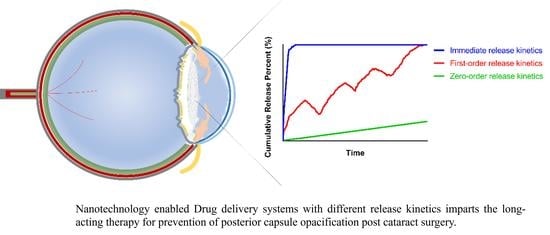
Clinical Translation of Long-Acting Drug Delivery Systems for Posterior Capsule Opacification Prophylaxis
Abstract
:1. Introduction
2. Drug Delivery Approach to PCO Prophylaxis
2.1. Conventional Delivery of Free Drug(s) Solution
| Reference | Drug Used | Treatment Dose and Length | Application Methods in the Capsular Bag (In Vivo Models) | Drug Action | Therapeutic Efficacy |
|---|---|---|---|---|---|
| [23] | Distilled water | 0.1 mL for 3 min | Dropping water with a modified syringe using fluid/air exchange technique (Human) | Hypoosmotic stress | Damaged LECs from the anterior capsule without damage to intraocular structures. |
| [24] | Sorbinil | Oral: 200 mg or 400 mg q.d for 7 days; Topical: 0.5 mg up to 14 h | Oral and topical (Human) | Aldose reductase inhibitor (anti-oxidation) | Length of treatment was too short to effect lens sugar or sugar alcohol levels. |
| [25] | 10 mg/kg | Intraperitoneal injection (Mice) | Inhibition of LEC EMT in mice. | ||
| [26] | Dexamethasone | Single dose: 4 mg/mL | Subconjunctival injection (Rabbit) | Anti-inflammation | Reduced LEC proliferation on the posterior capsule and effectively prevented PCO |
| Diclofenac | Single dose: 2.5 mg/mL | Injection with an anterior chamber cannula (Rabbit) | |||
| RGD peptide a | Single dose: 2.5 mg/mL | Anti-adhesion | |||
| EDTA a | Single dose: 8 mg/mL | ||||
| Mitomycin C | Single dose: 0.04 mg/mL | Antimetabolites | |||
| [27] | N-Acetylcysteine | Single dose: 25 μL, 10 mmol/L | Injection into the eye chamber (Mouse) | Antioxidant | Attenuated LEC EMT signaling. |
| [28] | EDTA | 1, 2.5, and 5 mg with a single dose | Intracameral injection (Rabbit) | MMP inhibitor | Reduced the degree of PCO by suppressing the matrix metalloproteinase activity. |
| [29] | Distilled water | / | Injection through SCI device (Rabbit) | Hypoosmotic stress | Reduced PCO development without toxicity to surrounding ocular tissues. |
| Mitomycin C | 0.4 mg/mL for 2 min | Antimetabolite | |||
| [30] | Actinomycin D a | 10 μg/mL for 5 min | Flush with the Perfect Capsule Device and sodium hyaluronate (Rabbit) | Anti-proliferation | Reduced the formation of visible capsular opacification. |
| Cycloheximide a | 25 μg/mL for 5 min | ||||
| [31] | Methotrexate a | 10 μM for 5 min | Human capsular rhexis specimens; Lens refilling (Rabbit) | Antimetabolite | Ablated viable LECs ex vivo, and delayed PCO formation in vivo. |
| Actinomycin D a | 10 μM for 5 min | Anti-proliferation | |||
| [32] | Sodium Hyaluronate | 2.3% for 5 min | Lens refilling (Monkey) | Influencing LEC growing pattern | No capsular bag fibrosis. |
| [33] | Sodium Hyaluronate | 1.4% for 3 min | Refilling (Rabbit) | Anti-proliferation | Distilled water and EDTA were most effective against PCO development. |
| Balanced salt solution | / | ||||
| Mitomycin C | 0.2 mg/mL for 3 min | ||||
| EDTA | 10 and 15 mM for 3 min | ||||
| 5-Fluorouacil | 33 mg/mL for 3 min | ||||
| Acetic acid | 3%, 0.3% and 0.003% for 3 min | ||||
| [34] | Dexamethasone | IOL incubated in 1 mg/mL | Implantation of IOL pre-soaked with the drug (Rabbit) | Anti-inflammation | Reduced postoperative inflammation. |
| [35] | Thapsigargin | IOL incubated in 0.2–2 μM | Insert into the capsular bag (Human) | Inhibitor of endoplasmic reticulum (Ca2+)-ATPase | Reduced LEC growth in the capsular bag. |
2.2. Nanotechnology-Based Drug Delivery
2.2.1. Surface Modification of IOL Materials
2.2.2. Development of Non-IOL Dosage Forms
| Drug-Loaded IOL | ||||||||
|---|---|---|---|---|---|---|---|---|
| Reference | Fabrication Method (Loaded Drug) | Part of IOL Modification | IOL Material | Drug Loading | Release Profile | Release Medium | Release Duration (t50 /t90 in Days) | Maximum Release% |
| [37] | IOL immersion into the BP-DOX solution via facial activation-immersion (Doxorubicin) | Non-optical | Hydrophobic acrylic | / | Photo-responsive | PBS (pH 7.4) | 16 days (104/1148) | 13% |
| [38] | Rapa@Ti3C2 was deposited onto the oxygen plasma-activated IOL with a spin-coater (Rapamycin) | Optical | Hydrophobic acrylic | / | NIR-triggered | Aqueous humor | 2 days (0.43/2.9) | 74% |
| [39] | DOX@Exos immobilized on the aminated IOL surface by electrostatic self-assembling (Doxorubicin) | Optical | Hydrophobic acrylic | / | A slow and continuous release | PBS (pH 7.4) | 3 days (6.3/264) | 40% |
| [40] | Fluorine ion beam-activating IOL was soaked in 5-Fu-CSNP suspension (5-Fluorouracil) | Optical | Hydrophobic PMMA | / | A burst release of the drug in 2 h followed by slow release | PBS (pH 7.2) | 4 days (0.085/2.8) | 100% |
| [41] | The activated IOL alternatively coated with heparin and drug-loaded NPs (CTDNPs by layer-by-layer assembly (Doxorubicin) | Optical | Hydrophobic acrylic | / | pH-responsive; no burst release | Acetate buffer (pH 5.5) | 7 days (4.4 × 104/9.6 × 107) | 7.2% |
| [42,43] | Drug-loaded PLGA was sprayed by ultrasonic coating system (Bromfenac or Indomethacin) | Plate haptics | Hydrophobic acrylic | 0.1 mg | Biphasic release profiles | PBS | Bromfenac: 14 days (0.93/5.9) Indomethacin: 56 days (12/89) | Bromfenac: 91% Indomethacin: 80% |
| [44] | Drug-loaded PLGA coating by spin-coating (Cyclosporin A) | Thin center and thick periphery | Hydrophobic acrylic | / | Four-phase release: (1) exponential; (2) linear; (3) burst; (4) plateaued. | PBS (pH 7.4) | 120 days (23/126) | 78% |
| [45] | Supercritical impregnation (Methotrexate) | Optical | Hydrophobic acrylic | 0.0069 mg | Sustained release | Aqueous humor (pH 7.2) | 87 days (31/143) | >90% |
| [46] | The aminated IOL chemically grafted with PPG followed by DOX immobilization via the reaction of epoxy and amino groups (Doxorubicin) | Optical | Hydrophobic acrylic | / | pH-responsive | Sodium acetate buffer (pH 5.5) | 7 days (4.8 × 104/1.6 × 108) | 8.1% (pH 5.5) |
| [47] | Drug-loaded polydopamine coating followed by MPC immobilization via immersion (Doxorubicin) | Optical | Hydrophobic acrylic | / | A burst release of 75% of drug in the first 24 h followed by drug sustained release | Sodium acetate buffer (pH 5.5) | 21 days (0.098/9.6) | >85% (PDA(DOX)-MPC) |
| [48] | Soaking IOLs in solution containing dual drugs (Moxifloxacin/ketorolac) | Optical | Hydrophobic G-free® and hydrophilic CI26Y | / | An extended release of dual drugs for 26 days | PBS | 26 days | CI26Y IOL: MOX: 52 μg ketorolac: 63 μg G-free® IOL: MOX: 6 μg ketorolac: 7 μg |
| Other DSS | ||||||||
| Reference | Drug Carrier | Fabrication Method | Drug Loading | Release Kinetics | Release Medium | Release Duration (t50 /t90 in Days) | Maximum Release% | |
| [49] | Capsular tension ring (DTX-CTR) | Porous PMMA via polyHIPE in combination with P(HEMA-co-MMA)-PMMA composite (Docetaxel) | / | Porous structure controlled sustained release | PBS (pH 7.4) | 6 weeks | 5.3 mg/g | |
| [50] | Nanoparticle–hydrogel composite (GenNLC-DEX-MOX hydrogel) | NPs were mixed with the gel solution (Combination of Genistein Moxifloxacin and Dexamethasone) | Gen: 10 mg DEX: 4 mg/mL MOX: 2 mg/mL | Multiple drug release with differential kinetics | PBS (pH 7.0–7.6) | Gen: 40 days (20/127) DEX: 40 days (6.4/24) MOX: 10 days (1.8/7.4) | 63% (Gen) 97% (DEX) 99% (MOX) | |
| [51] | PLGA microparticles (DXM-PLGA) | Oil-in-water (o/w) emulsion-solvent extraction method followed by bench-top pellet press (Dexamethasone) | 0.32 mg | Initial burst release followed by a sustained release | BSS (pH 7.4) | 22 days (18/45) | 53% | |
| [52] | NPs (MePEG-PCL DOX NPs) | Solvent evaporation (Doxorubicin) | 0.25 mg | Initial burst release followed by a sustained release of the drug | PBS (pH 7.4) | 10 days (4.1/99) | 75% | |
2.3. Pros and Cons of Conventional and Nanotechnology-Based Drug Delivery
3. Design Consideration of Anti-PCO DDS
3.1. Long-Acting DDS
3.1.1. Exploitation of the Intraocular Environment for Stimuli-Responsive Release
3.1.2. Issues of Burst Drug Release
3.1.3. Determination of Drug Loading Content
3.2. Delivery of Drug Combination
3.3. Long-Term Ocular Safety
4. Conclusions
Abbreviation
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Hashemi, H.; Pakzad, R.; Yekta, A.; Aghamirsalim, M.; Pakbin, M.; Ramin, S.; Khabazkhoob, M. Global and regional prevalence of age-related cataract: A comprehensive systematic review and meta-analysis. Eye 2020, 34, 1357–1370. [Google Scholar] [CrossRef]
- Sheeladevi, S.; Lawrenson, J.G.; Fielder, A.R.; Suttle, C.M. Global prevalence of childhood cataract: A systematic review. Eye 2016, 30, 1160–1169. [Google Scholar] [CrossRef] [Green Version]
- Yan, H. Binocular cataract surgery-yan hong’s viewpoint. Chin. Med. Clin. Ref. Modul. 2019, 1. [Google Scholar]
- Wormstone, I.M.; Wormstone, Y.M.; Smith, A.J.O.; Eldred, J.A. Posterior capsule opacification: What’s in the bag? Prog. Retin. Eye Res. 2021, 82, 100905. [Google Scholar] [CrossRef] [PubMed]
- Konopinska, J.; Mlynarczyk, M.; Dmuchowska, D.A.; Obuchowska, I. Posterior Capsule Opacification: A Review of Experimental Studies. J. Clin. Med. 2021, 10, 2847. [Google Scholar] [CrossRef]
- Wormstone, I.M.; Wang, L.; Liu, C.S. Posterior capsule opacification. Exp. Eye Res. 2009, 88, 257–269. [Google Scholar] [CrossRef]
- Eldred, J.A.; Zheng, J.; Chen, S.; Wormstone, I.M. An In Vitro Human Lens Capsular Bag Model Adopting a Graded Culture Regime to Assess Putative Impact of IOLs on PCO Formation. Investig. Ophthalmol. Vis. Sci. 2019, 60, 113–122. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ursell, P.G.; Dhariwal, M.; Majirska, K.; Ender, F.; Kalson-Ray, S.; Venerus, A.; Miglio, C.; Bouchet, C. Three-year incidence of Nd:YAG capsulotomy and posterior capsule opacification and its relationship to monofocal acrylic IOL biomaterial: A UK Real World Evidence study. Eye 2018, 32, 1579–1589. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vella, M.; Wickremasinghe, S.; Gupta, N.; Andreou, P.; Sinha, A. YAG laser capsulotomy, an unusual complication. Eye 2004, 18, 193–194. [Google Scholar] [CrossRef]
- Jinagal, J.; Sahu, S.; Gupta, G. Quantification of Inflammation Following Nd:YAG Laser Capsulotomy and Assessing the Anti-inflammatory Effects of Nepafenac 0.1% and Betamethasone 0.1. Ocul. Immunol. Inflamm. 2021, 29, 411–416. [Google Scholar] [CrossRef]
- Walker, T.D. Pharmacological attempts to reduce posterior capsule opacification after cataract surgery—A review. Clin. Exp. Ophthalmol. 2008, 36, 883–890. [Google Scholar] [CrossRef] [PubMed]
- Zhang, R.P.; Xie, Z.G. Research Progress of Drug Prophylaxis for Lens Capsule Opacification after Cataract Surgery. J. Ophthalmol. 2020, 2020, 2181685. [Google Scholar] [CrossRef]
- Nibourg, L.M.; Gelens, E.; Kuijer, R.; Hooymans, J.M.M.; van Kooten, T.G.; Koopmans, S.A. Prevention of posterior capsular opacification. Exp. Eye Res. 2015, 136, 100–115. [Google Scholar] [CrossRef]
- Awasthi, N.; Guo, S.; Wagner, B.J. Posterior capsular opacification: A problem reduced but not yet eradicated. Arch. Ophthalmol. 2009, 127, 555–562. [Google Scholar] [CrossRef] [Green Version]
- Meng, T.; Kulkarni, V.; Simmers, R.; Brar, V.; Xu, Q.G. Therapeutic implications of nanomedicine for ocular drug delivery. Drug Discov. Today 2019, 24, 1524–1538. [Google Scholar] [CrossRef]
- Kang-Mieler, J.J.; Rudeen, K.M.; Liu, W.; Mieler, W.F. Advances in ocular drug delivery systems. Eye 2020, 34, 1371–1379. [Google Scholar] [CrossRef]
- Gote, V.; Sikder, S.; Sicotte, J.; Pal, D. Ocular Drug Delivery: Present Innovations and Future Challenges. J. Pharmacol. Exp. Ther. 2019, 370, 602–624. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, S.; Amin, M.M.; Sayed, S. Ocular Drug Delivery: A Comprehensive Review. AAPS PharmSciTech 2023, 24, 66. [Google Scholar] [CrossRef]
- Han, H.; Li, S.; Xu, M.; Zhong, Y.; Fan, W.; Xu, J.; Zhou, T.; Ji, J.; Ye, J.; Yao, K. Polymer- and Lipid-Based Nanocarriers for Ocular Drug Delivery: Current Status and Future Perspectives. Adv. Drug Deliv. Rev. 2023, 196, 114770. [Google Scholar] [CrossRef] [PubMed]
- Donnenfeld, E.; Holland, E. Dexamethasone Intracameral Drug-Delivery Suspension for Inflammation Associated with Cataract Surgery: A Randomized, Placebo-Controlled, Phase III Trial. Ophthalmology 2018, 125, 799–806. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.T.; Cui, X.X.; Yang, X.T.; Li, B.; Ren, X.J.; Li, X.R.; Zhang, X.M. Utilizing dexamethasone intravitreal implant to control postoperative inflammation in refractory uveitis undergoing cataract surgery. Int. J. Ophthalmol. 2021, 14, 317–322. [Google Scholar] [CrossRef]
- Tan, D.T.; Chee, S.P.; Lim, L.; Theng, J.; Van Ede, M. Randomized clinical trial of Surodex steroid drug delivery system for cataract surgery: Anterior versus posterior placement of two Surodex in the eye. Ophthalmology 2001, 108, 2172–2181. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Huang, L.; Jin, H.; Li, J.; Zhao, P. Air/Fluid-Dropping Technique for Intracapsular Distilled Water Application: A Vitrectomy Approach for Selective Targeting of Lens Epithelial Cells. Retina 2019, 39, 364–370. [Google Scholar] [CrossRef] [PubMed]
- Crabbe, M.J.; Petchey, M.; Burgess, S.E.; Cheng, H. The penetration of Sorbinil, an aldose reductase inhibitor, into lens, aqueous humour and erythrocytes of patients undergoing cataract extraction. Exp. Eye Res. 1985, 40, 95–99. [Google Scholar] [CrossRef] [PubMed]
- Zukin, L.M.; Pedler, M.G.; Groman-Lupa, S.; Pantcheva, M.; Ammar, D.A.; Petrash, J.M. Aldose Reductase Inhibition Prevents Development of Posterior Capsular Opacification in an In Vivo Model of Cataract Surgery. Investig. Ophthalmol. Vis. Sci. 2018, 59, 3591–3598. [Google Scholar] [CrossRef] [Green Version]
- Inan, U.U.; Oztürk, F.; Kaynak, S.; Kurt, E.; Emiroğlu, L.; Ozer, E.; Ilker, S.S.; Güler, C. Prevention of posterior capsule opacification by intraoperative single-dose pharmacologic agents. J. Cataract. Refract. Surg. 2001, 27, 1079–1087. [Google Scholar] [CrossRef]
- Wei, Z.B.; Caty, J.; Whitson, J.; Zhang, A.D.; Srinivasagan, R.; Kavanagh, T.J.; Yan, H.; Fan, X.J. Reduced Glutathione Level Promotes Epithelial-Mesenchymal Transition in Lens Epithelial Cells via a Wnt/Chi-Catenin-Mediated Pathway Relevance for Cataract Therapy. Am. J. Pathol. 2017, 187, 2399–2412. [Google Scholar] [CrossRef] [Green Version]
- Hazra, S.; Guha, R.; Jongkey, G.; Palui, H.; Mishra, A.; Vemuganti, G.K.; Basak, S.K.; Mandal, T.K.; Konar, A. Modulation of matrix metalloproteinase activity by EDTA prevents posterior capsular opacification. Mol. Vis. 2012, 18, 1701–1711. [Google Scholar]
- Kim, S.Y.; Kim, J.H.; Choi, J.S.; Joo, C.K. Comparison of posterior capsule opacification in rabbits receiving either mitomycin-C or distilled water for sealed-capsule irrigation during cataract surgery. Clin. Exp. Ophthalmol. 2007, 35, 755–758. [Google Scholar] [CrossRef]
- Koopmans, S.A.; Terwee, T.; van Kooten, T.G. Prevention of capsular opacification after accommodative lens refilling surgery in rabbits. Biomaterials 2011, 32, 5743–5755. [Google Scholar] [CrossRef]
- Sternberg, K.; Terwee, T.; Stachs, O.; Guthoff, R.; Löbler, M.; Schmitz, K.P. Drug-induced secondary cataract prevention: Experimental ex vivo and in vivo results with disulfiram, methotrexate and actinomycin D. Ophthalmic Res. 2010, 44, 225–236. [Google Scholar] [CrossRef] [PubMed]
- Koopmans, S.A.; Terwee, T.; Hanssen, A.; Martin, H.; Langner, S.; Stachs, O.; van Kooten, T.G. Prevention of capsule opacification after accommodating lens refilling: Pilot study of strategies evaluated in a monkey model. J. Cataract. Refract. Surg. 2014, 40, 1521–1535. [Google Scholar] [CrossRef]
- Fernandez, V.; Fragoso, M.A.; Billotte, C.; Lamar, P.; Orozco, M.A.; Dubovy, S.; Willcox, M.; Parel, J.M. Efficacy of various drugs in the prevention of posterior capsule opacification: Experimental study of rabbit eyes. J. Cataract. Refract. Surg. 2004, 30, 2598–2605. [Google Scholar] [CrossRef]
- Kugelberg, M.; Shafiei, K.; Van Der Ploeg, I.; Zetterström, C. Intraocular lens as a drug delivery system for dexamethasone. Acta Ophthalmol. 2010, 88, 241–244. [Google Scholar] [CrossRef]
- Duncan, G.; Wormstone, I.M.; Liu, C.S.; Marcantonio, J.M.; Davies, P.D. Thapsigargin-coated intraocular lenses inhibit human lens cell growth. Nat. Med. 1997, 3, 1026–1028. [Google Scholar] [CrossRef]
- Maloof, A.; Neilson, G.; Milverton, E.J.; Pandey, S.K. Selective and specific targeting of lens epithelial cells during cataract surgery using sealed- capsule irrigation. J. Cataract. Refract. Surg. 2003, 29, 1566–1568. [Google Scholar] [CrossRef] [PubMed]
- Mao, Y.Y.; Li, M.; Wang, J.D.; Wang, K.J.; Zhang, J.S.; Chen, S.Y.; Liu, X.; Liang, Q.F.; Gao, F.; Wan, X.H. NIR-triggered drug delivery system for chemo-photothermal therapy of posterior capsule opacification. J. Control. Release 2021, 339, 391–402. [Google Scholar] [CrossRef]
- Ye, Z.; Huang, Y.; Li, J.; Ma, T.; Gao, L.; Hu, H.; He, Q.; Jin, H.; Li, Z. Two-dimensional ultrathin Ti(3)C(2) MXene nanosheets coated intraocular lens for synergistic photothermal and NIR-controllable rapamycin releasing therapy against posterior capsule opacification. Front. Bioeng. Biotechnol. 2022, 10, 989099. [Google Scholar] [CrossRef] [PubMed]
- Zhu, S.; Huang, H.; Liu, D.; Wen, S.; Shen, L.; Lin, Q. Augmented cellular uptake and homologous targeting of exosome-based drug loaded IOL for posterior capsular opacification prevention and biosafety improvement. Bioact. Mater. 2022, 15, 469–481. [Google Scholar] [CrossRef]
- Huang, X.; Wang, Y.; Cai, J.P.; Ma, X.Y.; Li, Y.; Cheng, J.W.; Wei, R.L. Sustained Release of 5-Fluorouracil from Chitosan Nanoparticles Surface Modified Intra Ocular Lens to Prevent Posterior Capsule Opacification: An In Vitro and In Vivo Study. J. Ocul. Pharmacol. Ther. 2013, 29, 208–215. [Google Scholar] [CrossRef] [PubMed]
- Han, Y.M.; Tang, J.M.; Xia, J.Y.; Wang, R.; Qin, C.; Liu, S.H.; Zhao, X.; Chen, H.; Lin, Q.K. Anti-Adhesive And Antiproliferative Synergistic Surface Modification Of Intraocular Lens For Reduced Posterior Capsular Opacification. Int. J. Nanomed. 2019, 14, 9047–9061. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, X.; Lai, K.; Li, S.; Wang, J.; Li, J.; Wang, W.; Ni, S.; Lu, B.; Grzybowski, A.; Ji, J.; et al. Drug-eluting intraocular lens with sustained bromfenac release for conquering posterior capsular opacification. Bioact. Mater. 2022, 9, 343–357. [Google Scholar] [CrossRef]
- Zhang, X.; Wang, J.; Xu, J.; Xu, W.; Zhang, Y.; Luo, C.; Ni, S.; Han, H.; Shentu, X.; Ye, J.; et al. Prophylaxis of posterior capsule opacification through autophagy activation with indomethacin-eluting intraocular lens. Bioact. Mater. 2023, 23, 539–550. [Google Scholar] [CrossRef]
- Lu, D.; Han, Y.; Liu, D.; Chen, S.; Qie, J.; Qu, J.; Lin, Q. Centrifugally concentric ring-patterned drug-loaded polymeric coating as an intraocular lens surface modification for efficient prevention of posterior capsular opacification. Acta Biomater. 2022, 138, 327–341. [Google Scholar] [CrossRef]
- Ongkasin, K.; Masmoudi, Y.; Wertheimer, C.M.; Hillenmayer, A.; Eibl-Lindner, K.H.; Badens, E. Supercritical fluid technology for the development of innovative ophthalmic medical devices: Drug loaded intraocular lenses to mitigate posterior capsule opacification. Eur. J. Pharm. Biopharm. 2020, 149, 248–256. [Google Scholar] [CrossRef] [PubMed]
- Xia, J.; Lu, D.; Han, Y.; Wang, J.; Hong, Y.; Zhao, P.; Fang, Q.; Lin, Q. Facile multifunctional IOL surface modification via poly(PEGMA-co-GMA) grafting for posterior capsular opacification inhibition. RSC Adv. 2021, 11, 9840–9848. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Zhao, X.; Tang, J.; Han, Y.; Lin, Q. Drug-Eluting Hydrophilic Coating Modification of Intraocular Lens via Facile Dopamine Self-Polymerization for Posterior Capsular Opacification Prevention. ACS Biomater. Sci. Eng. 2021, 7, 1065–1073. [Google Scholar] [CrossRef]
- Topete, A.; Tang, J.; Ding, X.; Filipe, H.P.; Saraiva, J.A.; Serro, A.P.; Lin, Q.; Saramago, B. Dual drug delivery from hydrophobic and hydrophilic intraocular lenses: In-vitro and in-vivo studies. J. Control. Release 2020, 326, 245–255. [Google Scholar] [CrossRef] [PubMed]
- Lei, M.; Peng, Z.; Dong, Q.; He, Y.; Zhang, Z.; Zhang, X.; Yan, M.; Zhao, C. A novel capsular tension ring as local sustained-release carrier for preventing posterior capsule opacification. Biomaterials 2016, 89, 148–156. [Google Scholar] [CrossRef]
- Yan, T.Y.; Ma, Z.X.; Liu, J.J.; Yin, N.; Lei, S.Z.; Zhang, X.X.; Li, X.D.; Zhang, Y.; Kong, J. Thermoresponsive GenisteinNLC-dexamethasone-moxifloxacin multi drug delivery system in lens capsule bag to prevent complications after cataract surgery. Sci. Rep. 2021, 11, 13. [Google Scholar] [CrossRef] [PubMed]
- Chennamaneni, S.R.; Mamalis, C.; Archer, B.; Oakey, Z.; Ambati, B.K. Development of a novel bioerodible dexamethasone implant for uveitis and postoperative cataract inflammation. J. Control. Release 2013, 167, 53–59. [Google Scholar] [CrossRef] [Green Version]
- Guha, R.; Chowdhury, S.; Palui, H.; Mishra, A.; Basak, S.; Mandal, T.K.; Hazra, S.; Konar, A. Doxorubicin-loaded MePEG-PCL nanoparticles for prevention of posterior capsular opacification. Nanomedicine 2013, 8, 1415–1428. [Google Scholar] [CrossRef] [PubMed]
- Urtti, A. Challenges and obstacles of ocular pharmacokinetics and drug delivery. Adv. Drug Deliv. Rev. 2006, 58, 1131–1135. [Google Scholar] [CrossRef]
- Freddo, T.F. Shifting the paradigm of the blood-aqueous barrier. Exp. Eye Res. 2001, 73, 581–592. [Google Scholar] [CrossRef]
- Shihan, M.H.; Novo, S.G.; Duncan, M.K. Cataract surgeon viewpoints on the need for novel preventative anti-inflammatory and anti-posterior capsular opacification therapies. Curr. Med. Res. Opin. 2019, 35, 1971–1981. [Google Scholar] [CrossRef]
- Zhang, Y.; Huo, M.; Zhou, J.; Zou, A.; Li, W.; Yao, C.; Xie, S. DDSolver: An add-in program for modeling and comparison of drug dissolution profiles. AAPS J. 2010, 12, 263–271. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mylona, I.; Tsinopoulos, I. A Critical Appraisal of New Developments in Intraocular Lens Modifications and Drug Delivery Systems for the Prevention of Cataract Surgery Complications. Pharmaceuticals 2020, 13, 448. [Google Scholar] [CrossRef] [PubMed]
- Beebe, D.C.; Shui, Y.B.; Siegfried, C.J.; Holekamp, N.M.; Bai, F. Preserve the (intraocular) environment: The importance of maintaining normal oxygen gradients in the eye. Jpn. J. Ophthalmol. 2014, 58, 225–231. [Google Scholar] [CrossRef]
- Hsueh, Y.J.; Chen, Y.N. The Pathomechanism, Antioxidant Biomarkers, and Treatment of Oxidative Stress-Related Eye Diseases. Int. J. Mol. Sci. 2022, 23, 1255. [Google Scholar] [CrossRef]
- Eaton, J.W. Is the lens canned? Free Radic. Biol. Med. 1991, 11, 207–213. [Google Scholar] [CrossRef]
- Jiang, J.; Shihan, M.H.; Wang, Y.; Duncan, M.K. Lens Epithelial Cells Initiate an Inflammatory Response Following Cataract Surgery. Investig. Ophthalmol. Vis. Sci. 2018, 59, 4986–4997. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mura, S.; Nicolas, J.; Couvreur, P. Stimuli-responsive nanocarriers for drug delivery. Nat. Mater. 2013, 12, 991–1003. [Google Scholar] [CrossRef]
- Nguyen, D.D.; Lai, J.-Y. Advancing the stimuli response of polymer-based drug delivery systems for ocular disease treatment. Polym. Chem. 2020, 11, 6988–7008. [Google Scholar] [CrossRef]
- Nguyen, D.D.; Lai, J.-Y. Synthesis, bioactive properties, and biomedical applications of intrinsically therapeutic nanoparticles for disease treatment. Chem. Eng. J. 2022, 435, 134970. [Google Scholar] [CrossRef]
- Wang, D.G.; Guo, D.D.; Bi, H.S.; Wu, Q.X.; Tian, Q.M.; Du, Y.X. Zinc oxide nanoparticles inhibit Ca2+-ATPase expression in human lens epithelial cells under UVB irradiation. Toxicol. Vitr. 2013, 27, 2117–2126. [Google Scholar] [CrossRef] [PubMed]
- Wu, Q.X.; Guo, D.D.; Du, Y.X.; Liu, D.M.; Wang, D.G.; Bi, H.S. UVB Irradiation Enhances TiO2 Nanoparticle-induced Disruption of Calcium Homeostasis in Human Lens Epithelial Cells. Photochem. Photobiol. 2014, 90, 1324–1331. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.; Brazel, C.S. On the importance and mechanisms of burst release in matrix-controlled drug delivery systems. J. Control. Release 2001, 73, 121–136. [Google Scholar] [CrossRef]
- Rhoades, W.; Kump, L.; Margalit, E. Anterior Chamber and Retina (Structure, Function and Immunology). In Neuroimmune Pharmacology; Ikezu, T., Gendelman, H., Eds.; Springer: Cham, Switzerland, 2017; pp. 39–54. [Google Scholar]
- Laracuente, M.L.; Yu, M.H.; McHugh, K.J. Zero-order drug delivery: State of the art and future prospects. J. Control. Release 2020, 327, 834–856. [Google Scholar] [CrossRef]
- Lamprogiannis, L.; Karamitsos, A.; Karagkiozaki, V.; Tsinopoulos, I.; Gioti, M.; Fatouros, D.G.; Dimitrakos, S.; Logothetidis, S. Design and fabrication of drug-eluting polymeric thin films for applications in ophthalmology. IET Nanobiotechnol. 2018, 12, 1074–1079. [Google Scholar] [CrossRef] [PubMed]
- Subrizi, A.; del Amo, E.M.; Korzhikov-Vlakh, V.; Tennikova, T.; Ruponen, M.; Urtti, A. Design principles of ocular drug delivery systems: Importance of drug payload, release rate, and material properties. Drug Discov. Today 2019, 24, 1446–1457. [Google Scholar] [CrossRef]
- Barry, P.; Cordovés, L.; Gardner, S. ESCRS Guidelines for Prevention and Treatment of Endophthalmitis Following Cataract Surgery; European Society of Cataract and Refractive Surgeons: Dublin, Ireland, 2020. [Google Scholar]
- Liu, Y.; Yang, G.; Jin, S.; Xu, L.; Zhao, C.X. Development of High-Drug-Loading Nanoparticles. Chempluschem 2020, 85, 2143–2157. [Google Scholar] [CrossRef]
- Peyman, G.A.; Hosseini, K. Combination therapies in ophthalmology: Implications for intravitreal delivery. J. Ophthalmic Vis. Res. 2011, 6, 36–46. [Google Scholar]
- Bertens, C.J.F.; Gijs, M.; Wolters, J.E.J.; Beckers, H.J.M.; Nuijts, R.M.M.A. Combination drug delivery approaches in ophthalmology. In Combination Drug Delivery Approach as an Effective Therapy for Various Diseases, 1st ed.; Academic Press: London, UK, 2022; pp. 47–63. [Google Scholar]
- Nguyen, D.D.; Luo, L.J.; Yang, C.J.; Lai, J.Y. Highly Retina-Permeating and Long-Acting Resveratrol/Metformin Nanotherapeutics for Enhanced Treatment of Macular Degeneration. ACS Nano 2023, 17, 168–183. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Mahesh, P.; Wang, Y.; Novo, S.G.; Shihan, M.H.; Hayward-Piatkovskyi, B.; Duncan, M.K. Spatiotemporal dynamics of canonical Wnt signaling during embryonic eye development and posterior capsular opacification (PCO). Exp. Eye Res. 2018, 175, 148–158. [Google Scholar] [CrossRef]
- Jampel, H.D.; Roche, N.; Stark, W.J.; Roberts, A.B. Transforming growth factor-beta in human aqueous humor. Curr. Eye Res. 1990, 9, 963–969. [Google Scholar] [CrossRef] [PubMed]
- Akhmetshina, A.; Palumbo, K.; Dees, C.; Bergmann, C.; Venalis, P.; Zerr, P.; Horn, A.; Kireva, T.; Beyer, C.; Zwerina, J.; et al. Activation of canonical Wnt signalling is required for TGF-β-mediated fibrosis. Nat. Commun. 2012, 3, 735. [Google Scholar] [CrossRef] [Green Version]
- Zhang, R.X.; Wong, H.L.; Xue, H.Y.; Eoh, J.Y.; Wu, X.Y. Nanomedicine of synergistic drug combinations for cancer therapy—Strategies and perspectives. J. Control. Release 2016, 240, 489–503. [Google Scholar] [CrossRef] [Green Version]
- Zhang, R.X.; Cai, P.; Zhang, T.; Chen, K.; Li, J.; Cheng, J.; Pang, K.S.; Adissu, H.A.; Rauth, A.M.; Wu, X.Y. Polymer-lipid hybrid nanoparticles synchronize pharmacokinetics of co-encapsulated doxorubicin-mitomycin C and enable their spatiotemporal co-delivery and local bioavailability in breast tumor. Nanomedicine 2016, 12, 1279–1290. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shuhendler, A.J.; Prasad, P.; Zhang, R.X.; Amini, M.A.; Sun, M.; Liu, P.P.; Bristow, R.G.; Rauth, A.M.; Wu, X.Y. Synergistic nanoparticulate drug combination overcomes multidrug resistance, increases efficacy, and reduces cardiotoxicity in a nonimmunocompromised breast tumor model. Mol. Pharm. 2014, 11, 2659–2674. [Google Scholar] [CrossRef] [PubMed]
- Thackaberry, E.A.; Lorget, F.; Farman, C.; Bantseev, V. The safety evaluation of long-acting ocular delivery systems. Drug Discov. Today 2019, 24, 1539–1550. [Google Scholar] [CrossRef]
- Ferrell Ramos, M.; Attar, M.; Stern, M.E.; Brassard, J.A.; Kim, A.S.; Matsumoto, S.; Vangyi, C. Chapter 29—Safety Evaluation of Ocular Drugs. In A Comprehensive Guide to Toxicology in Nonclinical Drug Development, 2nd ed.; Faqi, A.S., Ed.; Academic Press: Boston, MA, USA, 2017; pp. 757–811. [Google Scholar]
- Liu, Y.-C.; Wong, T.T.; Mehta, J.S. Intraocular lens as a drug delivery reservoir. Curr. Opin. Ophthalmol. 2013, 24, 53–59. [Google Scholar] [CrossRef] [PubMed]
- Luo, C.; Wang, H.; Chen, X.; Xu, J.; Yin, H.; Yao, K. Recent Advances of Intraocular Lens Materials and Surface Modification in Cataract Surgery. Front. Bioeng. Biotechnol. 2022, 10, 913383. [Google Scholar] [CrossRef] [PubMed]



 represents Ser/Thr phosphorylation, where the signaling pathways are activated upon phosphorylation.
represents Ser/Thr phosphorylation, where the signaling pathways are activated upon phosphorylation.
 represents Ser/Thr phosphorylation, where the signaling pathways are activated upon phosphorylation.
represents Ser/Thr phosphorylation, where the signaling pathways are activated upon phosphorylation.
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, X.; Liang, C.; Guo, Y.; Su, J.; Chen, X.; Macgregor, R.B., Jr.; Zhang, R.X.; Yan, H. Clinical Translation of Long-Acting Drug Delivery Systems for Posterior Capsule Opacification Prophylaxis. Pharmaceutics 2023, 15, 1235. https://doi.org/10.3390/pharmaceutics15041235
Li X, Liang C, Guo Y, Su J, Chen X, Macgregor RB Jr., Zhang RX, Yan H. Clinical Translation of Long-Acting Drug Delivery Systems for Posterior Capsule Opacification Prophylaxis. Pharmaceutics. 2023; 15(4):1235. https://doi.org/10.3390/pharmaceutics15041235
Chicago/Turabian StyleLi, Xinyang, Chen Liang, Yexuan Guo, Jing Su, Xi Chen, Robert B. Macgregor, Jr., Rui Xue Zhang, and Hong Yan. 2023. "Clinical Translation of Long-Acting Drug Delivery Systems for Posterior Capsule Opacification Prophylaxis" Pharmaceutics 15, no. 4: 1235. https://doi.org/10.3390/pharmaceutics15041235
APA StyleLi, X., Liang, C., Guo, Y., Su, J., Chen, X., Macgregor, R. B., Jr., Zhang, R. X., & Yan, H. (2023). Clinical Translation of Long-Acting Drug Delivery Systems for Posterior Capsule Opacification Prophylaxis. Pharmaceutics, 15(4), 1235. https://doi.org/10.3390/pharmaceutics15041235