Polysaccharide-Based Carriers for Pulmonary Insulin Delivery: The Potential of Coffee as an Unconventional Source
Abstract
:1. Introduction
2. Materials and Methods
2.1. Sample Preparation
2.2. Sugar Analysis
2.3. Microparticle Preparation & Characterisation
2.3.1. Spray Drying
2.3.2. Scanning Electron Microscopy (SEM)
2.4. In Vitro Insulin Release
2.5. Cytotoxicity Assay
2.6. Statistical Analysis
3. Results and Discussion
3.1. Samples Preparation and Characterization
3.2. Microparticles Formation and Characterization
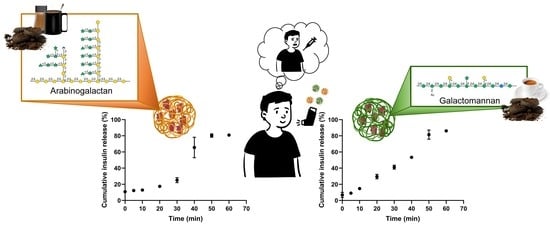
3.3. Insulin In Vitro Release
3.4. Cytotoxicity Assay
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- American Diabetes Association. Classification and Diagnosis of Diabetes: Standards of Medical Care in Diabetes-2019. Diabetes Care 2019, 42, S13–S28. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Easa, N.; Alany, R.G.; Carew, M.; Vangala, A. A review of non-invasive insulin delivery systems for diabetes therapy in clinical trials over the past decade. Drug Discov. Today 2019, 24, 440–451. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- El Maalouf, I.R.; Capoccia, K.; Priefer, R. Non-invasive ways of administering insulin. Diabetes Metab. Syndr. 2022, 16, 102478. [Google Scholar] [CrossRef] [PubMed]
- Valente, S.A.; Silva, L.M.; Lopes, G.R.; Sarmento, B.; Coimbra, M.A.; Passos, C.P. Polysaccharide-based formulations as potential carriers for pulmonary delivery—A review of their properties and fates. Carbohydr. Polym. 2022, 277, 118784. [Google Scholar] [CrossRef]
- Patton, J.S.; Byron, P.R. Inhaling medicines: Delivering drugs to the body through the lungs. Nat. Rev. Drug. Discov. 2007, 6, 67–74. [Google Scholar] [CrossRef] [PubMed]
- Byron, P.R. Prediction of drug residence times in regions of the human respiratory tract following aerosol inhalation. J. Pharm. Sci. 1986, 75, 433–438. [Google Scholar] [CrossRef]
- Marante, T.; Viegas, C.; Duarte, I.; Macedo, A.S.; Fonte, P. An Overview on Spray-Drying of Protein-Loaded Polymeric Nanoparticles for Dry Powder Inhalation. Pharmaceutics 2020, 12, 1032. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Chen, F.; Fonseca, N.A.; He, Y.; Fujita, M.; Nakagawa, H.; Zhang, Z.; Brazma, A.; Creighton, C.J. High-coverage whole-genome analysis of 1220 cancers reveals hundreds of genes deregulated by rearrangement-mediated cis-regulatory alterations. Nat. Commun. 2020, 11, 736. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pinés Corrales, P.J.; Bellido Castañeda, V.; Ampudia-Blasco, F.J. Update on postprandial hyperglycaemia: The pathophysiology, prevalence, consequences and implications of treating diabetes. Rev. Clin. Esp. 2020, 220, 57–68. [Google Scholar] [CrossRef]
- Gatto, N.M.; Bracken, M.B.; Kolitsopoulos, F.; Duggan, W.T.; Koch, G.G.; Wise, R.A.; Jackson, N.C. Pulmonary and cardiovascular safety of inhaled insulin in routine practice: The Exubera Large Simple Trial (VOLUME). Contemp. Clin. Trials Commun. 2020, 18, 100427. [Google Scholar] [CrossRef]
- Klonoff, D.C. Afrezza inhaled insulin: The fastest-acting FDA-approved insulin on the market has favorable properties. J. Diabetes Sci. Technol. 2014, 8, 1071–1073. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zamboulis, A.; Michailidou, G.; Koumentakou, I.; Bikiaris, D.N. Polysaccharide 3D Printing for Drug Delivery Applications. Pharmaceutics 2022, 14, 145. [Google Scholar] [CrossRef]
- Barclay, T.G.; Day, C.M.; Petrovsky, N.; Garg, S. Review of polysaccharide particle-based functional drug delivery. Carbohydr. Polym. 2019, 221, 94–112. [Google Scholar] [CrossRef]
- Alves, A.D.; Cavaco, J.S.; Guerreiro, F.; Lourenco, J.P.; Rosa da Costa, A.M.; Grenha, A. Inhalable Antitubercular Therapy Mediated by Locust Bean Gum Microparticles. Molecules 2016, 21, 702. [Google Scholar] [CrossRef] [Green Version]
- Bradbury, A.G.W.; Halliday, D.J. Chemical structures of green coffee bean polysaccharides. J. Agric. Food Chem. 1990, 38, 389–392. [Google Scholar] [CrossRef]
- FDA. Section 184.1343—Locust (carob) Bean Gum. Title 21—Food and Drugs, Part 184—Direct Food Substances Affirmed as Generally Recognized as Safe; FDA: Silver Spring, MD, USA, 2023. [Google Scholar]
- FDA. Section 172.610—Arabinogalactan. Title 21—Food and Drugs, Part 172—Food Addictives Permitted for Direct Addition to Food for Human Consumption; FDA: Silver Spring, MD, USA, 2023. [Google Scholar]
- EFSA Panel on Food Additives and Nutrient Sources added to Food (ANS); Mortensen, A.; Aguilar, F.; Crebelli, R.; Di Domenico, A.; Frutos, M.J.; Galtier, P.; Gott, D.; Gundert-Remy, U.; Lambré, C.; et al. Re-evaluation of acacia gum (E 414) as a food additive. EFSA J. 2017, 15, e04741. [Google Scholar] [CrossRef]
- EFSA Panel on Food Additives and Nutrient Sources added to Food (ANS); Mortensen, A.; Aguilar, F.; Crebelli, R.; Di Domenico, A.; Frutos, M.J.; Galtier, P.; Gott, D.; Gundert-Remy, U.; Lambré, C.; et al. Re-evaluation of tara gum (E 417) as a food additive. EFSA J. 2017, 15, e04863. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yadav, H.; Maiti, S. Research progress in galactomannan-based nanomaterials: Synthesis and application. Int. J. Biol. Macromol. 2020, 163, 2113–2126. [Google Scholar] [CrossRef] [PubMed]
- Froelich, A.; Jakubowska, E.; Jadach, B.; Gadziński, P.; Osmałek, T. Natural Gums in Drug-Loaded Micro- and Nanogels. Pharmaceutics 2023, 15, 759. [Google Scholar] [CrossRef]
- Klingel, T.; Kremer, J.I.; Gottstein, V.; Rajcic de Rezende, T.; Schwarz, S.; Lachenmeier, D.W. A Review of Coffee By-Products Including Leaf, Flower, Cherry, Husk, Silver Skin, and Spent Grounds as Novel Foods within the European Union. Foods 2020, 9, 665. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, G.; Passos, C.P.; Ferreira, P.; Coimbra, M.A.; Gonçalves, I. Coffee by-products and their suitability for developing active food packaging materials. Foods 2021, 10, 683. [Google Scholar] [CrossRef]
- Murthy, P.S.; Madhava Naidu, M. Sustainable management of coffee industry by-products and value addition—A review. Resour. Conserv. Recycl. 2012, 66, 45–58. [Google Scholar] [CrossRef]
- Nunes, F.M.; Coimbra, M.A. Chemical Characterization of Galactomannans and Arabinogalactans from Two Arabica Coffee Infusions As Affected by the Degree of Roast. J. Agric. Food Chem. 2002, 50, 1429–1434. [Google Scholar] [CrossRef] [PubMed]
- Simões, J.; Nunes, F.M.; Domingues, M.R.; Coimbra, M.A. Extractability and structure of spent coffee ground polysaccharides by roasting pre-treatments. Carbohydr. Polym. 2013, 97, 81–89. [Google Scholar] [CrossRef] [PubMed]
- Passos, C.P.; Coimbra, M.A. Microwave superheated water extraction of polysaccharides from spent coffee grounds. Carbohydr. Polym. 2013, 94, 626–633. [Google Scholar] [CrossRef] [PubMed]
- Passos, C.P.; Rudnitskaya, A.; Neves, J.M.M.G.C.; Lopes, G.R.; Evtuguin, D.V.; Coimbra, M.A. Structural features of spent coffee grounds water-soluble polysaccharides: Towards tailor-made microwave assisted extractions. Carbohydr. Polym. 2019, 214, 53–61. [Google Scholar] [CrossRef]
- Passos, C.P.; Moreira, A.S.P.; Domingues, M.R.M.; Evtuguin, D.V.; Coimbra, M.A. Sequential microwave superheated water extraction of mannans from spent coffee grounds. Carbohydr. Polym. 2014, 103, 333–338. [Google Scholar] [CrossRef] [PubMed]
- Passos, C.P.; Costa, R.M.; Ferreira, S.S.; Lopes, G.R.; Cruz, M.T.; Coimbra, M.A. Role of coffee caffeine and chlorogenic acids adsorption to polysaccharides with impact on brew immunomodulation effects. Foods 2021, 10, 378. [Google Scholar] [CrossRef] [PubMed]
- Lopes, G.R.; Ferreira, A.S.; Pinto, M.; Passos, C.P.; Coelho, E.; Rodrigues, C.; Figueira, C.; Rocha, S.M.; Nunes, F.M.; Coimbra, M.A. Carbohydrate content, dietary fibre and melanoidins: Composition of espresso from single-dose coffee capsules. Food Res. Inter. 2016, 89, 989–996. [Google Scholar] [CrossRef]
- Nunes, F.M.; Coimbra, M.A.; Duarte, A.C.; Delgadillo, I. Foamability, Foam Stability, and Chemical Composition of Espresso Coffee As Affected by the Degree of Roast. J. Agric. Food Chem. 1997, 45, 3238–3243. [Google Scholar] [CrossRef]
- Grenha, A.; Alves, A.D.; Guerreiro, F.; Pinho, J.; Simões, S.; Almeida, A.J.; Gaspar, M.M. Inhalable locust bean gum microparticles co-associating isoniazid and rifabutin: Therapeutic assessment in a murine model of tuberculosis infection. Eur. J. Pharm. Biopharm. 2020, 147, 38–44. [Google Scholar] [CrossRef]
- Schneider, C.A.; Rasband, W.S.; Eliceiri, K.W. NIH Image to ImageJ: 25 years of image analysis. Nat. Methods 2012, 9, 671–675. [Google Scholar] [CrossRef]
- Amidi, M.; Pellikaan, H.C.; de Boer, A.H.; Crommelin, D.J.A.; Hennink, W.E.; Jiskoot, W. Preparation and physicochemical characterization of supercritically dried insulin-loaded microparticles for pulmonary delivery. Eur. J. Pharm. Biopharm. 2008, 68, 191–200. [Google Scholar] [CrossRef]
- Rodrigues, S.; Alves, A.D.; Cavaco, J.S.; Pontes, J.F.; Guerreiro, F.; da Costa, A.M.R.; Buttini, F.; Grenha, A. Dual antibiotherapy of tuberculosis mediated by inhalable locust bean gum microparticles. Int. J. Pharm. 2017, 529, 433–441. [Google Scholar] [CrossRef]
- Guerreiro, F.; Pontes, J.F.; da Costa, A.M.R.; Grenha, A. Spray-drying of konjac glucomannan to produce microparticles for an application as antitubercular drug carriers. Powder Technol. 2019, 342, 246–252. [Google Scholar] [CrossRef]
- Jiang, J.; Ren, H.Y.; Geng, G.J.; Mi, Y.J.; Liu, Y.; Li, N.; Yang, S.Y.; Shen, D.Y. Oncogenic activity of insulin in the development of non-small cell lung carcinoma. Oncol. Lett. 2018, 15, 447–452. [Google Scholar] [CrossRef] [PubMed]
- O'Brien, J.; Wilson, I.; Orton, T.; Pognan, F. Investigation of the Alamar Blue (resazurin) fluorescent dye for the assessment of mammalian cell cytotoxicity. Eur. J. Biochem. 2000, 267, 5421–5426. [Google Scholar] [CrossRef]
- Moreira, A.S.P.; Nunes, F.M.; Domingues, M.R.; Coimbra, M.A. Coffee melanoidins: Structures, mechanisms of formation and potential health impacts. Food Funct. 2012, 3, 903–915. [Google Scholar] [CrossRef]
- Passos, C.P.; Cepeda, M.R.; Ferreira, S.S.; Nunes, F.M.; Evtuguin, D.V.; Madureira, P.; Vilanova, M.; Coimbra, M.A. Influence of molecular weight on in vitro immunostimulatory properties of instant coffee. Food Chem. 2014, 161, 60–66. [Google Scholar] [CrossRef]
- Gniechwitz, D.; Brueckel, B.; Reichardt, N.; Blaut, M.; Steinhart, H.; Bunzel, M. Coffee Dietary Fiber Contents and Structural Characteristics As Influenced by Coffee Type and Technological and Brewing Procedures. J. Agric. Food Chem. 2007, 55, 11027–11034. [Google Scholar] [CrossRef] [PubMed]
- Cerqueira, M.A.; Pinheiro, A.C.; Souza, B.W.S.; Lima, Á.M.P.; Ribeiro, C.; Miranda, C.; Teixeira, J.A.; Moreira, R.A.; Coimbra, M.A.; Gonçalves, M.P.; et al. Extraction, purification and characterization of galactomannans from non-traditional sources. Carbohydr. Polym. 2009, 75, 408–414. [Google Scholar] [CrossRef] [Green Version]
- Passos, C.P.; Rudnitskaya, A.; Neves, J.M.M.G.C.; Lopes, G.R.; Coimbra, M.A. Data on yields, sugars and glycosidic-linkage analyses of coffee arabinogalactan and galactomannan mixtures and optimization of their microwave assisted extraction from spent coffee grounds. Data Brief 2019, 24, 103931. [Google Scholar] [CrossRef]
- Mortensen, N.P.; Durham, P.; Hickey, A.J. The role of particle physico-chemical properties in pulmonary drug delivery for tuberculosis therapy. J. Microencapsul. 2014, 31, 785–795. [Google Scholar] [CrossRef] [PubMed]
- Chaurasiya, B.; Zhao, Y.-Y. Dry Powder for Pulmonary Delivery: A Comprehensive Review. Pharmaceutics 2021, 13, 31. [Google Scholar] [CrossRef] [PubMed]
- Balducci, A.G.; Cagnani, S.; Sonvico, F.; Rossi, A.; Barata, P.; Colombo, G.; Colombo, P.; Buttini, F. Pure insulin highly respirable powders for inhalation. Eur. J. Pharm. Sci. 2014, 51, 110–117. [Google Scholar] [CrossRef] [PubMed]
- FDA. AFREZZA® (Insulin Human) Inhalation Powder. 2014. Available online: https://www.accessdata.fda.gov/drugsatfda_docs/label/2014/022472lbl.pdf (accessed on 24 February 2023).
- Lopes, G.R.; Passos, C.P.; Petronilho, S.; Rodrigues, C.; Teixeira, J.A.; Coimbra, M.A. Carbohydrates as targeting compounds to produce infusions resembling espresso coffee brews using quality by design approach. Food Chem. 2021, 344, 128613. [Google Scholar] [CrossRef]
- Mark, Q.G.; Xinzhong, H.; Changlu, W.; Lianzhong, A. Polysaccharides: Structure and solubility. In Solubility of Polysaccharides; Zhenbo, X., Ed.; IntechOpen: Rijeka, Croatia, 2017; p. Ch. 2. [Google Scholar]
- Yoo, J.; Won, Y.-Y. Phenomenology of the Initial Burst Release of Drugs from PLGA Microparticles. ACS Biomater. Sci. Eng. 2020, 6, 6053–6062. [Google Scholar] [CrossRef]
- Mozzillo, E.; Franceschi, R.; Di Candia, F.; Ricci, A.; Leonardi, L.; Girardi, M.; Rosanio, F.M.; Marcovecchio, M.L. Optimal Prandial Timing of Insulin Bolus in Youths with Type 1 Diabetes: A Systematic Review. J. Pers. Med. 2022, 12, 2058. [Google Scholar] [CrossRef]
- Wang, Y.; Fu, M.; Wang, Z.; Zhu, X.X.; Guan, Y.; Zhang, Y. A sustained zero-order release carrier for long-acting, peakless basal insulin therapy. J. Mater. Chem. B 2020, 8, 1952–1959. [Google Scholar] [CrossRef] [PubMed]
- Young, S.H.; Robinson, V.A.; Barger, M.; Whitmer, M.; Porter, D.W.; Frazer, D.G.; Castranova, V. Exposure to particulate 1→3-β-glucans induces greater pulmonary toxicity than soluble 1→3-β-glucans in rats. J. Toxicol. Environ. Health Part A 2003, 66, 25–38. [Google Scholar] [CrossRef]








Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Valente, S.A.; Lopes, G.R.; Ferreira, I.; Galrinho, M.F.; Almeida, M.; Ferreira, P.; Cruz, M.T.; Coimbra, M.A.; Passos, C.P. Polysaccharide-Based Carriers for Pulmonary Insulin Delivery: The Potential of Coffee as an Unconventional Source. Pharmaceutics 2023, 15, 1213. https://doi.org/10.3390/pharmaceutics15041213
Valente SA, Lopes GR, Ferreira I, Galrinho MF, Almeida M, Ferreira P, Cruz MT, Coimbra MA, Passos CP. Polysaccharide-Based Carriers for Pulmonary Insulin Delivery: The Potential of Coffee as an Unconventional Source. Pharmaceutics. 2023; 15(4):1213. https://doi.org/10.3390/pharmaceutics15041213
Chicago/Turabian StyleValente, Sara A., Guido R. Lopes, Isabel Ferreira, Miguel F. Galrinho, Margarida Almeida, Paula Ferreira, Maria T. Cruz, Manuel A. Coimbra, and Cláudia P. Passos. 2023. "Polysaccharide-Based Carriers for Pulmonary Insulin Delivery: The Potential of Coffee as an Unconventional Source" Pharmaceutics 15, no. 4: 1213. https://doi.org/10.3390/pharmaceutics15041213
APA StyleValente, S. A., Lopes, G. R., Ferreira, I., Galrinho, M. F., Almeida, M., Ferreira, P., Cruz, M. T., Coimbra, M. A., & Passos, C. P. (2023). Polysaccharide-Based Carriers for Pulmonary Insulin Delivery: The Potential of Coffee as an Unconventional Source. Pharmaceutics, 15(4), 1213. https://doi.org/10.3390/pharmaceutics15041213