Antioxidant Efficacy and “In Vivo” Safety of a Bentonite/Vitamin C Hybrid
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
2.2.1. Preparation of Raw Clay
2.2.2. Toxicological Studies
2.2.3. Preparation of Clay/Drug Composite
2.2.4. Physical–Chemical Characterization
2.2.5. Antioxidant Capacity
3. Results and Discussion
3.1. In Vivo Toxicity Studies
3.2. Characterization of Raw and Composite Materials
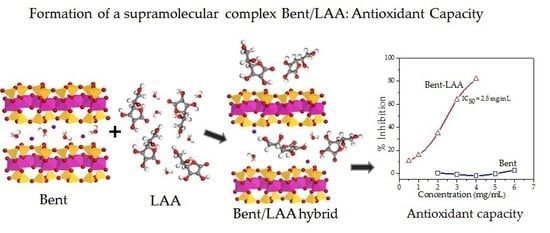
3.3. Antioxidant Activity
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ravetti, S.; Clemente, C.; Brignone, S.; Hergert, L.; Allemandi, D.; Palma, S. Ascorbic Acid in Skin Health. Cosmetics 2019, 6, 58. [Google Scholar] [CrossRef] [Green Version]
- Davey, M.W.; Montagu, M.V.; Inzé, D.; Sanmartin, M.; Kanellis, A.; Smirnoff, N.; Benzie, I.J.J.; Strain, J.J.; Favell, D.; Fletcher, J. Plant L-ascorbic acid: Chemistry, function, metabolism, bioavailability and effects of processing. J. Sci. Food Agr. 2000, 80, 825–860. [Google Scholar] [CrossRef]
- Johnston, C.S.; Steinberg, F.M.; Rucke, R.B. Handbook of Vitamins, 4th ed.; Taylor & Francis Group: Abingdon, UK, 2007. [Google Scholar]
- Baschieri, A.; Amorati, R.; Benelli, T.; Mazzocchetti, L.; D’Angelo, E.; Valgimigli, L. Enhanced Antioxidant Activity under Biomimetic Settings of Ascorbic Acid Included in Halloysite Nanotubes. Antioxidants 2019, 8, 30. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lin, J.-Y.; Selim, M.; Shea, C.R.; Grichnik, J.M.; Omar, M.M.; Monteiro-Riviere, N.; Pinnell, S.R. UV photoprotection by combination topical antioxidants vitamin C and vitamin E. J. Am. Acad. Dermatol. 2003, 48, 866–874. [Google Scholar] [CrossRef] [Green Version]
- Murad, S.; Grove, D.; Lindberg, K.A.; Reynolds, G.; Sivarajah, A.; Pinnell, S.R. Regulation of collagen synthesis by ascorbic acid. Proc. Natl. Acad. Sci. USA 1981, 78, 2879–2882. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Caritá, A.C.; Fonseca-Santos, B.; Shultz, J.D.; Michniak-Kohn, B.; Chorilli, M.; Leonardi, G.R. Vitamin C: One compound, several uses. Advances for delivery, efficiency and stability. Nanomed. Nanotechnol. Biol. Med. 2020, 24, 102117. [Google Scholar] [CrossRef]
- Lee, Y.-H.; Chen, B.-Y.; Lin, K.-Y.; Lin, K.-F.; Lin, F.-H. Feasibility study of using montmorillonite for stability enhancement of l-ascorbic acid. J. Chin. Inst. Chem. Eng. 2008, 39, 219–226. [Google Scholar] [CrossRef]
- Corvis, Y.; Menet, M.-C.; Négrier, P.; Lazerges, M.; Espeau, P. The role of stearic acid in ascorbic acid protection from degradation: A heterogeneous system for homogeneous thermodynamic data. New J. Chem. 2012, 37, 761–768. [Google Scholar] [CrossRef] [Green Version]
- Yuan, J.-P.; Chen, F. Degradation of Ascorbic Acid in Aqueous Solution. J. Agric. Food Chem. 1998, 46, 5078–5082. [Google Scholar] [CrossRef]
- Hernández, D.; Lazo, L.; Valdés, L.; de Ménorval, L.; Rozynek, Z.; Rivera, A. Synthetic clay mineral as nanocarrier of sulfamethoxazole and trimethoprim. Appl. Clay Sci. 2018, 161, 395–403. [Google Scholar] [CrossRef]
- Rivera, A.; Valdés, L.; Jiménez, J.; Pérez, I.; Lam, A.; Altshuler, E.; de Ménorval, L.; Fossum, J.; Hansen, E.; Rozynek, Z. Smectite as ciprofloxacin delivery system: Intercalation and temperature-controlled release properties. Appl. Clay Sci. 2016, 124–125, 150–156. [Google Scholar] [CrossRef] [Green Version]
- Valdés, L.; Hernández, D.; de Ménorval, L.C.; Pérez, I.; Altshuler, E.; Fossum, J.O.; Rivera, A. Incorporation of tramadol drug into Li-Fuorohectorite clay: A preliminary study of a medical nanofuid. Eur. Phys. J. Spec. Top. 2016, 225, 767–771. [Google Scholar] [CrossRef] [Green Version]
- Valdés, L.; Martín, S.A.; Hernández, D.; Lazo, L.; de Ménorval, L.C.; Rivera, A. Glycopeptide-Clay Nanocomposite: Chemical-Physical Characterization. Rev. Cuba. Fis. 2017, 34, 35–37. [Google Scholar]
- Valdés, L.; Pérez, I.; De Ménorval, L.C.; Altshuler, E.; Fossum, J.O.; Rivera, A. A simple way for targeted delivery of an antibiotic: In vitro evaluation of a nanoclay-based composite. PLoS ONE 2017, 12, e0187879. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hernández, D.; Quiñones, L.; Lazo, L.; Charnay, C.; Velázquez, M.; Altshuler, E.; Rivera, A. Removal of an emergent contaminant by a palygorskite from Pontezuela/Cuban region. J. Porous Mater. 2022, 1–13. [Google Scholar] [CrossRef]
- Martín, S.; Pérez, I.; Rivera, A. Hosting of the antibiotic Vancomycin by bentonite: Characterization and slow release study. Appl. Clay Sci. 2021, 202, 105965. [Google Scholar] [CrossRef]
- Martín, S.A.; Valdés, L.; Mérida, F.; de Ménorval, L.C.; Velázquez, M.; Rivera, A. Natural clay from Cuba for environmental remediation. Clay Miner. 2018, 53, 193–201. [Google Scholar] [CrossRef]
- Massaro, M.; Ciani, R.; Cinà, G.; Colletti, C.G.; Leone, F.; Riela, S. Antimicrobial Nanomaterials Based on Halloysite Clay Mineral: Research Advances and Outlook. Antibiotics 2022, 11, 1761. [Google Scholar] [CrossRef]
- Carretero, M.I.; Pozo, M. Clay and non-clay minerals in the pharmaceutical and cosmetic industries Part II. Active ingredients. Appl. Clay Sci. 2010, 47, 171–181. [Google Scholar] [CrossRef]
- Massaro, M.; Borrego-Sánchez, A.; Sánchez-Espejo, R.; Viseras, C.; Cavallaro, G.; García-Villén, F.; Guernelli, S.; Lazzara, G.; Miele, D.; Sainz-Díaz, I.; et al. Ciprofloxacin carrier systems based on hectorite/halloysite hybrid hydrogels for potential wound healing applications. Appl. Clay Sci. 2021, 215, 106310. [Google Scholar] [CrossRef]
- Viseras, C.; Carazo, E.; Borrego-Sánchez, A.; García-Villén, F.; Sánchez-Espejo, R.; Cerezo, P.; Aguzzi, C. Clay Minerals in Skin Drug Delivery. Clays Clay Miner. 2019, 67, 59–71. [Google Scholar] [CrossRef]
- Ghadiri, M.; Chrzanowski, W.; Rohanizadeh, R. Biomedical applications of cationic clay minerals. RSC Adv. 2015, 5, 29467–29481. [Google Scholar] [CrossRef]
- Anouar, F.; Elmchaouri, A.; Taoufik, N.; Rakhila, Y. Investigation of the ion exchange effect on surface properties and porous structure of clay: Application of ascorbic acid adsorption. J. Environ. Chem. Eng. 2019, 7, 103404. [Google Scholar] [CrossRef]
- Chen, B.-Y.; Lee, Y.-H.; Lin, W.-C.; Lin, F.-H.; Lin, K.-F. Understanding the characteristics of L-ascorbic acid-montmorillonite nanocomposite: Chemical structure and biotoxicity. Biomed. Eng. Appl. Basis Commun. 2006, 18, 30–36. [Google Scholar] [CrossRef] [Green Version]
- USP30-NF25; The United States Pharmacopeia-National Formulary. The United States Pharmacopeial Convention Inc.: Rockville, MD, USA, 2007.
- Organization for Economic Cooperation and Development. Test No. 405: Acute Eye Irritation/Corrosion, OECD Guideline for Testing of Chemicals; Section 4; OECD Publishing: Paris, France, 2002. [Google Scholar]
- Organization for Economic Cooperation and Development. Test No. 402: Acute Dermal Toxicity: Fixed Dose Procedure, OECD Guideline for Testing of Chemicals; Section 4; OECD Publishing: Paris, France, 2017. [Google Scholar]
- Rusche, B. The 3Rs and Animal Welfare—Conflict or the Way Forward? Altex 2003, 20, 63–76. [Google Scholar]
- Draize, J.H.; Woodard, G.; Calvery, H.O. Methods for the study of irritation and toxicity of substances applied topically to the skin and mucous membranes. J. Pharmacol. Exp. Ther. 1944, 82, 377. [Google Scholar]
- Njus, D.; Kelley, P.M.; Tu, Y.-J.; Schlegel, H.B. Ascorbic acid: The chemistry underlying its antioxidant properties. Free Radic. Biol. Med. 2020, 159, 37–43. [Google Scholar] [CrossRef]
- Brand-Williams, W.; Cuvelier, M.E.; Berset, C. Use of a free radical method to evaluate antioxidant activity. LWT Food Sci. Technol. 1995, 28, 25–30. [Google Scholar] [CrossRef]
- Garcia, E.J.; Oldoni, T.; de Alencar, S.M.; Reis, A.; Loguercio, A.D.; Grande, R.H.M. Antioxidant activity by DPPH assay of potential solutions to be applied on bleached teeth. Braz. Dent. J. 2012, 23, 22–27. [Google Scholar] [CrossRef]
- Moffat, A.C.; Osselton, M.D.; Widdop, B.; Watts, J. Clarke’ss Analysis of Drugs and Poisons, 3rd ed.; Pharmaceutical Press: London, UK, 2005. [Google Scholar]
- Mineralogy-Database. Available online: http://webmineral.com/ (accessed on 22 January 2020).
- Jendrzejewska, I.; Zajdel, P.; Pietrasik, E.; Barsova, Z.; Goryczka, T. Application of X-ray powder diffraction and differential scanning calorimetry for identification of counterfeit drugs. Monatsh. Chem. 2018, 149, 977–985. [Google Scholar] [CrossRef] [Green Version]
- Armour Research Foundation of Illinois Institute of Technology. Crystallographic Data-11. Ascorbic Acid. Anal. Chem. 1948, 20, 986. [Google Scholar]
- Sun, L.; Tanskanen, J.T.; Hirvi, J.T.; Kasa, S.; Schatz, T.; Pakkanen, T.A. Molecular dynamics study of montmorillonite crystalline swelling: Roles of interlayer cation species and water content. Chem. Phys. 2015, 455, 23–31. [Google Scholar] [CrossRef]
- Meleshyn, A.; Bunnenberg, C. The gap between crystalline and osmotic swelling of Na-montmorillonite: A Monte Carlo study. J. Chem. Phys. 2005, 122, 034705. [Google Scholar] [CrossRef] [PubMed]
- Diamond, S.; Kinter, E.B. Characterization of montmorillonite saturated with short-chain amine cations: 1. Interpretation of basal spacing measurements. Clays Clay Miner. 1961, 10, 163–173. [Google Scholar] [CrossRef]
- Mineralogy-Database. Available online: http://webmineral.com/data/Montmorillonite.shtml (accessed on 14 March 2023).
- Milanesio, M.; Bianchi, R.; Ugliengo, P.; Roetti, C.; Viterbo, D. Vitamin C at 120K: Experimental and theoretical study of the charge density. J. Mol. Struct. Theochem. 1997, 419, 139–154. [Google Scholar] [CrossRef]
- da Rocha, M.C.; de Araujo Braz, E.M.; Castro Honório, L.M.; Trigueiro, P.; Fonseca, M.G.; Cavalcanti Silva-Filho, E.; Medina Carrasco, S.; Sánchez Polo, M.; Viseras Iborra, C.; Anteveli Osajima, J. Understanding the effect of UV light in systems containing clay minerals and tetracycline. Appl. Clay Sci. 2019, 183, 105311. [Google Scholar] [CrossRef]
- Poch, O.; Jaber, M.; Stalport, F.; Nowak, S.; Georgelin, T.; Lambert, J.-F.; Szopa, C.; Coll, P. Effect of Nontronite Smectite Clay on the Chemical Evolution of Several Organic Molecules under Simulated Martian Surface Ultraviolet Radiation Conditions. Astrobiology 2015, 15, 221–237. [Google Scholar] [CrossRef] [Green Version]
- Madejová, J. FTIR techniques in clay mineral studies. Vib. Spectrosc. 2003, 31, 1–10. [Google Scholar] [CrossRef]
- Perelomov, L.; Sarkar, B.; Rahman, M.M.; Goryacheva, A.; Naidu, R. Uptake of lead by Na-exchanged and Al-pillared bentonite in the presence of organic acids with different functional groups. Appl. Clay Sci. 2016, 119, 417–423. [Google Scholar] [CrossRef]
- Caglar, B.; Afsin, B.; Tabak, A.; Eren, E. Characterization of the cation-exchanged bentonites by XRPD, ATR, DTA/TG analyses and BET measurement. Chem. Eng. J. 2009, 149, 242–248. [Google Scholar] [CrossRef]
- Panicker, C.Y.; Varghese, H.T.; Philip, D. FT-IR, FT-Raman and SERS spectra of Vitamin C. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2006, 65, 802–804. [Google Scholar] [CrossRef] [PubMed]
- Zapata, F.; López-Fernández, A.; Ortega-Ojeda, F.; Quintanilla, G.; García-Ruiz, C.; Montalvo, G. Introducing ATR-FTIR Spectroscopy through Analysis of Acetaminophen Drugs: Practical Lessons for Interdisciplinary and Progressive Learning for Undergraduate Students. J. Chem. Educ. 2021, 98, 2675–2686. [Google Scholar] [CrossRef] [PubMed]
- Madhusha, C.; Munaweera, I.; Karunaratne, V.; Kottegoda, N. Facile Mechanochemical Approach to Synthesizing Edible Food Preservation Coatings Based On Alginate/Ascorbic Acid-Layered Double Hydroxide Bio-Nanohybrids. J. Agric. Food Chem. 2020, 68, 8962–8975. [Google Scholar] [CrossRef] [PubMed]
- Zuzana, D.; Annamária, M.; Silvia, D.; Jaroslav, B. Effect of thermal treatment on the bentonite properties. Arh. Teh. Nauk. 2012, 4, 49–56. [Google Scholar]
- Anirudhan, T.; Jalajamony, S.; Sreekumari, S. Adsorption of heavy metal ions from aqueous solutions by amine and carboxylate functionalised bentonites. Appl. Clay Sci. 2012, 65–66, 67–71. [Google Scholar] [CrossRef]
- Zhang, Y.; Liu, Q.; Wu, Z.; Zhang, Y. Thermal behavior analysis of two bentonite samples selected from China. J. Therm. Anal. Calorim. 2015, 121, 1287–1295. [Google Scholar] [CrossRef]
- Kök, M.V. The Chemistry, Kinetics, and Potential Utilization of Different Origins of Bentonite Samples. Energy Sources Part A Recover. Util. Environ. Eff. 2013, 36, 173–183. [Google Scholar] [CrossRef]
- Stagnaro, S.M.; Rueda, M.; Volzone, C.; Ortiga, J. Structural Modification of a Lamellar Solid by Thermal Treatment. Effect on the Cd and Pb Adsorptions from Aqueous Solution. Procedia Mater. Sci. 2012, 1, 180–184. [Google Scholar] [CrossRef] [Green Version]
- Stagnaro, S.Y.M.; Volzone, C.; Rueda, M.L. Influence of thermal treatment on bentonite used as adsorbent for Cd, Pb, Zn retention from mono-solute and poly-solute aqueous solutions. Mater. Res. 2012, 15, 549–553. [Google Scholar] [CrossRef] [Green Version]
- Mackenzie, R.C. The Montmorillonite differential thermal curve. I. General Variability in the Dehydroxylation Region. In Bulletin du Groupe Français des Argiles; Editions du Centre National de la Recherche Scientifique: Paris, France, 1957; Volume 9, pp. 7–15. [Google Scholar]
- Földvári, M. Handbook of Thermogravimetric System of Minerals and Its Use in Geological Practice; Fancsik, T., Ed.; Geological Institute of Hungary—Kiadja a Magyar Állami Földtani Intézet: Budapest, Hungary, 2011. [Google Scholar]
- ymankowska-Kumon, S.; Holtzer, M.; Grabowski, G. Thermal analysis of foundry bentonites. Arch. Foundry Eng. 2011, 11, 209–213. [Google Scholar]
- Yılmaz, M.S.; Kalpaklı, Y.; Pis¸kin, S. Thermal behavior and dehydroxylation kinetics of naturally occurring sepiolite and bentonite. J. Therm. Anal. Calorim. 2013, 114, 1191–1199. [Google Scholar] [CrossRef]
- Sternik, D.; Galaburda, M.; Bogatyrov, V.M.; Gun’Ko, V.M. Influence of the Synthesis Method on the Structural Characteristics of Novel Hybrid Adsorbents Based on Bentonite. Colloids Interfaces 2019, 3, 18. [Google Scholar] [CrossRef] [Green Version]
- Jingyan, S.; Yuwen, L.; Zhiyong, W.; Cunxin, W. Investigation of thermal decomposition of ascorbic acid by TG-FTIR and thermal kinetics analysis. J. Pharm. Biomed. Anal. 2013, 77, 116–119. [Google Scholar] [CrossRef] [PubMed]
- Souza, V.C.; Oliveira, J.E.; Lima, S.J.G.; Silva, L.B. Influence of Vitamin C on Morphological and Thermal Behaviour of Biomedical UHMWPE. Macromol. Symp. 2014, 344, 8–13. [Google Scholar] [CrossRef]
- Zaka, E.E.; Güler, C. The effects of electrolyte concentration, ion species and ph on the zeta potential and electrokinetic charge density of montmorillonite. Clay Miner. 2006, 41, 853–861. [Google Scholar]
- Duc, M.; Gaboriaud, F.; Thomas, F. Sensitivity of the acid–base properties of clays to the methods of preparation and measurement. J. Colloid Interface Sci. 2005, 289, 139–147. [Google Scholar] [CrossRef]
- White, J.L.; Hem, S.L. Pharmaceutical aspects of clay-organic interactions. Ind. Eng. Chem. Prod. Res. Dev. 1983, 22, 665–671. [Google Scholar] [CrossRef]
- Yang, J.-H.; Lee, J.-H.; Ryu, H.-J.; Elzatahry, A.A.; Alothman, Z.A.; Choy, J.-H. Drug-clay nanohybrids as sustained delivery systems. Appl. Clay Sci. 2016, 130, 20–32. [Google Scholar] [CrossRef]







| Sample | SiO2 | Al2O3 | Fe2O3 | Na2O | MgO | CaO | TiO2 | K2O | MnO | LOI 1 |
|---|---|---|---|---|---|---|---|---|---|---|
| Bent | 48.86 | 19.05 | 9.42 | 2.81 | 2.26 | 1.71 | 0.82 | 0.21 | 0.07 | 12.68 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hernández, D.; Montalvo, A.; Pérez, I.; Charnay, C.; Sánchez-Espejo, R.; Cerezo, P.; Viseras, C.; Riela, S.; Cinà, G.; Rivera, A. Antioxidant Efficacy and “In Vivo” Safety of a Bentonite/Vitamin C Hybrid. Pharmaceutics 2023, 15, 1171. https://doi.org/10.3390/pharmaceutics15041171
Hernández D, Montalvo A, Pérez I, Charnay C, Sánchez-Espejo R, Cerezo P, Viseras C, Riela S, Cinà G, Rivera A. Antioxidant Efficacy and “In Vivo” Safety of a Bentonite/Vitamin C Hybrid. Pharmaceutics. 2023; 15(4):1171. https://doi.org/10.3390/pharmaceutics15041171
Chicago/Turabian StyleHernández, Dayaris, Anaela Montalvo, Irela Pérez, Clarence Charnay, Rita Sánchez-Espejo, Pilar Cerezo, César Viseras, Serena Riela, Giuseppe Cinà, and Aramis Rivera. 2023. "Antioxidant Efficacy and “In Vivo” Safety of a Bentonite/Vitamin C Hybrid" Pharmaceutics 15, no. 4: 1171. https://doi.org/10.3390/pharmaceutics15041171
APA StyleHernández, D., Montalvo, A., Pérez, I., Charnay, C., Sánchez-Espejo, R., Cerezo, P., Viseras, C., Riela, S., Cinà, G., & Rivera, A. (2023). Antioxidant Efficacy and “In Vivo” Safety of a Bentonite/Vitamin C Hybrid. Pharmaceutics, 15(4), 1171. https://doi.org/10.3390/pharmaceutics15041171