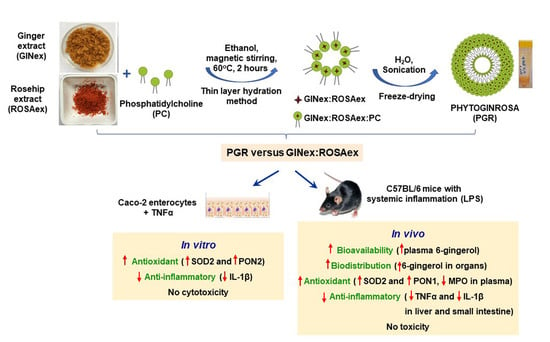
Formulation of Phytosomes with Extracts of Ginger Rhizomes and Rosehips with Improved Bioavailability, Antioxidant and Anti-Inflammatory Effects In Vivo
Abstract
:1. Introduction
2. Materials and Methods
2.1. Reagents
2.2. Preparation and Characterization of Ginger Extracts
2.3. Preparation and Characterization of Rosehip Extract
2.4. Preparation of Phytosomes
2.5. Characterization of PGR and PG Phytosomes
2.5.1. Size and Zeta Potential
2.5.2. Surface Morphology
2.5.3. Encapsulation Efficiency and Drug Loading
2.5.4. In Vitro Bioaccesibility Study
2.5.5. Physical Storage Stability Test
2.5.6. Structure Determination by Nuclear Magnetic Resonance (NMR) Spectroscopy
2.6. In Vitro Evaluation of PGR
2.6.1. Cell Culture
2.6.2. Selection of the Optimal Formulation and Concentration of PGR Depending on the Antioxidant and Anti-Inflammatory Properties
Quantitative Real-Time PCR
2.6.3. Evaluation of the PGR 0.5:0.5:1 Cytotoxicity
Estimation of Cells’ Viability
Measurement of the Energetic Status of the Cells
2.7. In Vivo Evaluation of PGR 0.5:0.5:1
2.7.1. Animal Model
Measurement of the Free 6-Gingerol Levels in the Plasma and Organs of Mice
- Bioavailability of free 6-gingerol in the plasma
- Biodistribution of free 6-gingerol in organs
- Sample preparation for the measurement of free 6-gingerol
- UHPLC determination of free 6-gingerol from plasma and organs
- Assessment of the antioxidant and anti-inflammatory effects of PGR 0.5:0.5:1
- Measurement of PON1 enzymatic activity in serum
- Measurement of TNFα levels in serum
- Measurement of MPO enzymatic activity in serum
- Western blotting analysis
- Evaluation of the PGR 0.5:0.5:1 toxicity in vivo
- Measurement of ALT activity
- Measurement of creatinine level
2.8. Statistical Analysis
3. Results
3.1. Characterization of GINex and ROSAex
3.2. Characterization of PGR
3.2.1. Size and Zeta Potential of PGR
3.2.2. Surface Morphology
3.2.3. Encapsulation Efficiency and Drug Loading
3.2.4. In Vitro Bioaccesibility
3.2.5. Physical Storage Stability Test
3.2.6. Structure Determination by Nuclear Magnetic Resonance (NMR) Spectroscopy
3.3. Selection of the Optimal Formulation and Concentration of PGR Depending on the Antioxidant and Anti-Inflammatory Properties Determined In Vitro
3.4. Evaluation of the PGR 0.5:0.5:1 Cytotoxicity
3.5. Biodistribution of 6-Gingerol from PGR 0.5:0.5:1 in the Plasma and Organs of Mice
3.6. Assessment of the Antioxidant Effects of PGR 0.5:0.5:1 In Vivo
3.7. Assessment of the Anti-Inflammatory Effects of PGR 0.5:0.5:1 In Vivo
3.8. Evaluation of the PGR 0.5:0.5:1 Toxicity In Vivo
4. Discussion
5. Conclusion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Vona, R.; Gambardella, L.; Cittadini, C.; Straface, E.; Pietraforte, D. Biomarkers of Oxidative Stress in Metabolic Syndrome and Associated Diseases. Oxid. Med. Cell Longev. 2019, 2019, 8267234. [Google Scholar] [CrossRef] [Green Version]
- Maloberti, A.; Vallerio, P.; Triglione, N.; Occhi, L.; Panzeri, F.; Bassi, I.; Pansera, F.; Piccinelli, E.; Peretti, A.; Garatti, L.; et al. Vascular Aging and Disease of the Large Vessels: Role of Inflammation. High Blood Press Cardiovasc. Prev. 2019, 26, 175–182. [Google Scholar] [CrossRef]
- Neha, K.; Haider, M.R.; Pathak, A.; Yar, M.S. Medicinal prospects of antioxidants: A review. Eur. J. Med. Chem. 2019, 178, 687–704. [Google Scholar] [CrossRef] [PubMed]
- Azzi, A. Antioxidants: Wonder drugs or quackery? Biofactors 2017, 43, 785–788. [Google Scholar] [CrossRef] [PubMed]
- Forman, H.J.; Zhang, H. Targeting oxidative stress in disease: Promise and limitations of antioxidant therapy. Nat. Rev. Drug Discov. 2021, 20, 689–709. [Google Scholar] [CrossRef]
- de Lima, R.M.T.; Dos Reis, A.C.; de Menezes, A.P.M.; Santos, J.V.O.; Filho, J.; Ferreira, J.R.O.; de Alencar, M.; da Mata, A.; Khan, I.N.; Islam, A.; et al. Protective and therapeutic potential of ginger (Zingiber officinale) extract and [6]-gingerol in cancer: A comprehensive review. Phytother. Res. 2018, 32, 1885–1907. [Google Scholar] [CrossRef] [PubMed]
- Kiyama, R. Nutritional implications of ginger: Chemistry, biological activities and signaling pathways. J. Nutr. Biochem. 2020, 86, 108486. [Google Scholar] [CrossRef]
- Hosseinzadeh, A.; Bahrampour Juybari, K.; Fatemi, M.J.; Kamarul, T.; Bagheri, A.; Tekiyehmaroof, N.; Sharifi, A.M. Protective Effect of Ginger (Zingiber officinale Roscoe) Extract against Oxidative Stress and Mitochondrial Apoptosis Induced by Interleukin-1beta in Cultured Chondrocytes. Cells Tissues Organs 2017, 204, 241–250. [Google Scholar] [CrossRef]
- Ballester, P.; Cerdá, B.; Arcusa, R.; Marhuenda, J.; Yamedjeu, K.; Zafrilla, P. Effect of Ginger on Inflammatory Diseases. Molecules 2022, 27, 7223. [Google Scholar] [CrossRef]
- Carnuta, M.G.; Deleanu, M.; Barbalata, T.; Toma, L.; Raileanu, M.; Sima, A.V.; Stancu, C.S. Zingiber officinale extract administration diminishes steroyl-CoA desaturase gene expression and activity in hyperlipidemic hamster liver by reducing the oxidative and endoplasmic reticulum stress. Phytomedicine 2018, 48, 62–69. [Google Scholar] [CrossRef]
- Barbalata, T.; Deleanu, M.; Carnuta, M.G.; Niculescu, L.S.; Raileanu, M.; Sima, A.V.; Stancu, C.S. Hyperlipidemia Determines Dysfunctional HDL Production and Impedes Cholesterol Efflux in the Small Intestine: Alleviation by Ginger Extract. Mol. Nutr. Food Res. 2019, 63, e1900029. [Google Scholar] [CrossRef]
- Ebrahimzadeh, A.; Ebrahimzadeh, A.; Mirghazanfari, S.M.; Hazrati, E.; Hadi, S.; Milajerdi, A. The effect of ginger supplementation on metabolic profiles in patients with type 2 diabetes mellitus: A systematic review and meta-analysis of randomized controlled trials. Complement Ther. Med. 2022, 65, 102802. [Google Scholar] [CrossRef] [PubMed]
- Zadorozhna, M.; Mangieri, D. Mechanisms of Chemopreventive and Therapeutic Proprieties of Ginger Extracts in Cancer. Int. J. Mol. Sci. 2021, 22, 6599. [Google Scholar] [CrossRef] [PubMed]
- Mohamed, O.I.; El-Nahas, A.F.; El-Sayed, Y.S.; Ashry, K.M. Ginger extract modulates Pb-induced hepatic oxidative stress and expression of antioxidant gene transcripts in rat liver. Pharm. Biol. 2016, 54, 1164–1172. [Google Scholar] [CrossRef]
- Khan, A.; Azam, M.; Allemailem, K.S.; Alrumaihi, F.; Almatroudi, A.; Alhumaydhi, F.A.; Ahmad, H.I.; Khan, M.U.; Khan, M.A. Coadministration of Ginger Extract and Fluconazole Shows a Synergistic Effect in the Treatment of Drug-Resistant Vulvovaginal Candidiasis. Infect. Drug Resist. 2021, 14, 1585–1599. [Google Scholar] [CrossRef]
- Mao, Q.-Q.; Xu, X.-Y.; Cao, S.-Y.; Gan, R.-Y.; Corke, H.; Beta, T.; Li, H.-B. Bioactive Compounds and Bioactivities of Ginger (Zingiber officinale Roscoe). Foods 2019, 8, 185. [Google Scholar] [CrossRef] [Green Version]
- Zhu, J.; Chen, H.; Song, Z.; Wang, X.; Sun, Z. Effects of Ginger (Zingiber officinale Roscoe) on Type 2 Diabetes Mellitus and Components of the Metabolic Syndrome: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Evid. Based Complement. Alternat. Med. 2018, 2018, 5692962. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Santos Braga, S. Ginger: Panacea or Consumer’s Hype? Appl. Sci. 2019, 9, 1570. [Google Scholar] [CrossRef] [Green Version]
- Palachai, N.; Wattanathorn, J.; Muchimapura, S.; Thukham-Mee, W. Phytosome Loading the Combined Extract of Mulberry Fruit and Ginger Protects against Cerebral Ischemia in Metabolic Syndrome Rats. Oxid. Med. Cell Longev. 2020, 2020, 5305437. [Google Scholar] [CrossRef] [PubMed]
- Rondanelli, M.; Riva, A.; Allegrini, P.; Faliva, M.A.; Naso, M.; Peroni, G.; Nichetti, M.; Gasparri, C.; Spadaccini, D.; Iannello, G.; et al. The Use of a New Food-Grade Lecithin Formulation of Highly Standardized Ginger (Zingiber officinale) and Acmella oleracea Extracts for the Treatment of Pain and Inflammation in a Group of Subjects with Moderate Knee Osteoarthritis. J. Pain Res. 2020, 13, 761–770. [Google Scholar] [CrossRef] [Green Version]
- Chrubasik, C.; Duke, R.K.; Chrubasik, S. The evidence for clinical efficacy of rose hip and seed: A systematic review. Phytother. Res. 2006, 20, 1–3. [Google Scholar] [CrossRef] [PubMed]
- Ayati, Z.; Amiri, M.S.; Ramezani, M.; Delshad, E.; Sahebkar, A.; Emami, S.A. Phytochemistry, Traditional Uses and Pharmacological Profile of Rose Hip: A Review. Curr. Pharm. Des. 2018, 24, 4101–4124. [Google Scholar] [CrossRef] [PubMed]
- Gruenwald, J.; Uebelhack, R.; Moré, M.I. Rosa canina—Rose hip pharmacological ingredients and molecular mechanics counteracting osteoarthritis—A systematic review. Phytomedicine 2019, 60, 152958. [Google Scholar] [CrossRef] [PubMed]
- Boscaro, V.; Rivoira, M.; Sgorbini, B.; Bordano, V.; Dadone, F.; Gallicchio, M.; Pons, A.; Benetti, E.; Rosa, A.C. Evidence-Based Anti-Diabetic Properties of Plant from the Occitan Valleys of the Piedmont Alps. Pharmaceutics 2022, 14, 2371. [Google Scholar] [CrossRef]
- Li, J.; Wang, X.; Zhang, T.; Wang, C.; Huang, Z.; Luo, X.; Deng, Y. A review on phospholipids and their main applications in drug delivery systems. Asian J. Pharm. Sci. 2015, 10, 81–98. [Google Scholar] [CrossRef]
- Dehnad, D.; Emadzadeh, B.; Ghorani, B.; Rajabzadeh, G.; Kharazmi, M.S.; Jafari, S.M. Nano-vesicular carriers for bioactive compounds and their applications in food formulations. Crit. Rev. Food Sci. Nutr. 2022, 1–20. [Google Scholar] [CrossRef]
- Toma, L.; Raileanu, M.; Deleanu, M.; Stancu, C.S.; Sima, A.V. Novel molecular mechanisms by which ginger extract reduces the inflammatory stress in TNFα—Activated human endothelial cells; decrease of Ninjurin-1, TNFR1 and NADPH oxidase subunits expression. J. Funct. Foods 2018, 48, 654–664. [Google Scholar] [CrossRef]
- Barba, A.I.O.; Hurtado, M.C.; Mata, M.C.S.; Ruiz, V.F.; Tejada, M.L.S.d. Application of a UV–vis detection-HPLC method for a rapid determination of lycopene and β-carotene in vegetables. Food Chem. 2006, 95, 328–336. [Google Scholar] [CrossRef]
- Gnananath, K.; Sri Nataraj, K.; Ganga Rao, B. Phospholipid Complex Technique for Superior Bioavailability of Phytoconstituents. Adv. Pharm. Bull. 2017, 7, 35–42. [Google Scholar] [CrossRef] [Green Version]
- Fuior, E.V.; Deleanu, M.; Constantinescu, C.A.; Rebleanu, D.; Voicu, G.; Simionescu, M.; Calin, M. Functional Role of VCAM-1 Targeted Flavonoid-Loaded Lipid Nanoemulsions in Reducing Endothelium Inflammation. Pharmaceutics 2019, 11, 391. [Google Scholar] [CrossRef] [Green Version]
- Brodkorb, A.; Egger, L.; Alminger, M.; Alvito, P.; Assunção, R.; Ballance, S.; Bohn, T.; Bourlieu-Lacanal, C.; Boutrou, R.; Carrière, F.; et al. INFOGEST static in vitro simulation of gastrointestinal food digestion. Nat. Protoc. 2019, 14, 991–1014. [Google Scholar] [CrossRef] [PubMed]
- Wanjiru, J.; Gathirwa, J.; Sauli, E.; Swai, H.S. Formulation, Optimization, and Evaluation of Moringa oleifera Leaf Polyphenol-Loaded Phytosome Delivery System against Breast Cancer Cell Lines. Molecules 2022, 27, 4430. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.G.; Shin, B.S.; Choi, Y.; Ryu, J.K.; Shin, S.W.; Choo, H.W.; Yoo, S.D. Determination and pharmacokinetics of [6]-gingerol in mouse plasma by liquid chromatography-tandem mass spectrometry. Biomed. Chromatogr. 2012, 26, 660–665. [Google Scholar] [CrossRef] [PubMed]
- Fuior, E.V.; Mocanu, C.A.; Deleanu, M.; Voicu, G.; Anghelache, M.; Rebleanu, D.; Simionescu, M.; Calin, M. Evaluation of VCAM-1 Targeted Naringenin/Indocyanine Green-Loaded Lipid Nanoemulsions as Theranostic Nanoplatforms in Inflammation. Pharmaceutics 2020, 12, 1066. [Google Scholar] [CrossRef] [PubMed]
- Rozenberg, O.; Shih, D.M.; Aviram, M. Paraoxonase 1 (PON1) attenuates macrophage oxidative status: Studies in PON1 transfected cells and in PON1 transgenic mice. Atherosclerosis 2005, 181, 9–18. [Google Scholar] [CrossRef]
- Schumann, G.; Bonora, R.; Ceriotti, F.; Férard, G.; Ferrero, C.A.; Franck, P.F.; Gella, F.J.; Hoelzel, W.; Jørgensen, P.J.; Kanno, T.; et al. IFCC primary reference procedures for the measurement of catalytic activity concentrations of enzymes at 37 degrees C. International Federation of Clinical Chemistry and Laboratory Medicine. Part 4. Reference procedure for the measurement of catalytic concentration of alanine aminotransferase. Clin. Chem. Lab. Med. 2002, 40, 718–724. [Google Scholar] [CrossRef]
- Junge, W.; Wilke, B.; Halabi, A.; Klein, G. Determination of reference intervals for serum creatinine, creatinine excretion and creatinine clearance with an enzymatic and a modified Jaffé method. Clin. Chim. Acta 2004, 344, 137–148. [Google Scholar] [CrossRef]
- Ding, S.H.; An, K.J.; Zhao, C.P.; Li, Y.; Guo, Y.H.; Wang, Z.F. Effect of drying methods on volatiles of Chinese ginger (Zingiber officinale Roscoe). Food Bioprod. Process. 2012, 90, 515–524. [Google Scholar] [CrossRef]
- Chumroenphat, T.; Khanprom, I.; Butkhup, L. Stability of Phytochemicals and Antioxidant Properties in Ginger (Zingiber officinale Roscoe) Rhizome with Different Drying Methods. J. Herbs Spices Med. Plants 2011, 17, 361–374. [Google Scholar] [CrossRef]
- Mustafa, I.; Chin, N.L. Antioxidant Properties of Dried Ginger (Zingiber officinale Roscoe) var. Bentong. Foods 2023, 12, 178. [Google Scholar] [CrossRef]
- Medveckienė, B.; Kulaitienė, J.; Jarienė, E.; Vaitkevičienė, N.; Hallman, E. Carotenoids, Polyphenols, and Ascorbic Acid in Organic Rosehips (Rosa spp.) Cultivated in Lithuania. Appl. Sci. 2020, 10, 5337. [Google Scholar] [CrossRef]
- Clogston, J.D.; Patri, A.K. Zeta potential measurement. Methods Mol. Biol. 2011, 697, 63–70. [Google Scholar] [CrossRef] [PubMed]
- Tiwari, R.; Tiwari, G.; Sharma, S.; Ramachandran, V. An Exploration of herbal extracts loaded phyto-phospholipid complexes (Phytosomes) against polycystic ovarian syndrome: Formulation considerations. Pharm. Nanotechnol. 2022, 11, 44–55. [Google Scholar] [CrossRef]
- Ed Nignpense, B.; Francis, N.; Blanchard, C.; Santhakumar, A.B. Bioaccessibility and Bioactivity of Cereal Polyphenols: A Review. Foods 2021, 10, 1595. [Google Scholar] [CrossRef]
- Grgić, J.; Šelo, G.; Planinić, M.; Tišma, M.; Bucić-Kojić, A. Role of the Encapsulation in Bioavailability of Phenolic Compounds. Antioxidants 2020, 9, 923. [Google Scholar] [CrossRef] [PubMed]
- Giori, A.; Franceschi, F. Phospholipid Complexes of Curcumin Having Improved Bioavailability. EP 1 837 030 A1, 29 September 2007. [Google Scholar]
- Toma, L.; Sanda, G.M.; Niculescu, L.S.; Deleanu, M.; Sima, A.V.; Stancu, C.S. Phenolic Compounds Exerting Lipid-Regulatory, Anti-Inflammatory and Epigenetic Effects as Complementary Treatments in Cardiovascular Diseases. Biomolecules 2020, 10, 641. [Google Scholar] [CrossRef] [Green Version]
- Palachai, N.; Wattanathorn, J.; Muchimapura, S.; Thukham-mee, W. Antimetabolic Syndrome Effect of Phytosome Containing the Combined Extracts of Mulberry and Ginger in an Animal Model of Metabolic Syndrome. Oxidative Med. Cell. Longev. 2019, 2019, 5972575. [Google Scholar] [CrossRef] [Green Version]
- Wattanathorn, J.; Palachai, N.; Thukham-Mee, W.; Muchimapura, S. Memory-Enhancing Effect of a Phytosome Containing the Combined Extract of Mulberry Fruit and Ginger in an Animal Model of Ischemic Stroke with Metabolic Syndrome. Oxid. Med. Cell. Longev. 2020, 2020, 3096826. [Google Scholar] [CrossRef]
- Al-Samydai, A.; Qaraleh, M.A.; Alshaer, W.; Al-Halaseh, L.K.; Issa, R.; Alshaikh, F.; Abu-Rumman, A.; Al-Ali, H.; Al-Dujaili, E.A.S. Preparation, Characterization, Wound Healing, and Cytotoxicity Assay of PEGylated Nanophytosomes Loaded with 6-Gingerol. Nutrients 2022, 14, 5170. [Google Scholar] [CrossRef]
- Cheng, B.C.; Fu, X.Q.; Guo, H.; Li, T.; Wu, Z.Z.; Chan, K.; Yu, Z.L. The genus Rosa and arthritis: Overview on pharmacological perspectives. Pharmacol. Res. 2016, 114, 219–234. [Google Scholar] [CrossRef]
- Shah, B.R.; Zhang, C.; Li, Y.; Li, B. Bioaccessibility and antioxidant activity of curcumin after encapsulated by nano and Pickering emulsion based on chitosan-tripolyphosphate nanoparticles. Food Res. Int. 2016, 89, 399–407. [Google Scholar] [CrossRef]
- Alharbi, W.S.; Almughem, F.A.; Almehmady, A.M.; Jarallah, S.J.; Alsharif, W.K.; Alzahrani, N.M.; Alshehri, A.A. Phytosomes as an Emerging Nanotechnology Platform for the Topical Delivery of Bioactive Phytochemicals. Pharmaceutics 2021, 13, 1475. [Google Scholar] [CrossRef] [PubMed]
- Mukkavilli, R.; Yang, C.; Tanwar, R.S.; Saxena, R.; Gundala, S.R.; Zhang, Y.; Ghareeb, A.; Floyd, S.D.; Vangala, S.; Kuo, W.W.; et al. Pharmacokinetic-pharmacodynamic correlations in the development of ginger extract as an anticancer agent. Sci. Rep. 2018, 8, 3056. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Arcusa, R.; Villaño, D.; Marhuenda, J.; Cano, M.; Cerdà, B.; Zafrilla, P. Potential Role of Ginger (Zingiber officinale Roscoe) in the Prevention of Neurodegenerative Diseases. Front. Nutr. 2022, 9, 809621. [Google Scholar] [CrossRef] [PubMed]
- Chrubasik, C.; Roufogalis, B.D.; Müller-Ladner, U.; Chrubasik, S. A systematic review on the Rosa canina effect and efficacy profiles. Phytother. Res. 2008, 22, 725–733. [Google Scholar] [CrossRef]
- Brahma Naidu, P.; Uddandrao, V.V.; Ravindar Naik, R.; Suresh, P.; Meriga, B.; Begum, M.S.; Pandiyan, R.; Saravanan, G. Ameliorative potential of gingerol: Promising modulation of inflammatory factors and lipid marker enzymes expressions in HFD induced obesity in rats. Mol. Cell. Endocrinol. 2016, 419, 139–147. [Google Scholar] [CrossRef]









| Phytosomes (Mass Ratio) | GINex (mg) | ROSAex (mg) | PC (mg) | EtOH (mL) | Temperature (°C) | Reflux Time (h) |
|---|---|---|---|---|---|---|
| PGR 0.5:0.5:1 | 500 | 500 | 1000 | 125 | 60 | 2 |
| PGR 0.75:0.25:1 | 750 | 250 | 1000 | 125 | 60 | 2 |
| PGR 0.9:0.1:1 | 900 | 100 | 1000 | 125 | 60 | 2 |
| PG 1:1 | 1000 | - | 1000 | 125 | 60 | 2 |
| Bioactive Compounds | Grams/100 grams GINex |
|---|---|
| Total polyphenols (eq. gallic acid) | 40.88 ± 0.48 |
| Total flavonoids (eq. quercetin) | 1.70 ± 0.17 |
| 6-gingerol (UHPLC) | 3.00 ± 0.38 |
| 6-shogaol (UHPLC) | 0.29 ± 0.01 |
| Total gingerols (UHPLC) | 9.00 ± 0.09 |
| Bioactive Compounds | Grams/100 grams ROSAex |
|---|---|
| Total polyphenols | 5.42 ± 0.09 |
| Total flavonoids | 0.041 ± 0.005 |
| Total carotenoids (UHPLC) | 0.342 ± 0.031 |
| β-carotene (UHPLC) | 0.297 ± 0.092 |
| Sample | Z-Average (nm) | PDI | Zeta Potential (mV) |
|---|---|---|---|
| PC | 103.78 ± 6.39 | 0.32 ± 0.02 | −19.02 ± 1.34 |
| PG | 119.95 ± 8.64 | 0.39 ± 0.02 | −22.66 ± 2.44 |
| PGR 0.9:0.1:1 | 193.72 ± 39.48 | 0.42 ± 0.05 | −18.17 ± 1.85 |
| PGR 0.75:0.25:1 | 335.78 ± 61.18 | 0.56 ± 0.06 | −21.10 ± 0.84 |
| PGR 0.5:0.5:1 | 783.14 ± 219.26 | 0.50 ± 0.09 | −18.90 ± 1.48 |
| Grams/100 grams | PGR 0.5:0.5:1 | EE% | PGR 0.75:0.25:1 | EE% | PGR 0.9:0.1:1 | EE% | PG 1:1 | EE% |
|---|---|---|---|---|---|---|---|---|
| 6-gingerol | 0.500 ± 0.120 | 83.3 ± 3.4 | 0.844 ± 0.101 | 89.2 ± 2.7 | 1.020 ± 0.021 | 86.3 ± 3.1 | 1.14 ± 0.13 | 91.3 ± 3.0 |
| β-carotene | 0.074 ± 0.009 | 94.3 ± 4.5 | 0.030 ± 0.002 | 91.3 ± 3.1 | 0.010 ± 0.001 | 85.4 ± 2.1 | - | - |
| Time at 4 °C (months) | Freeze-Dried GINex (6-Gingerol,%) | Freeze-Dried PGR (6-Gingerol,%) |
|---|---|---|
| 0 | 3.00 ± 0.38 | 0.500 ± 0.120 |
| 1 | 2.82 ± 0.25 | 0.521 ± 0.098 |
| 3 | 2.67 ± 0.18 | 0.517 ± 0.081 |
| 6 | 2.37 ± 0.25 | 0.508 ± 0.046 |
| 12 | 2.19 ± 0.18 | 0.509 ± 0.058 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Deleanu, M.; Toma, L.; Sanda, G.M.; Barbălată, T.; Niculescu, L.Ş.; Sima, A.V.; Deleanu, C.; Săcărescu, L.; Suciu, A.; Alexandru, G.; et al. Formulation of Phytosomes with Extracts of Ginger Rhizomes and Rosehips with Improved Bioavailability, Antioxidant and Anti-Inflammatory Effects In Vivo. Pharmaceutics 2023, 15, 1066. https://doi.org/10.3390/pharmaceutics15041066
Deleanu M, Toma L, Sanda GM, Barbălată T, Niculescu LŞ, Sima AV, Deleanu C, Săcărescu L, Suciu A, Alexandru G, et al. Formulation of Phytosomes with Extracts of Ginger Rhizomes and Rosehips with Improved Bioavailability, Antioxidant and Anti-Inflammatory Effects In Vivo. Pharmaceutics. 2023; 15(4):1066. https://doi.org/10.3390/pharmaceutics15041066
Chicago/Turabian StyleDeleanu, Mariana, Laura Toma, Gabriela Maria Sanda, Teodora Barbălată, Loredan Ştefan Niculescu, Anca Volumnia Sima, Calin Deleanu, Liviu Săcărescu, Alexandru Suciu, Georgeta Alexandru, and et al. 2023. "Formulation of Phytosomes with Extracts of Ginger Rhizomes and Rosehips with Improved Bioavailability, Antioxidant and Anti-Inflammatory Effects In Vivo" Pharmaceutics 15, no. 4: 1066. https://doi.org/10.3390/pharmaceutics15041066
APA StyleDeleanu, M., Toma, L., Sanda, G. M., Barbălată, T., Niculescu, L. Ş., Sima, A. V., Deleanu, C., Săcărescu, L., Suciu, A., Alexandru, G., Crişan, I., Popescu, M., & Stancu, C. S. (2023). Formulation of Phytosomes with Extracts of Ginger Rhizomes and Rosehips with Improved Bioavailability, Antioxidant and Anti-Inflammatory Effects In Vivo. Pharmaceutics, 15(4), 1066. https://doi.org/10.3390/pharmaceutics15041066