] [CrossRef]
- Hungerford, G.; Benesch, J.; Mano, J.F.; Reis, R.L. Effect of the labelling ratio on the photophysics of fluorescein isothiocyanate (FITC) conjugated to bovine serum albumin. Photochem. Photobiol. Sci. 2007, 6, 152–158. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Breen, C.J.; Raverdeau, M.; Voorheis, H.P. Development of a quantitative fluorescence-based ligand-binding assay. Sci. Rep. 2016, 6, 25769. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rekowska, N.; Arbeiter, D.; Seitz, H.; Mau, R.; Riess, A.; Eickner, T.; Grabow, N.; Teske, M. The influence of PEGDA’s molecular weight on its mechanical properties in the context of biomedical applications. Curr. Dir. Biomed. Eng. 2022, 8, 181–184. [Google Scholar] [CrossRef]
- Anthony, M. Lowman and Nikolaos, A. Peppas. Analysis of the Complexation/Decomplexation Phenomena in Graft Copolymer Networks. Macromolecules 1997, 30, 4959–4965. [Google Scholar] [CrossRef]
- Yang, X.; Dargaville, B.L.; Hutmacher, D.W. Elucidating the Molecular Mechanisms for the Interaction of Water with Polyethylene Glycol-Based Hydrogels: Influence of Ionic Strength and Gel Network Structure. Polymers 2021, 13, 845. [Google Scholar] [CrossRef]
- Della Sala, F.; Biondi, M.; Guarnieri, D.; Borzacchiello, A.; Ambrosio, L.; Mayol, L. Mechanical behavior of bioactive poly(ethylene glycol) diacrylate matrices for biomedical application. J. Mech. Behav. Biomed. Mater. 2020, 110, 103885. [Google Scholar] [CrossRef]
- Karoyo, A.H.; Wilson, L.D. A Review on the Design and Hydration Properties of Natural Polymer-Based Hydrogels. Materials 2021, 14, 1095. [Google Scholar] [CrossRef]
- Ju, H.; McCloskey, B.D.; Sagle, A.C.; Kusuma, V.A.; Freeman, B.D. Preparation and characterization of crosslinked poly(ethylene glycol) diacrylate hydrogels as fouling-resistant membrane coating materials. J. Membr. Sci. 2009, 330, 180–188. [Google Scholar] [CrossRef]
- Peris, E.; Bañuls, M.-J.; Maquieira, Á.; Puchades, R. Photopolymerization as a promising method to sense biorecognition events. Trends Analyt. Chem. 2012, 41, 86–104. [Google Scholar] [CrossRef]
- Karamchand, L.; Makeiff, D.; Gao, Y.; Azyat, K.; Serpe, M.J.; Kulka, M. Biomaterial inks and bioinks for fabricating 3D biomimetic lung tissue: A delicate balancing act between biocompatibility and mechanical printability. Bioprinting 2023, 29, e00255. [Google Scholar] [CrossRef]
- Zandrini, T.; Liaros, N.; Jiang, L.J.; Lu, Y.F.; Fourkas, J.T.; Osellame, R.; Baldacchini, T. Effect of the resin viscosity on the writing properties of two-photon polymerization. Opt. Mater. Express 2019, 9, 2601. [Google Scholar] [CrossRef]
- Marcinkowska, A.; Andrzejewska, E. Viscosity effects in the photopolymerization of two-monomer systems. J. Appl. Polym. Sci. 2010, 116, 280–287. [Google Scholar] [CrossRef]
- Lin, J.-T.; Liu, H.-W.; Chen, K.-T.; Cheng, D.-C. Modeling the Kinetics, Curing Depth, and Efficacy of Radical-Mediated Photopolymerization: The Role of Oxygen Inhibition, Viscosity, and Dynamic Light Intensity. Front. Chem. 2019, 7, 760. [Google Scholar] [CrossRef] [PubMed]
- Wozniak, M.; de Hazan, Y.; Graule, T.; Kata, D. Rheology of UV curable colloidal silica dispersions for rapid prototyping applications. J. Eur. Ceram. Soc. 2011, 31, 2221–2229. [Google Scholar] [CrossRef]
- Hinczewski, C.; Corbel, S.; Chartier, T. Ceramic suspensions suitable for stereolithography. J. Eur. Ceram. Soc. 1998, 18, 583–590. [Google Scholar] [CrossRef]
- Shahzadi, L.; Li, F.; Alejandro, F.; Breadmore, M.; Thickett, S. Chapter 4 Resin design in stereolithography 3D printing for microfluidic applications. In 3D Printing with Light; Xiao, P., Zhang, J., Eds.; De Gruyter: Berlin, Germany; Boston, MA, USA, 2021. [Google Scholar] [CrossRef]
- Komissarenko, D.A.; Sokolov, P.S.; Evstigneeva, A.D.; Shmeleva, I.A.; Dosovitsky, A.E. Rheological and Curing Behavior of Acrylate-Based Suspensions for the DLP 3D Printing of Complex Zirconia Parts. Materials 2018, 11, 2350. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, X.; Xie, B.; Jin, J.; Chai, Y.; Chen, Y. 3D printing temporary crown and bridge by temperature controlled mask image projection stereolithography. Procedia Manuf. 2018, 26, 1023–1033. [Google Scholar] [CrossRef]
- Omelczuk, M.O.; McGinity, J.W. The influence of polymer glass transition temperature and molecular weight on drug release from tablets containing poly(DL-lactic acid). Pharm. Res. 1992, 9, 26–32. [Google Scholar] [CrossRef]
- Chen, J.; Yuan, T.; Liu, Z. Supramolecular medical antibacterial tissue adhesive prepared based on natural small molecules. Biomater. Sci. 2020, 8, 6235–6245. [Google Scholar] [CrossRef] [PubMed]
- Jadhav, N.; Gaikwad, V.; Nair, K.; Kadam, H. Glass transition temperature: Basics and application in pharmaceutical sector. Asian J. Pharm. 2009, 3, 82. [Google Scholar] [CrossRef]
- Comyn, J. What are adhesives and sealants and how do they work? In Adhesive Bonding: Science, Technology and Applications; Adams, R.D., Ed.; Woodhead Publishing an Imprint of Elsevier: Duxford, UK; Cambridge, MA, USA; Kidlington, UK, 2021; pp. 41–78. ISBN 9780128199541. [Google Scholar]
- Guo, F.; Zhang, W.; Pei, X.; Shen, X.; Yan, Q.; Li, H.; Yun, J.; Yang, G. Biodegradable star-shaped polycyclic ester elastomers: Preparation, degradability, protein release, and biocompatibility in vitro. J. Bioact. Compat. Polym. 2017, 32, 178–195. [Google Scholar] [CrossRef]
- Li, Z.; Jiang, Y.; Wüst, K.; Callari, M.; Stenzel, M.H. Crosslinking of Self-Assembled Protein–Polymer Conjugates with Divanillin. Aust. J. Chem. 2020, 73, 1034–1041. [Google Scholar] [CrossRef]
- Bao, W.; Zhang, X.; Wu, H.; Chen, R.; Guo, S. Synergistic Effect of Ultrasound and Polyethylene Glycol on the Mechanism of the Controlled Drug Release from Polylactide Matrices. Polymers 2019, 11, 880. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Browning, M.B.; Cereceres, S.N.; Luong, P.T.; Cosgriff-Hernandez, E.M. Determination of the in vivo degradation mechanism of PEGDA hydrogels. J. Biomed. Mater. Res. A 2014, 102, 4244–4251. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yoo, J.; Won, Y.-Y. Phenomenology of the Initial Burst Release of Drugs from PLGA Microparticles. ACS Biomater. Sci. Eng. 2020, 6, 6053–6062. [Google Scholar] [CrossRef] [PubMed]








Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rekowska, N.; Wulf, K.; Koper, D.; Senz, V.; Seitz, H.; Grabow, N.; Teske, M. Influence of PEGDA Molecular Weight and Concentration on the In Vitro Release of the Model Protein BSA–FITC from Photo Crosslinked Systems. Pharmaceutics 2023, 15, 1039. https://doi.org/10.3390/pharmaceutics15041039
Rekowska N, Wulf K, Koper D, Senz V, Seitz H, Grabow N, Teske M. Influence of PEGDA Molecular Weight and Concentration on the In Vitro Release of the Model Protein BSA–FITC from Photo Crosslinked Systems. Pharmaceutics. 2023; 15(4):1039. https://doi.org/10.3390/pharmaceutics15041039
Chicago/Turabian StyleRekowska, Natalia, Katharina Wulf, Daniela Koper, Volkmar Senz, Hermann Seitz, Niels Grabow, and Michael Teske. 2023. "Influence of PEGDA Molecular Weight and Concentration on the In Vitro Release of the Model Protein BSA–FITC from Photo Crosslinked Systems" Pharmaceutics 15, no. 4: 1039. https://doi.org/10.3390/pharmaceutics15041039
APA StyleRekowska, N., Wulf, K., Koper, D., Senz, V., Seitz, H., Grabow, N., & Teske, M. (2023). Influence of PEGDA Molecular Weight and Concentration on the In Vitro Release of the Model Protein BSA–FITC from Photo Crosslinked Systems. Pharmaceutics, 15(4), 1039. https://doi.org/10.3390/pharmaceutics15041039