Oral Mucosa Models to Evaluate Drug Permeability
Abstract
:1. Introduction
2. Ex Vivo Models
Limits of Ex Vivo Oral Mucosa Models
3. In Vitro Models
Limits of In Vitro Oral Mucosa Models
4. Oral Mucosa Equivalents
5. Conclusions and Future Perspectives for Oral Mucosa Models
- (1)
- Replacement: the animal species utilized in the study are those with the lowest neurological development;
- (2)
- Reduction: the study utilized the minimum number of animals;
- (3)
- Refinement: the method is optimized to reduce animal suffering during the execution of the procedures.
- (1)
- Reduce the number of animals sacrificed;
- (2)
- Reduce experimental costs;
- (3)
- Focus on specific issues related to drug delivery due to the absence of in vivo complexity [111].
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Abdelaziz, H.; Gaber, M.; Abd-Elwakil, M.M.; Mabrouk, M.T.; Elgohary, M.; Kamel, N.M.; Kabary, D.M.; Freag, M.S.; Samaha, M.W.; Mortada, S.M.; et al. Inhalable particulate drug delivery systems for lung cancer therapy: Nanoparticles, microparticles, nanocomposites and nanoaggregates. J. Control. Release 2018, 269, 374–392. [Google Scholar] [CrossRef] [PubMed]
- Jumelle, C.; Gholizadeh, S.; Annabi, N.; Dana, R. Advances and limitations of drug delivery systems formulated as eye drops. J. Control. Release 2020, 321, 1–22. [Google Scholar] [CrossRef] [PubMed]
- Sudhakar, Y.; Kuotsu, K.; Bandyopadhyay, A.K. Buccal bioadhesive drug delivery a promising option for orally less efficient drugs. J. Control. Release 2006, 114, 15–40. [Google Scholar] [CrossRef] [PubMed]
- Patel, V.F.; Liu, F.; Brown, M.B. Advances in oral transmucosal drug delivery. J. Control. Release 2011, 153, 106–116. [Google Scholar] [CrossRef] [PubMed]
- Jacobsen, J.; Pedersen, M.; Rassing, M.R. TR146 cells as a model for human buccal epithelium. II Optimisation and use of a cellular sensitivity MTS/PMS assay. Int. J. Pharm. 1996, 141, 217–225. [Google Scholar] [CrossRef]
- Hua, S. Advances in Nanoparticulate Drug Delivery Approaches for Sublingual and Buccal Administration. Front. Pharmacol. 2019, 10, 1328. [Google Scholar] [CrossRef] [PubMed]
- Macedo, A.S.; Castro, P.M.; Roque, L.; Thomé, N.G.; Reis, C.P.; Pintado, M.E.; Fonte, P. Novel and revisited approaches in nanoparticle systems for buccal drug delivery. J. Control. Release 2020, 320, 125–141. [Google Scholar] [CrossRef]
- Wertz, P.W. Roles of Lipids in the Permeability Barriers of Skin and Oral Mucosa. Int. J. Mol. Sci. 2021, 22, 5229. [Google Scholar] [CrossRef]
- Sattar, M.; Lane, M.E. Oral Transmucosal Drug Delivery in Drug Delivery Approaches: Perspectives from Pharmacokinetics and Pharmacodynamics; Wiley Online Library: Hoboken, NJ, USA, 2021; pp. 333–353. [Google Scholar]
- Sung, Y.K.; Kim, S.W. Recent advances in polymeric drug delivery systems. Biomater. Res. 2020, 24, 12. [Google Scholar] [CrossRef]
- Borandeh, S.; van Bochove, B.; Teotia, A.; Seppälä, J. Polymeric drug delivery systems by additive manufacturing. Adv. Drug Deliv. Rev. 2021, 173, 349–373. [Google Scholar] [CrossRef]
- Adepu, S.; Ramakrishna, S. Controlled Drug Delivery Systems: Current Status and Future Directions. Molecules 2021, 26, 5905. [Google Scholar] [CrossRef] [PubMed]
- Chong, L.; Jiancheng, W.; Yiguang, W.; Huile, G.; Gang, W.; Yongzhuo, H.; Haijun, Y.; Yong, G.; Yongjun, W.; Lin, M.; et al. Recent progress in drug delivery. Acta Pharm. Sin. B 2019, 9, 1145–1162. [Google Scholar]
- Heng, P.W.S. Controlled release drug delivery system. Pharm. Dev. Technol. 2018, 23, 833. [Google Scholar] [CrossRef]
- Czech, T.; Lalani, R.; Oyewumi, M.O. Delivery Systems as Vital Tools in Drug Repurposing. AAPS PharmSciTech 2019, 20, 116. [Google Scholar] [CrossRef] [PubMed]
- Angellotti, G.; Di Prima, G.; Scarpaci, A.G.; D’Agostino, F.; Campisi, G.; De Caro, V. Spray-Dried Cytisine-Loaded Matrices: Development of Transbuccal Sustained-Release Tablets as a Promising Tool in Smoking Cessation Therapy. Pharmaceutics 2022, 14, 1583. [Google Scholar] [CrossRef]
- Lin, G.C.; Leitgeb, T.; Vladetic, A.; Friedl, H.P.; Rhodes, N.; Rossi, A.; Roblegg, E.; Neuhaus, W. Optimization of an oral mucosa in-vitro model based on cell line TR146. Tissue Barriers 2020, 8, 1748459. [Google Scholar] [CrossRef]
- Selin Seda Timura, S.S.; Yüksela, S.; Akcab, G.; Şenela, S. Localized drug delivery with mono and bilayered mucoadhesive films and wafers for oral mucosal infections. Int. J. Pharm. 2019, 559, 102–112. [Google Scholar] [CrossRef]
- Suharyani, I.; Fouad, A.M.A.; Muchtaridi, M.; Wathoni, N.; Abdassah, M. Evolution of Drug Delivery Systems for Recurrent Aphthous Stomatitis. Drug Des. Devel. Ther. 2021, 1, 4071–4089. [Google Scholar] [CrossRef]
- Uzunoğlu, B.; Wilson, C.G.; Sağıroğlu, M.; Yüksel, S.; Şenel, S. Mucoadhesive bilayered buccal platform for antifungal drug delivery into the oral cavity. Drug Deliv. Transl. Res. 2021, 11, 318–327. [Google Scholar] [CrossRef]
- Jin, B.Z.; Dong, X.Q.; Xu, X.; Zhang, F.H. Development and in vitro evaluation of mucoadhesive patches of methotrexate for targeted delivery in oral cancer. Oncol. Lett. 2018, 15, 2541–2549. [Google Scholar] [CrossRef]
- Paderni, C.; Compilato, D.; Giannola, L.I.; Campisi, G. Oral local drug delivery and new perspectives in oral drug formulation. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. 2012, 114, e25–e34. [Google Scholar] [CrossRef]
- Bierbaumer, L.; Schwarze, U.Y.; Gruber, R.; Neuhaus, W. Cell culture models of oral mucosal barriers: A review with a focus on applications, culture conditions and barrier properties. Tissue Barriers 2018, 6, 1479568. [Google Scholar] [CrossRef] [PubMed]
- Patel, V.F.; Liu, F.; Brown, M.B. Modeling the oral cavity: In-vitro and in vivo evaluations of buccal drug delivery systems. J. Control. Release 2012, 161, 746–756. [Google Scholar] [CrossRef] [PubMed]
- Tavakoli-Saberi, M.R.; Audus, K.L. Cultured buccal epithelium: An in-vitro model derived from the hamster pouch for studying drug transport and metabolism. Pharm. Res. 1989, 6, 160–166. [Google Scholar] [CrossRef]
- Sa, G.; Xiong, X.; Wu, T.; Yang, J.; He, S.; Zhao, Y. Histological features of oral epithelium in seven animal species: As a reference for selecting animal models. Eur. J. Pharm. Sci. 2016, 81, 10–17. [Google Scholar] [CrossRef] [PubMed]
- Le Brun, P.P.H.; Fox, P.L.A.; De Vries, M.E.; Bodd’e, H.E. In-vitro penetration of some β-adrenoreceptor blocking drugs through porcine buccal mucosa. Int. J. Pharm. 1989, 49, 141–145. [Google Scholar] [CrossRef]
- Hansen, L.B.; Christrup, L.L.; Bundgaard, H. Enhanced delivery of ketobemidone through porcine buccal mucosa in-vitro via more lipophilic ester prodrugs. Int. J. Pharm. 1992, 88, 237–242. [Google Scholar] [CrossRef]
- Castro, P.; Madureira, R.; Sarmento, B.; Pintado, M. Tissue-based in-vitro and ex-vivo models for buccal permeability studies. In Concepts and Models for Drug Permeability Studies: Cell and Tissue Based In-Vitro Culture Models; Elsevier Ltd.: Amsterdam, The Netherlands, 2016; pp. 189–202. [Google Scholar]
- Davies, M.; Peramuhendige, P.; King, L.; Golding, M.; Kotian, A.; Penney, M.; Shah, S.; Manevski, N. Evaluation of In Vitro Models for Assessment of Human Intestinal Metabolism in Drug Discovery. Drug Metab. Dispos. 2020, 48, 1169–1182. [Google Scholar] [CrossRef]
- Majid, H.; Bartel, A.; Bjoern, B. Burckhardt. Development, validation and standardization of oromucosal ex-vivo permeation studies for implementation in quality-controlled environments. J. Pharm. Biomed. Anal. 2021, 194, 113769. [Google Scholar] [CrossRef] [PubMed]
- Obradovic, T.; Hidalgo, I.J. In-vitro models for investigations of buccal drug permeation and metabolism. In Drug Absorption Studies: In Situ, In-Vitro and In Silico Models, 1st ed.; Kim, K.J., Ehrhardt, C., Eds.; Springer: Berlin/Heidelberg, Germany, 2008; pp. 167–181. [Google Scholar]
- Wang, S.; Liu, L.; Meng, S.; Wang, Y.; Liu, D.; Gao, Z.; Zuo, A.; Guo, J. A method for evaluating drug penetration and absorption through isolated buccal mucosa with highly accuracy and reproducibility. Drug Deliv. Transl. Res. 2022, 12, 2875–2892. [Google Scholar] [CrossRef]
- Kah, M.; Brown, C.D. LogD: Lipophilicity for ionisable compounds. Chemosphere 2008, 72, 1401–1408. [Google Scholar] [CrossRef] [PubMed]
- Kokate, A.; Li, X.; Jasti, B. Effect of drug lipophilicity and ionization on permeability across the buccal mucosa: A technical note. AAPS PharmSciTech 2008, 9, 501–504. [Google Scholar] [CrossRef]
- Holm, R.; Meng-Lund, E.; Andersen, M.B.; Jespersen, M.L.; Karlsson, J.J.; Garmer, M.; Jørgensen, E.B.; Jacobsen, J. In-vitro, ex-vivo and in vivo examination of buccal absorption of metoprolol with varying pH in TR146 cell culture, porcine buccal mucosa and Göttingen minipigs. Eur. J. Pharm. Sci. 2013, 49, 117–124. [Google Scholar] [CrossRef] [PubMed]
- Meng-Lund, E.; Marxen, E.; Pedersen, A.M.L.; Müllertz, A.; Hyrup, B.; Holm, R.; Jacobsen, J. Ex-vivo correlation of the permeability of metoprolol across human and porcine buccal mucosa. J. Pharm. Sci. 2014, 103, 2053–2061. [Google Scholar] [CrossRef] [PubMed]
- Consuelo, I.D.; Falson, F.; Guy, R.H.; Jacques, Y. Transport of fentanyl through pig buccal and esophageal epithelia in-vitro. Influence of concentration and vehicle pH. Pharm. Res. 2005, 22, 1525–1529. [Google Scholar] [CrossRef] [PubMed]
- Deneer, V.H.; Drese, G.B.; Roemelé, P.E.; Verhoef, J.C.; Lie-A-Huen, L.; Kingma, J.H.; Brouwers, J.R.; Junginger, H.E. Buccal transport of flecainide and sotalol: Effect of a bile salt and ionization state. Int. J. Pharm. 2002, 241, 127–134. [Google Scholar] [CrossRef]
- Wang, Y.; Zuo, Z.; Chow, M.S.S. HO-1-u-1 model for screening sublingual drug delivery–influence of pH, osmolarity and permeation enhancer. Int. J. Pharm. 2009, 370, 68–74. [Google Scholar] [CrossRef]
- Pinto, S.; Pintado, M.E.; Sarmento, B. In vivo, ex vivo and in vitro assessment of buccal permeation of drugs from delivery systems. Expert Opin. Drug Deliv. 2020, 17, 33–48. [Google Scholar] [CrossRef]
- Imbert, D.; Cullander, C. Buccal mucosa in-vitro experiments. I. Confocal imaging of vital staining and MTT assays for the determination of tissue viability. J. Control. Release 1999, 58, 39–50. [Google Scholar] [CrossRef]
- Zanoni, I.; Crosera, M.; Pavoni, E.; Adami, G.; Mauro, M.; Costa, A.L.; Lead, J.R.; Larese Filon, F. Use of single particle ICP-MS to estimate silver nanoparticle penetration through baby porcine mucosa. Nanotoxicology 2021, 15, 1005–1015. [Google Scholar] [CrossRef]
- Brayden, D.J.; Stuettgen, V. Sodium glycodeoxycholate and sodium deoxycholate as epithelial permeation enhancers: In vitro and ex vivo intestinal and buccal bioassays. Eur. J. Pharm. Sci. 2021, 159, 105737. [Google Scholar] [CrossRef] [PubMed]
- Wanasathop, A.; Patel, P.B.; Choi, H.A.; Li, S.K. Permeability of Buccal Mucosa. Pharmaceutics 2021, 13, 1814. [Google Scholar] [CrossRef] [PubMed]
- Wong, C.Y.; Al-Salami, H.; Dass, C.R. Current status and applications of animal models in pre-clinical development of orally administered insulin-loaded nanoparticles. J. Drug Target. 2020, 28, 882–903. [Google Scholar] [CrossRef] [PubMed]
- Sohi, H.; Ahuja, A.; Ahmad, F.J.; Khar, R.K. Critical evaluation of permeation enhancers for oral mucosal drug delivery. Drug Dev. Ind. Pharm. 2010, 36, 254–282. [Google Scholar] [CrossRef]
- Kokate, A.; Li, X.; Jasti, B. HPLC detection of marker compounds during buccal permeation enhancement studies. J. Pharm. Biomed. Anal. 2008, 47, 190–194. [Google Scholar] [CrossRef]
- Kolli, C.S.; Pather, I. Characterization methods for Oral mucosal drug delivery. In Oral Mucosal Drug Delivery and Therapy; Rathbone, M.J., Senel, S., Pather, I., Eds.; Springer: New York, NY, USA; Heidelberg, Germany; Dordrecht, The Netherlands; London, UK, 2015; pp. 125–148. [Google Scholar]
- Quintanilha, N.P.; Dos Santos Miranda Costa, I.; Freiman de Souza Ramos, M.; Campos de Oliveira Miguel, N.; Riemma Pierre, M.B. α-Bisabolol improves 5-aminolevulinic acid retention in buccal tissues: Potential application in the photodynamic therapy of oral cancer. J. Photochem. Photobiol. B 2017, 174, 298–305. [Google Scholar] [CrossRef]
- Costa Idos, S.; Abranches, R.P.; Garcia, M.T.; Pierre, M.B. Chitosan-based mucoadhesive films containing 5-aminolevulinic acid for buccal cancer’s treatment. J. Photochem. Photobiol. B 2014, 140, 266–275. [Google Scholar] [CrossRef]
- Li, C.; Yu, D.G.; Williams, G.R.; Wang, Z.H. Fast-Dissolving Core-Shell Composite Microparticles of Quercetin Fabricated Using a Coaxial Electrospray Process. PLoS ONE 2014, 9, e92106. [Google Scholar] [CrossRef]
- Chaudhary, B.; Verma, S. Preparation and evaluation of novel in situ gels containing acyclovir for the treatment of oral herpes simplex virus infections. Sci. World J. 2014, 2014, 280928. [Google Scholar] [CrossRef]
- El-Nabarawi, M.A.; Ali, A.A.; Aboud, H.M.; Hassan, A.H.; Godah, A.H. Transbuccal delivery of betahistine dihydrochloride from mucoadhesive tablets with a unidirectional drug flow: In-vitro, ex-vivo and in vivo evaluation. Drug Des. Dev. Ther. 2016, 10, 4031–4045. [Google Scholar] [CrossRef]
- Franz-Montan, M.; Baroni, D.; Brunetto, G.; Sobral, V.R.; da Silva, C.M.; Venâncio, P.; Zago, P.W.; Cereda, C.M.; Volpato, M.C.; de Araújo, D.R.; et al. Liposomal lidocaine gel for topical use at the oral mucosa: Characterization, in-vitro assays and in vivo anaesthetic efficacy in humans. J. Liposome Res. 2015, 25, 11–19. [Google Scholar] [CrossRef] [PubMed]
- Giannola, L.I.; De Caro, V.; Giandalia, G.; Siragusa, M.G.; Paderni, C.; Campisi, G.; Florena, A.M. 5-Fluorouracil buccal tablets for locoregional chemotherapy of oral squamous cell carcinoma: Formulation, drug release and histological effects on reconstituted human oral epithelium and porcine buccal mucosa. Curr. Drug Deliv. 2010, 7, 109–117. [Google Scholar] [CrossRef] [PubMed]
- Handler, A.M.; Marxen, E.; Jacobsen, J.; Janfelt, C. Visualization of the penetration modifying mechanism of laurocapram by Mass Spectrometry Imaging in buccal drug delivery. Eur. J. Pharm. Sci. 2019, 127, 276–281. [Google Scholar] [CrossRef]
- Heemstra, L.B.; Finnin, B.C.; Nicolazzo, J.A. The buccal mucosa as an alternative route for the systemic delivery of risperidone. J. Pharm. Sci. 2010, 99, 4584–4592. [Google Scholar] [CrossRef] [PubMed]
- Itin, C.; Barasch, D.; Domb, A.J.; Hoffman, A. Prolonged oral transmucosal delivery of highly lipophilic drug cannabidiol. Int. J. Pharm. 2020, 581, 119276. [Google Scholar] [CrossRef]
- Kroth, R.; Argenta, D.F.; Conte, J.; Amaral, B.R.; Caon, T. Transbuccal Delivery of Isoniazid: Ex-vivo Permeability and Drug-Surfactant Interaction Studies. AAPS PharmSciTech 2020, 21, 289. [Google Scholar] [CrossRef]
- Marxen, E.; Jin, L.; Jacobsen, J.; Janfelt, C.; Hyrup, B.; Nicolazzo, J.A. Effect of Permeation Enhancers on the Buccal Permeability of Nicotine: Ex-vivo Transport Studies Complemented by MALDI MS Imaging. Pharm. Res. 2018, 35, 70. [Google Scholar] [CrossRef]
- Meng-Lund, E.; Jacobsen, J.; Jin, L.; Janfelt, C.; Holm, R.; Müllertz, A.; Nicolazzo, J.A. Azone® decreases the buccal mucosal permeation of diazepam in a concentration-dependent manner via a reservoir effect. J. Pharm. Sci. 2014, 103, 1133–1141. [Google Scholar] [CrossRef]
- Mashru, R.; Sutariya, V.; Sankalia, M.; Sankalia, J. Transbuccal delivery of lamotrigine across porcine buccal mucosa: In-vitro determination of routes of buccal transport. J. Pharm. Sci. 2005, 8, 54–62. [Google Scholar]
- Trastullo, R.; Abruzzo, A.; Saladini, B.; Gallucci, M.C.; Cerchiara, T.; Luppi, B.; Bigucci, F. Design and evaluation of buccal films as paediatric dosage form for transmucosal delivery of ondansetron. Eur. J. Pharm. Biopharm. 2016, 105, 115–121. [Google Scholar] [CrossRef]
- Wróblewska, M.; Szymańska, E.; Szekalska, M.; Winnicka, K. Different Types of Gel Carriers as Metronidazole Delivery Systems to the Oral Mucosa. Polymers 2020, 12, 680. [Google Scholar] [CrossRef] [PubMed]
- Xu, L.; Sheybani, N.; Ren, S.; Bowlin, G.L.; Yeudall, W.A.; Yang, H. Semi-interpenetrating network (sIPN) co-electrospun gelatin/insulin fiber formulation for transbuccal insulin delivery. Pharm. Res. 2015, 32, 275–285. [Google Scholar] [CrossRef] [PubMed]
- Kalouta, K.; Stie, M.B.; Janfelt, C.; Chronakis, I.S.; Jacobsen, J.; Mørck Nielsen, H.; Foderà, V. Electrospun -lactalbumin nanofibers for site-specific and fast-onset delivery of nicotine in the oral cavity: An in-vitro, ex-vivo and tissue spatial distribution study. Mol. Pharm. 2020, 17, 4189–4200. [Google Scholar] [CrossRef]
- Bashyal, S.; Seo, J.E.; Keum, T.; Noh, G.; Lamichhane, S.; Kim, J.H.; Kim, C.H.; Choi, Y.W.; Lee, S. Facilitated Buccal Insulin Delivery via Hydrophobic Ion-Pairing Approach: In-vitro and ex-vivo Evaluation. Int. J. Nanomed. 2021, 16, 4677–4691. [Google Scholar] [CrossRef] [PubMed]
- Eleftheriadis, G.K.; Monou, P.K.; Bouropoulos, N.; Boetker, J.; Rantanen, J.; Jacobsen, J.; Vizirianakis, I.S.; Fatouros, D.G. Fabrication of Mucoadhesive Buccal Films for Local Administration of Ketoprofen and Lidocaine Hydrochloride by Combining Fused Deposition Modeling and Inkjet Printing. J. Pharm. Sci. 2020, 109, 2757–2766. [Google Scholar] [CrossRef]
- Sharifi, N.; Mortazavi, S.A.; Rabbani, S.; Torshabi, M.; Talimi, R.; Haeri, A. Fast dissolving nanofibrous mats for diclofenac sodium delivery: Effects of electrospinning polymer and addition of super-disintegrant. J. Drug Deliv. Sci. Technol. 2022, 73, 103356. [Google Scholar] [CrossRef]
- Ho, H.N.; Le, H.H.; Le, T.G.; Duong, T.H.A.; Ngo, V.Q.T.; Dang, C.T.; Nguyen, V.M.; Tran, T.H.; Nguyen, C.N. Formulation and characterization of hydroxyethyl cellulose-based gel containing metronidazole-loaded solid lipid nanoparticles for buccal mucosal drug delivery. Int. J. Biol. Macromol. 2022, 194, 1010–1018. [Google Scholar] [CrossRef]
- Silva-Abreu, M.; Espinoza, L.C.; Halbaut, L.; Espina, M.; García, M.L.; Calpen, A.C. Comparative Study of Ex Vivo Transmucosal Permeation of Pioglitazone Nanoparticles for the Treatment of Alzheimer’s Disease. Polymers 2018, 10, 316. [Google Scholar] [CrossRef]
- Dommisch, H.; Stolte, K.N.; Jager, J.; Vogel, K.; Müller Hedtrich, R.; Unbehauen, M.; Haag, R.; Danker, K. Characterization of an ester-based core-multishell (CMS) nanocarrier for the topical application at the oral mucosa. Clin. Oral Investig. 2021, 25, 5795–5805. [Google Scholar] [CrossRef]
- Chen, J.; Duan, H.; Pan, H.; Yang, X.; Pan, W. Two types of core/shell fibers based on carboxymethyl chitosan and Sodium carboxymethyl cellulose with self-assembled liposome for buccal delivery of carvedilol across TR146 cell culture and porcine buccal mucosa. Int. J. Biol. Macromol. 2019, 128, 700–709. [Google Scholar] [CrossRef]
- Hu, S.; Pei, X.; Duan, L.; Zhu, Z.; Liu, Y.; Chen, J.; Chen, T.; Ji, P.; Wan, Q.; Wang, J. A mussel-inspired film for adhesion to wet buccal tissue and efficient buccal drug delivery. Nat. Commun. 2021, 12, 1689. [Google Scholar] [CrossRef] [PubMed]
- Stie, M.B.; Oblom, H.; Nørgaard Hansen, H.C.; Jacobsen, J.; Chronakis, I.S.; Rantanen, J.; Mørck Nielsen, H.; Genina, N. Mucoadhesive chitosan- and cellulose derivative-based nanofiber-on-foam-on-film system for non-invasive peptide delivery. Carbohydr. Polym. 2023, 303, 120429. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Jiang, L.; Meng, S.; Liu, C.; Wang, H.; Gao, Z. Hollow mesoporous silica nanoparticles-loaded ion-crosslinked bilayer films with excellent mechanical properties and high bioavailability for buccal delivery. Int. J. Pharm. 2022, 624, 122056. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Gao, Z.; Liu, L.; Li, M.; Zuo, A. Preparation, in vitro and in vivo evaluation of chitosan-sodium alginate-ethyl cellulose polyelectrolyte film as a novel buccal mucosal delivery vehicle. Eur. J. Pharm. Sci. 2022, 168, 106085. [Google Scholar] [CrossRef] [PubMed]
- Serpe, L.; Jain, A.; de Macedo, C.G.; Volpato, M.C.; Groppo, F.C.; Gill, H.S.; Franz-Montan, M. Influence of salivary washout on drug delivery to the oral cavity using coated microneedles: An in-vitro evaluation. Eur. J. Pharm. Sci. 2016, 93, 215–223. [Google Scholar] [CrossRef]
- Majid, H.; Puzik, A.; Maier, T.; Merk, R.; Bartel, A.; Mueller, H.-C.; Burckhardt, B.B. Formulation Development of Sublingual Cyclobenzaprine Tablets Empowered by Standardized and Physiologically Relevant Ex Vivo Permeation Studies. Pharmaceutics 2021, 13, 1409. [Google Scholar] [CrossRef]
- Di Cesare, F.; Gioeni, D.; Ravasio, G.; Pellegrini, A.; Lucatello, L.; Bisutti, V.; Villa, R.; Cagnardi, P. Clinical pharmacokinetics of a dexmedetomidine-methadone combination in dogs undergoing routine anaesthesia after buccal or intramuscular administration. J. Vet. Pharmacol. Ther. 2019, 42, 392–400. [Google Scholar] [CrossRef]
- Garren, K.W.; Repta, A.J. Buccal absorption. II. In-vitro diffusion across the hamster cheek pouch. J. Pharm. Sci. 1989, 78, 160–162. [Google Scholar] [CrossRef]
- Rupniak, H.T.; Rowlatt, C.; Lane, E.B.; Steele, J.G.; Trejdosiewicz, L.K.; Laskiewicz, B.; Povey, S.; Hill, B.T. Characteristics of four new human cell lines derived from squamous cell carcinomas of the head and neck. J. Natl. Cancer Inst. 1985, 75, 621–635. [Google Scholar]
- Jacobsen, J.; van Deurs, B.; Pedersen, M.; Rassing, M.R. TR146 cells grown on filters as a model for human buccal epithelium: I. Morphology, growth, barrier properties, and permeability. Int. J. Pharm. 1995, 125, 165–184. [Google Scholar] [CrossRef]
- Jacobsen, J.; Bjerregaard, S.; Pedersen, M. Cyclodextrin inclusion complexes of antimycotics intended to act in the oral cavity–drug supersaturation, toxicity on TR146 cells and release from a delivery system. Eur. J. Pharm. Biopharm. 1999, 48, 217–224. [Google Scholar] [CrossRef] [PubMed]
- Nielsen, H.M.; Rassing, M.R. TR146 cells grown on filters as a model of human buccal epithelium: V. Enzyme activity of the TR146 cell culture model, human buccal epithelium and porcine buccal epithelium, and permeability of leu-enkephalin. Int. J. Pharm. 2000, 200, 261–270. [Google Scholar] [CrossRef] [PubMed]
- Sander, C.; Mørck Nielsen, H.; Jacobsen, J. Buccal delivery of metformin: TR146 cell culture model evaluating the use of bioadhesive chitosan discs for drug permeability enhancement International. J. Pharm. 2013, 458, 254–261. [Google Scholar] [CrossRef] [PubMed]
- Portero, A.; Remuñán-López, C.; Nielsen, H.M. The potential of chitosan in enhancing peptide and protein absorption across the TR146 cell culture model-an in-vitro model of the buccal epithelium. Pharm. Res 2002, 19, 169–174. [Google Scholar] [CrossRef]
- Klemetsrud, T.; Kjøniksen, A.L.; Hiorth, M.; Jacobsen, J.; Smistad, G. Polymer coated liposomes for use in the oral cavity—A study of the in-vitro toxicity, effect on cell permeability and interaction with mucin. J. Liposome Res. 2018, 28, 62–73. [Google Scholar] [CrossRef]
- Iyire, A.; Alayedi, M.; Mohammed, A.R. Pre-formulation and systematic evaluation of amino acid assisted permeability of insulin across in-vitro buccal cell layers. Sci. Rep. 2016, 6, 32498. [Google Scholar] [CrossRef]
- Teubl, B.J.; Absenger, M.; Fröhlich, E.; Leitinger, G.; Zimmer, A.; Roblegg, E. The oral cavity as a biological barrier system: Design of an advanced buccal in-vitro permeability model. Eur. J. Pharm. Biopharm. 2013, 84, 386–393. [Google Scholar] [CrossRef]
- O’Callaghan, K.; Palagano, E.; Butini, S.; Campiani, G.; Williams, D.C.; Zisterer, D.M.; O’Sullivan, J. Induction of apoptosis in oral squamous carcinoma cells by pyrrolo-1,5-benzoxazepines. Mol. Med. Rep. 2015, 12, 3748–3754. [Google Scholar] [CrossRef]
- Eirheim, H.U.; Bundgaard, C.; Nielsen, H.M. Evaluation of different toxicity assays applied to proliferating cell and to stratified epithelium in relation to permeability enhancement with glycocholate. Toxicol. In Vitro 2004, 18, 649–657. [Google Scholar] [CrossRef]
- Benson, K.; Cramer, S.; Galla, H.J. Impedance-based cell monitoring: Barrier properties and beyond. Fluids Barriers CNS 2013, 10, 5. [Google Scholar] [CrossRef]
- Yadev, N.P.; Murdoch, C.; Saville, S.P.; Thornhill, M.H. Evaluation of tissue engineered models of the oral mucosa to investigate oral candidiasis. Microb. Pathog. 2011, 50, 278–285. [Google Scholar] [CrossRef] [PubMed]
- Alopaeus, J.F.; Hellfritzsch, M.; Gutowski, T.; Scherließ, R.; Almeida, A.; Sarmento, B.; Škalko-Basnet, N.; Tho, I. Mucoadhesive buccal films based on a graft co-polymer—A mucin-retentive hydrogel scaffold. Eur. J. Pharm. Sci. 2020, 142, 105142. [Google Scholar] [CrossRef] [PubMed]
- Park, H.Y.; Kweon, D.K.; Kim, J.K. Molecular weight-dependent hyaluronic acid permeability and tight junction modulation in human buccal TR146 cell monolayers. Int. J. Biol. Macromol. 2023, 227, 182–192. [Google Scholar] [CrossRef] [PubMed]
- Batista, P.; Castro, P.M.; Madureira, A.R.; Sarmento, B.; Pintado, M. Preparation, Characterization and Evaluation of Guar Films Impregnated with Relaxing Peptide Loaded into Chitosan Microparticles. Appl. Sci. 2021, 11, 9849. [Google Scholar] [CrossRef]
- Jeitler, R.; Glader, C.; Tetyczka, C.; Zeiringer, S.; Absenger-Novak, M.; Selmani, A.; Fröhlich, E.; Roblegg, E. Investigation of Cellular Interactions of Lipid-Structured Nanoparticles With Oral Mucosal Epithelial Cells. Front. Mol. Biosci. 2022, 9, 917921. [Google Scholar] [CrossRef]
- Masson-Meyers, D.S.; Bertassoni, L.E.; Tayebi, L. Oral mucosa equivalents, prevascularization approaches, and potential applications. Connect. Tissue Res. 2022, 63, 514–529. [Google Scholar] [CrossRef]
- Jennings, L.R.; Colley, H.E.; Ong, J.; Panagakos, F.; Masters, J.G.; Trivedi, H.M.; Murdoch, C.; Whawell, S. Development and Characterization of In-vitro Human Oral Mucosal Equivalents Derived from Immortalized Oral Keratinocytes. Tissue Eng. Part C Methods 2016, 22, 1108–1117. [Google Scholar] [CrossRef]
- Tabatabaei, F.; Moharamzadeh, K.; Tayebi, L. Three-Dimensional In Vitro Oral Mucosa Models of Fungal and Bacterial Infections. Tissue Eng. Part B Rev. 2020, 26, 443–460. [Google Scholar] [CrossRef]
- Sapkota, D.; Bruland, O.; Parajuli, H.; Osman, T.A.; Teh, M.T.; Johannessen, A.C.; Costea, D.E. S100A16 promotes differentiation and contributes to a less aggressive tumor phenotype in oral squamous cell carcinoma. BMC Cancer 2015, 15, 631. [Google Scholar] [CrossRef]
- Nishiyama, K.; Akagi, T.; Iwai, S.; Akashi, M. Construction of vascularized oral mucosa equivalents using a layer-by-layer cell coating technology. Tissue Eng.-Part C Methods 2019, 25, 262–275. [Google Scholar] [CrossRef]
- Said, Z.; Murdoch, C.; Hansen, J.; Siim Madsen, L.; Colley, H.E. Corticosteroid delivery using oral mucosa equivalents for the treatment of inflammatory mucosal diseases. Eur. J. Oral Sci. 2021, 129, e12761. [Google Scholar] [CrossRef] [PubMed]
- Buskermolen, J.K.; Reijnders, C.M.; Spiekstra, S.W.; Steinberg, T.; Kleverlaan, C.J.; Feilzer, A.J.; Bakker, A.D.; Gibbs, S. Development of a Full-Thickness Human Gingiva Equivalent Constructed from Immortalized Keratinocytes and Fibroblasts. Tissue Eng. Part C Methods 2016, 22, 781–791. [Google Scholar] [CrossRef] [PubMed]
- Marxen, E.; Mosgaard, M.D.; Pedersen, A.M.L.; Jacobsen, J. Mucin dispersions as a model for the oromucosal mucus layer in in vitro and ex vivo buccal permeability studies of small molecules. Eur. J. Pharm. Biopharm. 2017, 121, 121–128. [Google Scholar] [CrossRef] [PubMed]
- Ployon, S.; Belloir, C.; Bonnotte, A.; Lherminier, J.; Canon, F.; Morzel, M. The membrane-associated MUC1 improves adhesion of salivary MUC5B on buccal cells. Application to development of an in vitro cellular model of oral epithelium. Arch. Oral Biol. 2016, 61, 149–155. [Google Scholar] [CrossRef] [PubMed]
- Hubrecht, R.C.; Carter, E. The 3Rs and humane experimental technique: Implementing change. Animals 2019, 10, 754. [Google Scholar] [CrossRef] [PubMed]
- Cheluvappa, R.; Scowen, P.; Eri, R. Ethics of animal research in human disease remediation, its institutional teaching; and alternatives to animal experimentation. Pharmacol. Res. Perspect. 2017, 5, e00332. [Google Scholar] [CrossRef]
- Klausner, M.; Handa, Y.; Aizawa, S. In vitro three-dimensional organotypic culture models of the oral mucosa. In Vitro Cell. Dev. Biol.-Anim. 2021, 57, 148–159. [Google Scholar] [CrossRef]


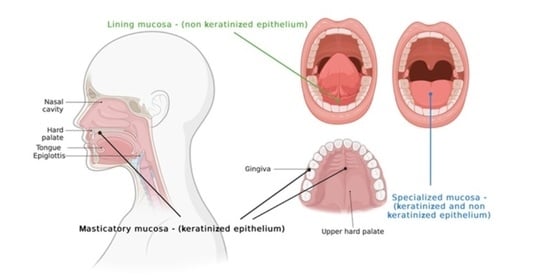
| Type of Mucosa | Localization | Characteristics |
|---|---|---|
| Masticatory mucosa (25% of the oral cavity’s surface) |
|
|
| Lining mucosa (60% of the oral cavity’s surface) |
|
|
| Animals | Kind of Epithelium | Advantages | Disadvantages |
|---|---|---|---|
| Rats | Keratinized | Different permeability [25] | |
| Hamsters | Keratinized | Different permeability [25] | |
| Rabbits | Non-keratinized or keratinized | The permeability is like the human mucosa | The amount of mucosa is significantly reduced [26] |
| Dogs | non-keratinized | The epithelium is thinner with respect to human one, thus the permeability is different [26] | |
| Monkeys | non-keratinized | The epithelium is thinner with respect to human one, thus the permeability is different [26] | |
| Pigs | Non-keratinized or keratinized | The permeability values are similar with respect to human mucosa [27,28] |
| Active Compounds | Vehicle | Excision Animal Tissues (Ex Vivo Models) |
|---|---|---|
| 5-Aminolevulinic acid | No | porcine buccal mucosa [50] |
| 5-aminolevulinic acid | Chitosan based mucoadhesive films | Pig buccal (Cheek) mucosa [51] |
| Quercetin | Core-Shell Composite Microparticles | Porcine sublingual mucosae [52] |
| Acyclovir | Gels | porcine oral mucosa [53] |
| Betahistine dihydrochloride | mucoadhesive tablets | Camel buccal mucosa [54] |
| Lidocaine | Gel | pig palatal mucosa [55] |
| 5-Fluorouracil | Tablets | porcine buccal mucosa [56] |
| Diazepan | No | Porcine buccal mucosa [57] |
| Risperidone | mucoadhesive gel formulation | Porcine buccal mucosa [58] |
| Cannabidiol | No | Pig buccal tissues [59] |
| Isoniazid | micelles | Porcine buccal mucosa [60] |
| Nicotine | enhancer | Porcine buccal mucosa [61] |
| Diazepan | No | porcine buccal mucosa [62] |
| Lamotrigine | No | Porcine buccal tissue [63] |
| Ondansetron | Film | Porcine oral mucosa [64] |
| Metronidazole | Gel | Porcine buccal mucosa [65] |
| Insulin | Fibers | porcine cheek tissues [66] |
| Isoniazid | micelles | Human buccal mucosa [60] |
| Nicotine | Nanofibers | Porcine buccal mucosa [67] |
| Insulin | hydrophobic ion-pairing (HIP)-nano complexes | Porcine buccal mucosa [68] |
| Ketoprofen and lidocaine | Film | Porcine buccal mucosa [69] |
| Diclofenac | nanofibers | sheep buccal mucosa [70] |
| metronidazole | hydroxyethyl cellulose-based gel containing metronidazole-loaded solid lipid nanoparticles | Porcine oral mucosa [71] |
| Pioglitazone | PLGA-PEG Nanoparticles | different ex vivo mucosal systems: buccal, sublingual, nasal, and intestinal [72] |
| fluorescence-labeled nanoparticles to investigate penetration efficiency to oral mucosal tissues | ester-based core-multishell nanoparticles | porcine masticatory and lining mucosa [73] |
| Carvedilol | self-assembled liposomes and core/shell fibers | Pig buccal mucosa [74] |
| DOPA 3,4-dihydroxy-D-phenylalanine | PLGA NPs | porcine buccal tissue [75] |
| Peptide | multi-layered nanofiber-on-foam-on-film | porcine buccal mucosa [76] |
| furosemide | hollow mesoporous silica nanoparticles | Porcine buccal mucosa [77] |
| Zolmitriptan and Etodolac | film comprising chitosan, sodium alginate, and ethyl cellulose | rabbit buccal mucosae [78] |
| Active Compounds | Vehicle |
|---|---|
| Furosemide | Mucoadhesive buccal films based on a graft co-polymer—A mucin-retentive hydrogel scaffold [96] |
| Furosenimide | Hollow mesoporous silica nanoparticles [78] |
| leu-enkephalin | No vehicles, the goal was the comparison between TR146 and human buccal epithelium [86] |
| Testosterone | No vehicle [84] |
| Metformin | Bio adhesive chitosan discs [87] |
| Carvedilol, | Self-assembled liposomes and core/shell fibers [74] |
| low molecular weight Hyaluronic Acid <100 kDa and >500 kDa | No vehicle, the goal was to study the dependence of permeability by molecular weight hyaluronic acid and tight junction modulation in human buccal TR146 [97] |
| Zolmitriptan and Etodolac | Film comprising chitosan, sodium alginate, and ethyl cellulose [78] |
| Peptides | Oral guar films entrapping peptide-containing chitosan microparticles TR146 [98] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mazzinelli, E.; Favuzzi, I.; Arcovito, A.; Castagnola, R.; Fratocchi, G.; Mordente, A.; Nocca, G. Oral Mucosa Models to Evaluate Drug Permeability. Pharmaceutics 2023, 15, 1559. https://doi.org/10.3390/pharmaceutics15051559
Mazzinelli E, Favuzzi I, Arcovito A, Castagnola R, Fratocchi G, Mordente A, Nocca G. Oral Mucosa Models to Evaluate Drug Permeability. Pharmaceutics. 2023; 15(5):1559. https://doi.org/10.3390/pharmaceutics15051559
Chicago/Turabian StyleMazzinelli, Elena, Ilaria Favuzzi, Alessandro Arcovito, Raffaella Castagnola, Giorgia Fratocchi, Alvaro Mordente, and Giuseppina Nocca. 2023. "Oral Mucosa Models to Evaluate Drug Permeability" Pharmaceutics 15, no. 5: 1559. https://doi.org/10.3390/pharmaceutics15051559
APA StyleMazzinelli, E., Favuzzi, I., Arcovito, A., Castagnola, R., Fratocchi, G., Mordente, A., & Nocca, G. (2023). Oral Mucosa Models to Evaluate Drug Permeability. Pharmaceutics, 15(5), 1559. https://doi.org/10.3390/pharmaceutics15051559