Hyperbranched Copolymers of Methacrylic Acid and Lauryl Methacrylate H-P(MAA-co-LMA): Synthetic Aspects and Interactions with Biorelevant Compounds
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Synthesis of H-P(MAA-co-LMA) Copolymers
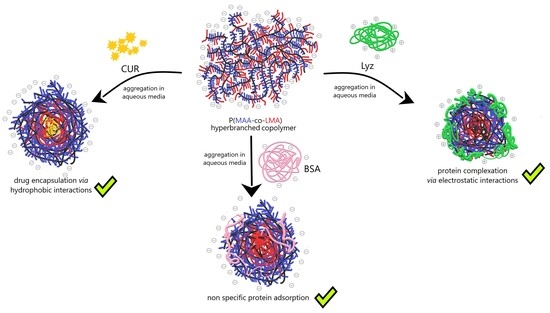
2.3. Preparation of Self-Assembled PNPs in Aqueous Solutions
2.4. Preparation of CUR-Loaded NPs
2.5. Preparation of HC Complexes with Lyz
2.6. Preparation of HC–BSA Co-Assemblies
2.7. Experimental Techniques
2.7.1. Size Exclusion Chromatography (SEC)
2.7.2. Proton Nuclear Magnetic Resonance Spectroscopy (1H-NMR)
2.7.3. Attenuated Total Reflectance–Fourier Transform Infrared (ATR–FTIR) Spectroscopy
2.7.4. Dynamic Light Scattering (DLS)
2.7.5. Electrophoretic Light Scattering (ELS)
2.7.6. Fluorescence Spectroscopy
2.7.7. UV-Vis Spectroscopy
3. Results & Discussion
3.1. HC Synthesis and Molecular Characterization
3.2. Self-Assembly Studies
3.2.1. CAC Determination
3.2.2. Composition Dependency and pH-Sensitivity
3.3. Lyz Complexation Studies
3.4. CUR Loading Study
3.5. HC–BSA Co-Assembly Studies
3.6. Stability Studies
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bader, H.; Ringsdorf, H.; Schmidt, B. Watersoluble Polymers in Medicine. Angew. Makromol. Chem. 1984, 123, 457–485. [Google Scholar] [CrossRef]
- Yokoyama, M. Polymeric Micelles as Drug Carriers: Their Lights and Shadows. J. Drug Target. 2014, 22, 576–583. [Google Scholar] [CrossRef]
- Osorno, L.L.; Brandley, A.N.; Maldonado, D.E.; Yiantsos, A.; Mosley, R.J.; Byrne, M.E. Review of Contemporary Self-Assembled Systems for the Controlled Delivery of Therapeutics in Medicine. Nanomaterials 2021, 11, 278. [Google Scholar] [CrossRef] [PubMed]
- Bodratti, A.M.; Alexandridis, P. Amphiphilic Block Copolymers in Drug Delivery: Advances in Formulation Structure and Performance. Expert Opin. Drug Deliv. 2018, 15, 1085–1104. [Google Scholar] [CrossRef] [PubMed]
- Kurmaz, S.V.; Ignatiev, V.M.; Emel’yanova, N.S.; Kurmaz, V.A.; Konev, D.V.; Balakina, A.A.; Terentyev, A.A. New Nanosized Systems Doxorubicin—Amphiphilic Copolymers of N-Vinylpyrrolidone and (Di)Methacrylates with Antitumor Activity. Pharmaceutics 2022, 14, 2572. [Google Scholar] [CrossRef]
- Lorenzo-Veiga, B.; Sigurdsson, H.H.; Loftsson, T.; Alvarez-Lorenzo, C. Cyclodextrin–Amphiphilic Copolymer Supramolecular Assemblies for the Ocular Delivery of Natamycin. Nanomaterials 2019, 9, 745. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Truong, D.H.; Tran, T.H.; Ramasamy, T.; Choi, J.Y.; Choi, H.G.; Yong, C.S.; Kim, J.O. Preparation and Characterization of Solid Dispersion Using a Novel Amphiphilic Copolymer to Enhance Dissolution and Oral Bioavailability of Sorafenib. Powder Technol. 2015, 283, 260–265. [Google Scholar] [CrossRef]
- Lin, W.; He, Y.; Zhang, J.; Wang, L.; Ji, F.; Chen, S. Highly hemocompatible zwitterionic micelles stabilized by reversible cross-linkage for anti-cancer drug delivery. Colloids Surf. B 2014, 115, 384–390. [Google Scholar] [CrossRef]
- Soleymani Abyaneh, H.; Vakili, M.R.; Zhang, F.; Choi, P.; Lavasanifar, A. Rationale design of block copolymer micelles to control burst drug release at a nanoscale dimension. Acta Biomater. 2015, 24, 127–139. [Google Scholar] [CrossRef] [PubMed]
- Hasannia, M.; Aliabadi, A.; Abnous, K.; Taghdisi, S.M.; Ramezani, M.; Alibolandi, M. Synthesis of Block Copolymers Used in Polymersome Fabrication: Application in Drug Delivery. J. Control. Release 2022, 341, 95–117. [Google Scholar] [CrossRef]
- Corrigan, N.; Jung, K.; Moad, G.; Hawker, C.J.; Matyjaszewski, K.; Boyer, C. Reversible-Deactivation Radical Polymerization (Controlled/Living Radical Polymerization): From Discovery to Materials Design and Applications. Prog. Polym. Sci. 2020, 111, 101311. [Google Scholar] [CrossRef]
- Gigmes, D.; Trimaille, T. Advances in Amphiphilic Polylactide/Vinyl Polymer Based Nano-Assemblies for Drug Delivery. Adv. Colloid Interface Sci. 2021, 294, 102483. [Google Scholar] [CrossRef] [PubMed]
- Mai, Y.; Eisenberg, A. Self-Assembly of Block Copolymers. Chem. Soc. Rev. 2012, 41, 5969–5985. [Google Scholar] [CrossRef]
- Kuperkar, K.; Tiwari, S.; Bahadur, P. Self-Assembled Block Copolymer Nanoaggregates for Drug Delivery Applications. In Applications of Polymers in Drug Delivery; Elsevier: Amsterdam, The Netherlands, 2021; pp. 423–447. ISBN 9780128196595. [Google Scholar]
- Terashima, T. Controlled Self-Assembly of Amphiphilic Random Copolymers into Folded Micelles and Nanostructure Materials. J. Oleo Sci. 2020, 69, 529–538. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Grayson, S.M. Approaches for the Preparation of Non-Linear Amphiphilic Polymers and Their Applications to Drug Delivery. Adv. Drug Deliv. Rev. 2012, 64, 852–865. [Google Scholar] [CrossRef]
- Cook, A.B.; Perrier, S. Branched and Dendritic Polymer Architectures: Functional Nanomaterials for Therapeutic Delivery. Adv. Funct. Mater. 2020, 30, 1901001. [Google Scholar] [CrossRef] [Green Version]
- Bej, R.; Rajdev, P.; Barman, R.; Ghosh, S. Hyperbranched Polydisulfides. Polym. Chem. 2020, 11, 990–1000. [Google Scholar] [CrossRef]
- Bera, S.; Barman, R.; Ghosh, S. Hyperbranched vs. Linear Poly(disulfide) for Intracellular Drug Delivery. Polym. Chem. 2022, 13, 5188–5192. [Google Scholar] [CrossRef]
- Tang, Q.; Cheng, F.; Lou, X.L.; Liu, H.J.; Chen, Y. Comparative Study of Thiol-Free Amphiphilic Hyperbranched and Linear Polymers for the Stabilization of Large Gold Nanoparticles in Organic Solvent. J. Colloid Interface Sci. 2009, 337, 485–491. [Google Scholar] [CrossRef]
- Li, Z.; Zhang, Z.; Liu, K.L.; Ni, X.; Li, J. Biodegradable Hyperbranched Amphiphilic Polyurethane Multiblock Copolymers Consisting of Poly(propylene glycol), Poly(ethylene glycol), and Polycaprolactone as in Situ Thermogels. Biomacromolecules 2012, 13, 3977–3989. [Google Scholar] [CrossRef]
- Namivandi-Zangeneh, R.; Kwan, R.J.; Nguyen, T.K.; Yeow, J.; Byrne, F.L.; Oehlers, S.H.; Wong, E.H.H.; Boyer, C. The Effects of Polymer Topology and Chain Length on the Antimicrobial Activity and Hemocompatibility of Amphiphilic Ternary Copolymers. Polym. Chem. 2018, 9, 1735–1744. [Google Scholar] [CrossRef]
- Martin, C.; Aibani, N.; Callan, J.F.; Callan, B. Recent Advances in Amphiphilic Polymers for Simultaneous Delivery of Hydrophobic and Hydrophilic Drugs. Ther. Deliv. 2016, 7, 15–31. [Google Scholar] [CrossRef]
- Karimi, M.; Eslami, M.; Sahandi-Zangabad, P.; Mirab, F.; Farajisafiloo, N.; Shafaei, Z.; Ghosh, D.; Bozorgomid, M.; Dashkhaneh, F.; Hamblin, M.R. PH-Sensitive Stimulus-Responsive Nanocarriers for Targeted Delivery of Therapeutic Agents. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnol. 2016, 8, 696–716. [Google Scholar] [CrossRef] [Green Version]
- Kavand, A.; Anton, N.; Vandamme, T.; Serra, C.A.; Chan-Seng, D. Synthesis and Functionalization of Hyperbranched Polymers for Targeted Drug Delivery. J. Control. Release 2020, 321, 285–311. [Google Scholar] [CrossRef] [PubMed]
- Ban, Q.; Sun, W.; Kong, J.; Wu, S. Hyperbranched Polymers with Controllable Topologies for Drug Delivery. Chem. Asian J. 2018, 13, 3341–3350. [Google Scholar] [CrossRef] [PubMed]
- Scheibe, P.; Barz, M.; Hemmelmann, M.; Zentel, R. Langmuir-Blodgett Films of Biocompatible Poly(HPMA)-Block-Poly(lauryl methacrylate) and Poly(HPMA)-Random-Poly(lauryl methacrylate): Influence of Polymer Structure on Membrane Formation and Stability. Langmuir 2010, 26, 5661–5669. [Google Scholar] [CrossRef] [PubMed]
- Klinger, D.; Landfester, K. Dual Stimuli-Responsive Poly(2-hydroxyethyl methacrylate-co-methacrylic acid) Microgels Based on Photo-Cleavable Cross-Linkers: PH-Dependent Swelling and Light-Induced Degradation. Macromolecules 2011, 44, 9758–9772. [Google Scholar] [CrossRef]
- Zhong, J.X.; Clegg, J.R.; Ander, E.W.; Peppas, N.A. Tunable Poly(methacrylic acid-co-acrylamide) Nanoparticles through Inverse Emulsion Polymerization. J. Biomed. Mater. Res. A 2018, 106, 1677–1686. [Google Scholar] [CrossRef]
- Kumar, A.; Lahiri, S.S.; Punyani, S.; Singh, H. Synthesis and Characterization of PH Sensitive Poly(PEGDMA-MAA) Copolymeric Microparticles for Oral Insulin Delivery. J. Appl. Polym. Sci. 2008, 107, 863–871. [Google Scholar] [CrossRef]
- Artar, M.; Terashima, T.; Sawamoto, M.; Meijer, E.W.; Palmans, A.R.A. Understanding the Catalytic Activity of Single-Chain Polymeric Nanoparticles in Water. J. Polym. Sci. A Polym. Chem. 2014, 52, 12–20. [Google Scholar] [CrossRef]
- Iatridi, Z.; Georgiadou, V.; Menelaou, M.; Dendrinou-Samara, C.; Bokias, G. Application of Hydrophobically Modified Water-Soluble Polymers for the Dispersion of Hydrophobic Magnetic Nanoparticles in Aqueous Media. Dalton Trans. 2014, 43, 8633–8643. [Google Scholar] [CrossRef] [PubMed]
- Sevimli, S.; Knight, F.C.; Gilchuk, P.; Joyce, S.; Wilson, J.T. Fatty Acid-Mimetic Micelles for Dual Delivery of Antigens and Imidazoquinoline Adjuvants. ACS Biomater. Sci. Eng. 2017, 3, 179–194. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Perrier, S. 50th Anniversary Perspective: RAFT Polymerization—A User Guide. Macromolecules 2017, 50, 7433–7447. [Google Scholar] [CrossRef]
- Smeets, N.M.B. Amphiphilic Hyperbranched Polymers from the Copolymerization of a Vinyl and Divinyl Monomer: The Potential of Catalytic Chain Transfer Polymerization. Eur. Polym. J. 2013, 49, 2528–2544. [Google Scholar] [CrossRef] [Green Version]
- Jiang, W.; Zhou, Y.; Yan, D. Hyperbranched Polymer Vesicles: From Self-Assembly, Characterization, Mechanisms, and Properties to Applications. Chem. Soc. Rev. 2015, 44, 3874–3889. [Google Scholar] [CrossRef] [PubMed]
- Rikkou-Kalourkoti, M.; Elladiou, M.; Patrickios, C.S. Synthesis and Characterization of Hyperbranched Amphiphilic Block Copolymers Prepared via Self-Condensing RAFT Polymerization. J. Polym. Sci. A Polym. Chem. 2015, 53, 1310–1319. [Google Scholar] [CrossRef]
- Liu, J.; Huang, W.; Pang, Y.; Zhu, X.; Zhou, Y.; Yan, D. Self-Assembled Micelles from an Amphiphilic Hyperbranched Copolymer with Polyphosphate Arms for Drug Delivery. Langmuir 2010, 26, 10585–10592. [Google Scholar] [CrossRef] [PubMed]
- Luzon, M.; Boyer, C.; Peinado, C.; Corrales, T.; Whittaker, M.; Tao, L.; Davis, T.P. Water-Soluble, Thermoresponsive, Hyperbranched Copolymers Based on PEG-Methacrylates: Synthesis, Characterization, and LCST Behavior. J. Polym. Sci. A Polym. Chem. 2010, 48, 2783–2792. [Google Scholar] [CrossRef]
- Weaver, J.V.M.; Williams, R.T.; Royles, B.J.L.; Findlay, P.H.; Cooper, A.I.; Rannard, S.P. PH-Responsive Branched Polymer Nanoparticles. Soft Matter 2008, 4, 985–992. [Google Scholar] [CrossRef]
- Neugebauer, D.; Mielańczyk, A.; Bielas, R.; Odrobińska, J.; Kupczak, M.; Niesyto, K. Ionic Polymethacrylate Based Delivery Systems: Effect of Carrier Topology and Drug Loading. Pharmaceutics 2019, 11, 337. [Google Scholar] [CrossRef] [Green Version]
- Domnina, Y.A.; Yeo, Y.; Tse, J.Y.; Bellas, E.; Kohane, D.S. Spray-dried lipid-hyaluronan-polymethacrylate microparticles for drug delivery in the peritoneum. J. Biomed. Mater. Res. A 2008, 87, 825–831. [Google Scholar] [CrossRef]
- Shi, X.; Ye, Y.; Wang, H.; Liu, F.; Wang, Z. Designing pH-Responsive Biodegradable Polymer Coatings for Controlled Drug Release via Vapor-Based Route. ACS Appl. Mater. Interfaces 2018, 10, 38449–38458. [Google Scholar] [CrossRef] [PubMed]
- Thakral, S.; Thakral, N.K.; Majumdar, D.K. Eudragit®: A technology evaluation. Expert Opin. Drug Deliv. 2012, 10, 131–149. [Google Scholar] [CrossRef] [PubMed]
- Hemmelmann, M.; Metz, V.V.; Koynov, K.; Blank, K.; Postina, R.; Zentel, R. Amphiphilic HPMA–LMA copolymers increase the transport of Rhodamine 123 across a BBB model without harming its barrier integrity. J. Control. Release 2012, 163, 170–177. [Google Scholar] [CrossRef] [PubMed]
- Sezgin-bayindir, Z.; Ergin, A.D.; Parmaksiz, M.; Elcin, A.E.; Elcin, Y.M.; Yuksel, N. Evaluation of Various Block Copolymers for Micelle Formation and Brain Drug Delivery: In Vitro Characterization and Cellular Uptake Studies. J. Drug Deliv. Sci. Technol. 2016, 36, 120–129. [Google Scholar] [CrossRef]
- Iborra, A.; Díaz, G.; López, D.; Giussi, J.M.; Azzaroni, O. Copolymer Based on Lauryl Methacrylate and Poly(ethylene glycol) Methyl Ether Methacrylate as Amphiphilic Macrosurfactant: Synthesis, Characterization and Their Application as Dispersing Agent for Carbon Nanotubes. Eur. Polym. J. 2017, 87, 308–317. [Google Scholar] [CrossRef]
- Kilicarislan Ozkan, C.; Yılmaz, O.; Cheaburu, C.N.; Ata Karavana, H.; Kılıçarislan Özkan, Ç.; Özgünay, H.; Yılmaz, C.N.; Yorgancıoğlu, A. Preparation of alkoxysilane functional water soluble block copolymers via raft polymerization. J. Int. Sci. Publ. 2017, 11, 274–286. [Google Scholar]
- Atayde, E.; Arco, S. Temperature-and PH-Dependent Drug Release of Block Copolymers of Methacrylic Acid and Poly(Ethylene Glycol) Methyl Ether Methacrylates. Philipp. J. Sci. 2018, 147, 363–372. [Google Scholar]
- Ashenagar, S.; Ziaee, F.; Jalilian, S.M. Calculation of Reactivity Ratios of Methacrylic Acid-Ethyl Acrylate Copolymer by on-Line Quantitative 1H NMR Spectroscopy. Iran. Polym. J. 2013, 22, 635–639. [Google Scholar] [CrossRef]
- González-Chomón, C.; Garamus, V.M.; Rangelov, S.; Ebdon, J.R.; Novakov, C.; Halacheva, S.S. Trimethoxysilyl End-Capped Hyperbranched Polyglycidol/Polycaprolactone Copolymers for Cell Delivery and Tissue Repair: Synthesis, Characterisation and Aqueous Solution Properties. Eur. Polym. J. 2019, 112, 648–659. [Google Scholar] [CrossRef]
- Cheng, D.B.; Yang, P.P.; Cong, Y.; Liu, F.H.; Qiao, Z.Y.; Wang, H. One-Pot Synthesis of PH-Responsive Hyperbranched Polymer-Peptide Conjugates with Enhanced Stability and Loading Efficiency for Combined Cancer Therapy. Polym. Chem. 2017, 8, 2462–2471. [Google Scholar] [CrossRef]
- Ray, P.; Alhalhooly, L.; Ghosh, A.; Choi, Y.; Banerjee, S.; Mallik, S.; Banerjee, S.; Quadir, M. Size-Transformable, Multifunctional Nanoparticles from Hyperbranched Polymers for Environment-Specific Therapeutic Delivery. ACS Biomater. Sci. Eng. 2019, 5, 1354–1365. [Google Scholar] [CrossRef] [PubMed]
- Topuzogullari, M.; Bulmus, V.; Dalgakiran, E.; Dincer, S. PH- and Temperature-Responsive Amphiphilic Diblock Copolymers of 4-Vinylpyridine and Oligoethyleneglycol Methacrylate Synthesized by RAFT Polymerization. Polymer 2014, 55, 525–534. [Google Scholar] [CrossRef] [Green Version]
- Lotocki, V.; Kakkar, A. Miktoarm Star Polymers: Branched Architectures in Drug Delivery. Pharmaceutics 2020, 12, 827. [Google Scholar] [CrossRef] [PubMed]
- Kapse, A.; Anup, N.; Patel, V.; Saraogi, G.K.; Mishra, D.K.; Tekade, R.K. Polymeric Micelles: A Ray of Hope among New Drug Delivery Systems. In Drug Delivery Systems; Elsevier: Amsterdam, The Netherlands, 2019; pp. 235–289. ISBN 9780128145081. [Google Scholar]
- Kim, B.; Shin, Y. PH-Sensitive Swelling and Release Behaviors of Anionic Hydrogels for Intelligent Drug Delivery System. J. Appl. Polym. Sci. 2007, 105, 3656–3661. [Google Scholar] [CrossRef]
- Ofridam, F.; Tarhini, M.; Lebaz, N.; Gagnière, E.; Mangin, D.; Elaïssari, A.; Gagnière, É.; Elaissari, A. PH-Sensitive Polymers: Classification and Some Fine Potential Applications. Polym. Adv. Technol. 2021, 32, 1455–1484. [Google Scholar] [CrossRef]
- Wu, F.G.; Jiang, Y.W.; Sun, H.Y.; Luo, J.J.; Yu, Z.W. Complexation of Lysozyme with Sodium Poly(styrenesulfonate) via the Two-State and Non-Two-State Unfoldings of Lysozyme. J. Phys. Chem. B 2015, 119, 14382–14392. [Google Scholar] [CrossRef] [PubMed]
- Steudle, A.; Pleiss, J. Modelling of Lysozyme Binding to a Cation Exchange Surface at Atomic Detail: The Role of Flexibility. Biophys. J. 2011, 100, 3016–3024. [Google Scholar] [CrossRef] [Green Version]
- Chernysheva, M.G.; Shnitko, A.V.; Ksenofontov, A.L.; Arutyunyan, A.M.; Petoukhov, M.V.; Badun, G.A. Structural Peculiarities of Lysozyme–PLURONIC Complexes at the Aqueous-Air and Liquid-Liquid Interfaces and in the Bulk of Aqueous Solution. Int. J. Biol. Macromol. 2020, 158, 721–731. [Google Scholar] [CrossRef] [PubMed]
- Sentoukas, T.; Pispas, S. Poly(2-(dimethylamino)ethyl methacrylate)-b-Poly(hydroxypropyl methacrylate) Copolymers/Bovine Serum Albumin Complexes in Aqueous Solutions. J. Polym. Sci. 2020, 58, 1241–1252. [Google Scholar] [CrossRef]
- Ben Amara, C.; Degraeve, P.; Oulahal, N.; Gharsallaoui, A. PH-Dependent Complexation of Lysozyme with Low Methoxyl (LM) Pectin. Food Chem. 2017, 236, 127–133. [Google Scholar] [CrossRef] [PubMed]
- Chang, T.; Trench, D.; Putnam, J.; Stenzel, M.H.; Lord, M.S. Curcumin-Loading-Dependent Stability of PEGMEMA-Based Micelles Affects Endocytosis and Exocytosis in Colon Carcinoma Cells. Mol. Pharm. 2016, 13, 924–932. [Google Scholar] [CrossRef]
- Yallapu, M.M.; Jaggi, M.; Chauhan, S.C. Curcumin Nanoformulations: A Future Nanomedicine for Cancer. Drug Discov. Today 2012, 17, 71–80. [Google Scholar] [CrossRef] [Green Version]
- Mondal, S.; Ghosh, S. Role of Curcumin on the Determination of the Critical Micellar Concentration by Absorbance, Fluorescence and Fluorescence Anisotropy Techniques. J. Photochem. Photobiol. B 2012, 115, 9–15. [Google Scholar] [CrossRef] [PubMed]
- Rani, S.; Mishra, S.; Sharma, M.; Nandy, A.; Mozumdar, S. Solubility and Stability Enhancement of Curcumin in Soluplus® Polymeric Micelles: A Spectroscopic Study. J. Dispers. Sci. Technol. 2020, 41, 523–536. [Google Scholar] [CrossRef]
- Wang, X.; Gao, Y. Effects of Length and Unsaturation of the Alkyl Chain on the Hydrophobic Binding of Curcumin with Tween Micelles. Food Chem. 2018, 246, 242–248. [Google Scholar] [CrossRef]
- Zhou, M.; Bi, Y.; Zhou, H.; Chen, X.; Zhang, F.; Li, Y.; Qu, X. Aggregation Behavior of Poly(acrylic acid-co-octadecyl methacrylate) and Bovine Serum Albumin in Aqueous Solutions. ChemistryOpen 2021, 10, 373–379. [Google Scholar] [CrossRef] [PubMed]
- Agnihotri, P.; Sangeeta; Aery, S.; Dan, A. Temperature- and PH-Responsive Poly(N-isopropylacrylamide-co-methacrylic acid) Microgels as a Carrier for Controlled Protein Adsorption and Release. Soft Matter 2021, 17, 9595–9606. [Google Scholar] [CrossRef]
- Mansuroglu, B.; Karaman, M.; Derman, S.A.; Akdeste, Z.M.; Kizilbey, K.; Mansuroglu, B.; Derman, S.; Budama, Y.; Mustafaeva Akdeste, B.Z. Conjugation of BSA Protein and VP/AA Copolymers Melanoma Hastalğını Oluşturan Sentetik Peptidlerin Poliakrilikasit ve Kopolimerleri Ile Konjugasyonu View Project Kafeik Asit Fenetil Ester Yüklü Nanopartiküllerin Üretimi ve Antimikrobiyal Aktivitelerinin İncelenmesi View Project Kadriye Kizilbey Yeni Yüzyil Üniversitesi Conjugation of BSA Protein and VP/AA Copolymers. Int. J. Nat. Eng. Sci. 2009, 3, 36–40. [Google Scholar]












| HC | Initial Feed Ratio c | Mw a (g/mol) (×104) | Mw/Mn a | %wt LMA Theoretical | %wt LMA b |
|---|---|---|---|---|---|
| HC 1 | [40.8]:[6]:[2]:[1]:[0.5] | 3.8 | 1.26 | 30 | 41 |
| HC 2 | [29]:[9.8]:[2]:[1]:[0.5] | 3.6 | 1.27 | 50 | 52 |
| HC Samples | I90° (a.u.) | Rh (nm) | PDI | ζp (mV) | CAC (g mL−1) |
|---|---|---|---|---|---|
| HC 1 pH3 | 2470 | 244 (71%)/67 (29%) | 0.43 | −7.3 | -- |
| HC 1 pH7 | 166 | 40 (92%)/5 (8%) | 0.44 | −17.8 | 4.66 × 10−6 |
| HC 1 pH10 | 176 | 33 (90%)/6 (10%) | 0.46 | −41.8 | -- |
| HC 2 pH3 | 16 | 65 | 0.66 | −6.1 | -- |
| HC 2 pH7 | 180 | 21 | 0.54 | −11.2 | 4.13 × 10−6 |
| HC 2 pH10 | 67 | 22 | 0.51 | −39.3 | -- |
| HC | HC:Lyz Ratio | Cpolymer (g/mL) | Clysozyme (g/mL) | I90° (a.u.) | PDI | Rh (nm) | ζp (mV) |
|---|---|---|---|---|---|---|---|
| 1 | HCNon complexed | 1.25 × 10−4 | - | 44 | 0.48 | 26 (almost bimodal) | −27.0 |
| 1 | HC:Lyz = 2:1 | 1.25 × 10−4 | 9.28 × 10−4 | 2580 | 0.21 | 70 (98%)/14 (2%) | +24 |
| 1 | HC:Lyz = 1.5:1 | 1.25 × 10−4 | 12.37 × 10−4 | 1355 | 0.21 | 87 (99%)/9 (1%) | +20 |
| 1 | HC:Lyz = 1:1 | 1.25 × 10−4 | 18.56 × 10−4 | 535 | 0.25 | 117 (85%)/38 (14%) | +19 |
| 1 | HC:Lyz = 1:1.5 | 1.25 × 10−4 | 27.83 × 10−4 | 104 | 0.41 | 94 (95%)/15 (5%) | +25 |
| 1 | HC:Lyz = 1:2 | 1.25 × 10−4 | 37.11 × 10−4 | 30 | 0.65 | 122 (90%)/6 (10%) | +17 |
| 2 | HCNon complexed | 0.75 × 10−4 | - | 3177 | 0.12 | 45 | −31.1 |
| 2 | HC:Lyz = 2:1 | 0.75 × 10−4 | 3.98 × 10−4 | 4250 | 0.10 | 55 (97%)/15 (3%) | +31.2 |
| 2 | HC:Lyz = 1.5:1 | 0.75 × 10−4 | 5.30 × 10−4 | 4000 | 0.10 | 54 | +26.8 |
| 2 | HC:Lyz = 1:1 | 0.75 × 10−4 | 7.95 × 10−4 | 4500 | 0.10 | 55 (91%)/25 (9%) | +35.5 |
| 2 | HC:Lyz = 1:1.5 | 0.75 × 10−4 | 11.93 × 10−4 | 4920 | 0.12 | 53 (97%)/17 (3%) | +28.2 |
| 2 | HC:Lyz = 1:2 | 0.75 × 10−4 | 15.91 × 10−4 | 2968 | 0.14 | 48 | +25.4 |
| HC Samples | I90° (a.u.) | Rh (nm) | PDI | ζp (mV) |
|---|---|---|---|---|
| HC 1 | 105 | 32 | 0.43 | −33.1 |
| HC 1/10% CUR | 125 | 40 | 0.48 | −35.8 |
| HC 2 | 93 | 20 | 0.50 | −27.1 |
| HC 2/10% CUR | 110 | 27 | 0.50 | −38.6 |
| HC Samples | I90° (a.u.) | Rh (nm) | PDI | ζp (mV) |
|---|---|---|---|---|
| HC 1 | 155 | 37 (96%)/3 (4%) | 0.43 | −7.2 |
| HC 1/20% BSA | 162 | 44 (83%)/7 (17%) | 0.49 | −11.8 |
| HC 1/50% BSA | 149 | 35 (bimodal) | 0.47 | −4.5 |
| BSACmin | 20 | 90 (72%)/4 (28%) | 0.50 | −19.3 |
| HC 2 | 135 | 20 | 0.50 | −7.0 |
| HC 2/20% BSA | 205 | 28 | 0.49 | −4.3 |
| HC 2/50% BSA | 210 | 20 | 0.51 | −8.4 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Balafouti, A.; Pispas, S. Hyperbranched Copolymers of Methacrylic Acid and Lauryl Methacrylate H-P(MAA-co-LMA): Synthetic Aspects and Interactions with Biorelevant Compounds. Pharmaceutics 2023, 15, 1198. https://doi.org/10.3390/pharmaceutics15041198
Balafouti A, Pispas S. Hyperbranched Copolymers of Methacrylic Acid and Lauryl Methacrylate H-P(MAA-co-LMA): Synthetic Aspects and Interactions with Biorelevant Compounds. Pharmaceutics. 2023; 15(4):1198. https://doi.org/10.3390/pharmaceutics15041198
Chicago/Turabian StyleBalafouti, Anastasia, and Stergios Pispas. 2023. "Hyperbranched Copolymers of Methacrylic Acid and Lauryl Methacrylate H-P(MAA-co-LMA): Synthetic Aspects and Interactions with Biorelevant Compounds" Pharmaceutics 15, no. 4: 1198. https://doi.org/10.3390/pharmaceutics15041198
APA StyleBalafouti, A., & Pispas, S. (2023). Hyperbranched Copolymers of Methacrylic Acid and Lauryl Methacrylate H-P(MAA-co-LMA): Synthetic Aspects and Interactions with Biorelevant Compounds. Pharmaceutics, 15(4), 1198. https://doi.org/10.3390/pharmaceutics15041198