Solid Lipid Nanoparticles vs. Nanostructured Lipid Carriers: A Comparative Review
Abstract
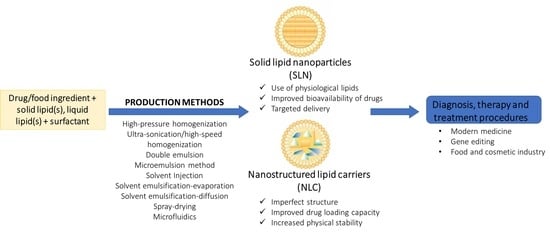
:1. Introduction
2. Structural Features of Solid Lipid Nanoparticles and Nanostructured Lipid Carriers
2.1. Solid Lipid Nanoparticles
2.2. Nanostructured Lipid Carriers
2.3. Comparison between Solid Lipid Nanoparticles and Nanostructured Lipid Carrier
3. Production Methods of Solid–lipid Nanoparticles and Nanostructured Lipid Carriers
3.1. High-Pressure Homogenization
3.2. Ultra-Sonication or High-Speed Homogenization
3.3. Double Emulsion
3.4. Microemulsion Method
3.5. Solvent Injection
3.6. Solvent Emulsification-Evaporation
3.7. Solvent Emulsification-Diffusion
3.8. Spray Drying
3.9. Microfluidics
4. In Vitro Performance of Solid Lipid Nanoparticles and Nanostructured Lipid Carrier
5. In Vivo Performance of Solid Lipid Nanoparticles and Nanostructured Lipid Carrier
6. Toxicity Concerns
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations/Nomenclature
| 18PA | 1,2-dioleoyl-sn-glycero-3-phosphate |
| BBB | Blood brain barrier |
| CLA | Conjugated linoleic acid |
| CPA | Cyproterone acetate |
| DODAP | 1,2-dioleoyl-3-dimethylammonium-propane |
| DOTAP | 1,2-dioleoyl-3-trimethylammonium-propane |
| DPPC | Dipalmitoylphosphatidylcholine |
| DSPE | 1,2-distearoyl-sn-glycero-3-phosphoethanolamine |
| EGFR-TK | Epidermal growth factor receptors |
| FA | Folic acid |
| GRAS | Generally recognized as safe |
| HPH | High-pressure homogenization |
| HSPC | Hydrogenated soybean phosphatidylcholine |
| NLC | Nanostructured lipid carriers |
| nNLC | Non-targeted nanostructured lipid carriers |
| PdI | Polydispersity index |
| pDNA | Plasmid DNA |
| PEG | Polyethylene glycol |
| PLGA | Poly Lac-tic-co-Glycolic Acid |
| PVA | Polyvinylalcohol |
| RH | Relative Humidity |
| ROS | Radical oxygen species |
| SLN | Solid–lipid nanoparticles |
| SLS | Sodium lauryl sulfate |
| SORT | Selective Organ Targeting |
| SORT LNPs | Selective organ targeting lipid nanoparticles |
| tNLC | Target nanostructured lipid carriers |
| VEGF | Vascular endothelial growth factor |
| VEGFR | Vascular endothelial growth factor receptor |
References
- Soares, S.; Sousa, J.; Pais, A.; Vitorino, C. Nanomedicine: Principles, Properties, and Regulatory Issues. Front. Chem. 2018, 6, 360. [Google Scholar] [CrossRef] [PubMed]
- Patra, J.K.; Das, G.; Fraceto, L.F.; Campos, E.V.R.; del Pilar Rodriguez-Torres, M.; Acosta-Torres, L.S.; Diaz-Torres, L.A.; Grillo, R.; Swamy, M.K.; Sharma, S.; et al. Nano based drug delivery systems: Recent developments and future prospects. J. Nanobiotechnol. 2018, 16, 71. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jaiswal, P.; Gidwani, B.; Vyas, A. Nanostructured lipid carriers and their current application in targeted drug delivery. Artif. Cells Nanomed. Biotechnol. 2016, 44, 27–40. [Google Scholar] [CrossRef] [PubMed]
- Alexis, F.; Rhee, J.-W.; Richie, J.P.; Radovic-Moreno, A.F.; Langer, R.; Farokhzad, O.C. New frontiers in nanotechnology for cancer treatment. Urol. Oncol. 2008, 26, 74–85. [Google Scholar] [CrossRef] [PubMed]
- ud Din, F.; Aman, W.; Ullah, I.; Qureshi, O.S.; Mustapha, O.; Shafique, S.; Zeb, A. Effective use of nanocarriers as drug delivery systems for the treatment of selected tumors. Int. J. Nanomed. 2017, 12, 7291–7309. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Akel, H.; Ismail, R.; Katona, G.; Sabir, F.; Ambrus, R.; Csóka, I. A comparison study of lipid and polymeric nanoparticles in the nasal delivery of meloxicam: Formulation, characterization, and in vitro evaluation. Int. J. Pharm. 2021, 604, 120724. [Google Scholar] [CrossRef]
- Ghasemiyeh, P.; Mohammadi-Samani, S.J.R.i.p.s. Solid lipid nanoparticles and nanostructured lipid carriers as novel drug delivery systems: Applications, advantages and disadvantages. Res. Pharm. Sci. 2018, 13, 288. [Google Scholar]
- Khosa, A.; Reddi, S.; Saha, R.N. Nanostructured lipid carriers for site-specific drug delivery. Biomed. Pharmacother. 2018, 103, 598–613. [Google Scholar] [CrossRef]
- Sgorla, D.; Bunhak, É.J.; Cavalcanti, O.A.; Fonte, P.; Sarmento, B. Exploitation of lipid-polymeric matrices at nanoscale for drug delivery applications. Expert Opin. Drug Deliv. 2016, 13, 1301–1309. [Google Scholar] [CrossRef]
- Liu, Y.; Jiang, Z.; Hou, X.; Xie, X.; Shi, J.; Shen, J.; He, Y.; Wang, Z.; Feng, N. Functional lipid polymeric nanoparticles for oral drug delivery: Rapid mucus penetration and improved cell entry and cellular transport. Nanomed. Nanotechnol. Biol. Med. 2019, 21, 102075. [Google Scholar] [CrossRef]
- Sgorla, D.; Lechanteur, A.; Almeida, A.; Sousa, F.; Melo, E.; Bunhak, É.; Mainardes, R.; Khalil, N.; Cavalcanti, O.; Sarmento, B. Development and characterization of lipid-polymeric nanoparticles for oral insulin delivery. Expert Opin. Drug Deliv. 2018, 15, 213–222. [Google Scholar] [CrossRef]
- Hallberg, D.; Holm, I.; Obel, A.L.; Schuberth, O.; Wretlind, A. Fat emulsions for complete intravenous nutrition. Postgrad. Med. J. 1967, 43, 307–316. [Google Scholar] [CrossRef] [Green Version]
- Bangham, A.D.; Standish, M.M.; Watkins, J.C. Diffusion of univalent ions across the lamellae of swollen phospholipids. J. Mol. Biol. 1965, 13, 238–252, IN26–IN27. [Google Scholar] [CrossRef]
- Bulbake, U.; Doppalapudi, S.; Kommineni, N.; Khan, W. Liposomal formulations in clinical use: An updated review. Pharmaceutics 2017, 9, 12. [Google Scholar] [CrossRef]
- Muller, R.H.; Radtke, M.; Wissing, S.A. Solid lipid nanoparticles (SLN) and nanostructured lipid carriers (NLC) in cosmetic and dermatological preparations. Adv. Drug Deliv. Rev. 2002, 54 (Suppl. S1), S131–S155. [Google Scholar] [CrossRef]
- Dudhipala, N.; Janga, K.Y.; Gorre, T. Comparative study of nisoldipine-loaded nanostructured lipid carriers and solid lipid nanoparticles for oral delivery: Preparation, characterization, permeation and pharmacokinetic evaluation. Artif. Cells Nanomed. Biotechnol. 2018, 46, 616–625. [Google Scholar] [CrossRef] [PubMed]
- Fonte, P.; Andrade, F.; Araújo, F.; Andrade, C.; das Neves, J.; Sarmento, B. Chitosan-coated solid lipid nanoparticles for insulin delivery. Methods Enzymol. 2012, 508, 295–314. [Google Scholar] [CrossRef] [PubMed]
- Müller, R.H.; Maaβen, S.; Weyhers, H.; Specht, F.; Lucks, J.S. Cytotoxicity of magnetite-loaded polylactide, polylactide/glycolide particles and solid lipid nanoparticles. Int. J. Pharm. 1996, 138, 85–94. [Google Scholar] [CrossRef]
- Eldem, T.; Speiser, P.; Hincal, A. Optimization of spray-dried and -congealed lipid micropellets and characterization of their surface morphology by scanning electron microscopy. Pharm. Res. 1991, 8, 47–54. [Google Scholar] [CrossRef] [PubMed]
- Domb, A.J. Long acting injectable oxytetracycline-liposphere formulations. Int. J. Pharm. 1995, 124, 271–278. [Google Scholar] [CrossRef]
- Patel, J. Liposomal doxorubicin: Doxil®. J. Oncol. Pharm. Pract. 1996, 2, 201–210. [Google Scholar] [CrossRef]
- Barenholz, Y. (Chezy) Doxil®—The first FDA-approved nano-drug: Lessons learned. J. Control Release 2012, 160, 117–134. [Google Scholar] [CrossRef]
- Haider, M.; Abdin, S.M.; Kamal, L.; Orive, G. Nanostructured lipid carriers for delivery of chemotherapeutics: A review. Pharmaceutics 2020, 12, 288. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jahangirian, H.; Lemraski, E.G.; Webster, T.J.; Rafiee-Moghaddam, R.; Abdollahi, Y. A review of drug delivery systems based on nanotechnology and green chemistry: Green nanomedicine. Int. J. Nanomed. 2017, 12, 2957–2978. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Barroso, L.; Viegas, C.; Vieira, J.; Ferreira-Pêgo, C.; Costa, J.; Fonte, P. Lipid-based carriers for food ingredients delivery. J. Food Eng. 2021, 295, 110451. [Google Scholar] [CrossRef]
- Mueller Rainer Prof, D.R.; Lucks, J.-S. Arzneistofftraeger Aus Festen Lipidteilchen-Feste Lipidnanosphaeren (SLN). DE4131562A1, 18 September 1991. [Google Scholar]
- Parhi, R.; Suresh, P. Preparation and characterization of solid lipid nanoparticles-a review. Curr. Drug Discov. Technol. 2012, 9, 2–16. [Google Scholar] [CrossRef]
- Morales, J.O.; Valdés, K.; Morales, J.; Oyarzun-Ampuero, F. Lipid nanoparticles for the topical delivery of retinoids and derivatives. Nanomedicine 2015, 10, 253–269. [Google Scholar] [CrossRef] [Green Version]
- Naseri, N.; Valizadeh, H.; Zakeri-Milani, P. Solid Lipid Nanoparticles and Nanostructured Lipid Carriers: Structure, Preparation and Application. Adv. Pharm. Bull. 2015, 5, 305–313. [Google Scholar] [CrossRef] [Green Version]
- Kumar, S.; Randhawa, J.K. High melting lipid based approach for drug delivery: Solid lipid nanoparticles. Mater. Sci. Eng. C Mater. Biol. Appl. 2013, 33, 1842–1852. [Google Scholar] [CrossRef]
- Souto, E.B.; Almeida, A.J.; Müller, R.H. Lipid Nanoparticles (SLN®, NLC®) for Cutaneous Drug Delivery:Structure, Protection and Skin Effects. J. Biomed. Nanotechnol. 2007, 3, 317–331. [Google Scholar] [CrossRef]
- Viegas, C.; Seck, F.; Fonte, P. An insight on lipid nanoparticles for therapeutic proteins delivery. J. Drug Deliv. Sci. Technol. 2022, 77, 103839. [Google Scholar] [CrossRef]
- zur Mühlen, A.; Schwarz, C.; Mehnert, W. Solid lipid nanoparticles (SLN) for controlled drug delivery—Drug release and release mechanism. Eur. J. Pharm. Biopharm. 1998, 45, 149–155. [Google Scholar] [CrossRef] [PubMed]
- Üner, M.; Yener, G. Importance of solid lipid nanoparticles (SLN) in various administration routes and future perspectives. Int. J. Nanomed. 2007, 2, 289–300. [Google Scholar]
- Borges, A.; De Freitas, V.; Mateus, N.; Fernandes, I.; Oliveira, J. Solid lipid nanoparticles as carriers of natural phenolic compounds. Antioxidants 2020, 9, 998. [Google Scholar] [CrossRef]
- Castellani, S.; Trapani, A.; Spagnoletta, A.; Di Toma, L.; Magrone, T.; Di Gioia, S.; Mandracchia, D.; Trapani, G.; Jirillo, E.; Conese, M. Nanoparticle delivery of grape seed-derived proanthocyanidins to airway epithelial cells dampens oxidative stress and inflammation. J. Transl. Med. 2018, 16, 140. [Google Scholar] [CrossRef] [PubMed]
- Maretti, E.; Costantino, L.; Buttini, F.; Rustichelli, C.; Leo, E.; Truzzi, E.; Iannuccelli, V. Newly synthesized surfactants for surface mannosylation of respirable SLN assemblies to target macrophages in tuberculosis therapy. Drug Deliv. Transl. Res. 2019, 9, 298–310. [Google Scholar] [CrossRef]
- Dai, Y.; Huang, J.; Xiang, B.; Zhu, H.; He, C. Antiproliferative and apoptosis triggering potential of paclitaxel-based targeted-lipid nanoparticles with enhanced cellular internalization by transferrin receptors—A study in leukemia cells. Nanoscale Res. Lett. 2018, 13, 271. [Google Scholar] [CrossRef]
- Tsai, M.-J.; Huang, Y.-B.; Wu, P.-C.; Fu, Y.-S.; Kao, Y.-R.; Fang, J.-Y.; Tsai, Y.-H. Oral Apomorphine Delivery from Solid Lipid Nanoparticles with Different Monostearate Emulsifiers: Pharmacokinetic and Behavioral Evaluations. J. Pharm. Sci. 2011, 100, 547–557. [Google Scholar] [CrossRef]
- Dhawan, S.; Kapil, R.; Singh, B. Formulation development and systematic optimization of solid lipid nanoparticles of quercetin for improved brain delivery. J. Pharm. Pharmacol. 2011, 63, 342–351. [Google Scholar] [CrossRef]
- Souza, C.; de Freitas, L.A.P.; Maia Campos, P.M.B.G. Topical formulation containing beeswax-based nanoparticles improved In Vivo skin barrier function. AAPS PharmSciTech 2017, 18, 2505–2516. [Google Scholar] [CrossRef]
- Rigon, R.; Fachinetti, N.; Severino, P.; Santana, M.; Chorilli, M. Skin Delivery and In Vitro biological evaluation of trans-resveratrol-loaded solid lipid nanoparticles for skin disorder therapies. Molecules 2016, 21, 116. [Google Scholar] [CrossRef] [Green Version]
- Das, S.; Das, J.; Samadder, A.; Paul, A.; Khuda-Bukhsh, A.R. Efficacy of PLGA-loaded apigenin nanoparticles in Benzo[a]pyrene and ultraviolet-B induced skin cancer of mice: Mitochondria mediated apoptotic signalling cascades. Food Chem. Toxicol. 2013, 62, 670–680. [Google Scholar] [CrossRef]
- Carlotti, M.E.; Sapino, S.; Ugazio, E.; Gallarate, M.; Morel, S. Resveratrol in solid lipid nanoparticles. J. Dispers. Sci. Technol. 2012, 33, 465–471. [Google Scholar] [CrossRef]
- Beloqui, A.; Solinís, M.Á.; Rodríguez-Gascón, A.; Almeida, A.J.; Préat, V. Nanostructured lipid carriers: Promising drug delivery systems for future clinics. Nanomed. Nanotechnol. Biol. Med. 2016, 12, 143–161. [Google Scholar] [CrossRef]
- Doktorovova, S.; Souto, E.B. Nanostructured lipid carrier-based hydrogel formulations for drug delivery: A comprehensive review. Expert Opin. Drug Deliv. 2009, 6, 165–176. [Google Scholar] [CrossRef]
- Gaba, B.; Fazil, M.; Ali, A.; Baboota, S.; Sahni, J.K.; Ali, J. Nanostructured lipid (NLCs) carriers as a bioavailability enhancement tool for oral administration. Drug Deliv. 2015, 22, 691–700. [Google Scholar] [CrossRef] [PubMed]
- Pathak, K.; Keshri, L.; Shah, M. Lipid nanocarriers: Influence of lipids on product development and pharmacokinetics. Crit. Rev. Ther. Drug Carr. Syst. 2011, 28, 357–393. [Google Scholar] [CrossRef] [PubMed]
- Liu, D.; Liu, Z.; Wang, L.; Zhang, C.; Zhang, N. Nanostructured lipid carriers as novel carrier for parenteral delivery of docetaxel. Colloids Surf. B 2011, 85, 262–269. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.-C.; Tsai, T.-H.; Huang, Z.-R.; Fang, J.-Y. Effects of lipophilic emulsifiers on the oral administration of lovastatin from nanostructured lipid carriers: Physicochemical characterization and pharmacokinetics. Eur. J. Pharm. Biopharm. 2010, 74, 474–482. [Google Scholar] [CrossRef]
- Zhuang, C.-Y.; Li, N.; Wang, M.; Zhang, X.-N.; Pan, W.-S.; Peng, J.-J.; Pan, Y.-S.; Tang, X. Preparation and characterization of vinpocetine loaded nanostructured lipid carriers (NLC) for improved oral bioavailability. Int. J. Pharm. 2010, 394, 179–185. [Google Scholar] [CrossRef]
- Malta, R.; Loureiro, J.B.; Costa, P.; Sousa, E.; Pinto, M.; Saraiva, L.; Amaral, M.H. Development of lipid nanoparticles containing the xanthone LEM2 for topical treatment of melanoma. J. Drug Deliv. Sci. Technol. 2021, 61, 102226. [Google Scholar] [CrossRef]
- Neves, A.R.; van der Putten, L.; Queiroz, J.F.; Pinheiro, M.; Reis, S. Transferrin-functionalized lipid nanoparticles for curcumin brain delivery. J. Biotechnol. 2021, 331, 108–117. [Google Scholar] [CrossRef] [PubMed]
- Murthy, A.; Rao Ravi, P.; Kathuria, H.; Malekar, S. Oral Bioavailability Enhancement of Raloxifene with Nanostructured Lipid Carriers. Nanomaterials 2020, 10, 1085. [Google Scholar] [CrossRef]
- Magalhães, J.; Chaves, L.L.; Vieira, A.C.; Santos, S.G.; Pinheiro, M.; Reis, S. Optimization of rifapentine-loaded lipid nanoparticles using a quality-by-design strategy. Pharmaceutics 2020, 12, 75. [Google Scholar] [CrossRef] [Green Version]
- Luan, J.; Zhang, D.; Hao, L.; Li, C.; Qi, L.; Guo, H.; Liu, X.; Zhang, Q. Design and characterization of Amoitone B-loaded nanostructured lipid carriers for controlled drug release. Drug Deliv. 2013, 20, 324–330. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cacicedo, M.L.; Ruiz, M.C.; Scioli-Montoto, S.; Ruiz, M.E.; Fernández, M.A.; Torres-Sanchez, R.M.; Baran, E.J.; Castro, G.R.; León, I.E. Lipid nanoparticles—Metvan: Revealing a novel way to deliver a vanadium compound to bone cancer cells. New J. Chem. 2019, 43, 17726–17734. [Google Scholar] [CrossRef]
- Galvão, J.G.; Santos, R.L.; Silva, A.R.S.T.; Santos, J.S.; Costa, A.M.B.; Chandasana, H.; Andrade-Neto, V.V.; Torres-Santos, E.C.; Lira, A.A.M.; Dolabella, S.; et al. Carvacrol loaded nanostructured lipid carriers as a promising parenteral formulation for leishmaniasis treatment. Eur. J. Pharm. Sci. 2020, 150, 105335. [Google Scholar] [CrossRef] [PubMed]
- Shidhaye, S.S.; Vaidya, R.; Sutar, S.; Patwardhan, A.; Kadam, V.J. Solid lipid nanoparticles and nanostructured lipid carriers—Innovative generations of solid lipid carriers. Curr. Drug Deliv. 2008, 5, 324–331. [Google Scholar] [CrossRef]
- Domingo, C.; Saurina, J. An overview of the analytical characterization of nanostructured drug delivery systems: Towards green and sustainable pharmaceuticals: A review. Anal. Chim. Acta 2012, 744, 8–22. [Google Scholar] [CrossRef]
- Pardeike, J.; Hommoss, A.; Müller, R.H. Lipid nanoparticles (SLN, NLC) in cosmetic and pharmaceutical dermal products. Int. J. Pharm. 2009, 366, 170–184. [Google Scholar] [CrossRef]
- Mehnert, W.; Mäder, K. Solid lipid nanoparticles Production, characterization and applications. Adv. Drug Deliv. Rev. 2001, 47, 165–196. [Google Scholar] [CrossRef]
- Li, Q.; Cai, T.; Huang, Y.; Xia, X.; Cole, S.P.C.; Cai, Y. A review of the structure, preparation, and application of NLCs, PNPs, and PLNs. Nanomaterials 2017, 7, 122. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Das, S.; Ng, W.K.; Tan, R.B.H. Are nanostructured lipid carriers (NLCs) better than solid lipid nanoparticles (SLNs): Development, characterizations and comparative evaluations of clotrimazole-loaded SLNs and NLCs? Eur. J. Pharm. Sci. 2012, 47, 139–151. [Google Scholar] [CrossRef]
- Abdelbary, G.; Haider, M. In vitro characterization and growth inhibition effect of nanostructured lipid carriers for controlled delivery of methotrexate. Pharm. Dev. Technol. 2013, 18, 1159–1168. [Google Scholar] [CrossRef]
- Esposito, E.; Drechsler, M.; Cortesi, R.; Nastruzzi, C. Encapsulation of cannabinoid drugs in nanostructured lipid carriers. Eur. J. Pharm. Biopharm. 2016, 102, 87–91. [Google Scholar] [CrossRef]
- Fang, C.L.; Al-Suwayeh, S.A.; Fang, J.Y. Nanostructured lipid carriers (NLCs) for drug delivery and targeting. Recent Pat. Nanotechnol. 2013, 7, 41–55. [Google Scholar] [CrossRef] [PubMed]
- Jaganathan, K.S.; Rao, Y.U.; Singh, P.; Prabakaran, D.; Gupta, S.; Jain, A.; Vyas, S.P. Development of a single dose tetanus toxoid formulation based on polymeric microspheres: A comparative study of poly(D,L-lactic-co-glycolic acid) versus chitosan microspheres. Int. J. Pharm. 2005, 294, 23–32. [Google Scholar] [CrossRef] [PubMed]
- Utada, A.S.; Lorenceau, E.; Link, D.R.; Kaplan, P.D.; Stone, H.A.; Weitz, D.A. Monodisperse double emulsions generated from a microcapillary device. Science 2005, 308, 537–541. [Google Scholar] [CrossRef] [Green Version]
- Gasco Maria, R. Method for Producing Solid Lipid Microspheres Having a Narrow Size Distribution. U.S. Patent 5,250,236A, 2 August 1991. [Google Scholar]
- Igartua, M.; Saulnier, P.; Heurtault, B.; Pech, B.; Proust, J.E.; Pedraz, J.L.; Benoit, J.P. Development and characterization of solid lipid nanoparticles loaded with magnetite. Int. J. Pharm. 2002, 233, 149–157. [Google Scholar] [CrossRef]
- Schubert, M.A.; Muller-Goymann, C.C. Solvent injection as a new approach for manufacturing lipid nanoparticles-evaluation of the method and process parameters. Eur. J. Pharm. Biopharm. 2003, 55, 125–131. [Google Scholar] [CrossRef]
- Shahgaldian, P.; Da Silva, E.; Coleman, A.W.; Rather, B.; Zaworotko, M.J. Para-acyl-calix-arene based solid lipid nanoparticles (SLNs): A detailed study of preparation and stability parameters. Int. J. Pharm. 2003, 253, 23–38. [Google Scholar] [CrossRef]
- Hu, F.Q.; Hong, Y.; Yuan, H. Preparation and characterization of solid lipid nanoparticles containing peptide. Int. J. Pharm. 2004, 273, 29–35. [Google Scholar] [CrossRef] [PubMed]
- Trotta, M.; Chirio, D.; Cavalli, R.; Peira, E. Hydrophilic microspheres from water-in-oil emulsions by the water diffusion technique. Pharm. Res. 2004, 21, 1445–1449. [Google Scholar] [CrossRef] [PubMed]
- Kaur, P.; Mishra, V.; Shunmugaperumal, T.; Goyal, A.K.; Ghosh, G.; Rath, G. Inhalable spray dried lipidnanoparticles for the co-delivery of paclitaxel and doxorubicin in lung cancer. J. Drug Deliv. Sci. Technol. 2020, 56, 101502. [Google Scholar] [CrossRef]
- Freitas, C.; Müller, R.H. Spray-drying of solid lipid nanoparticles (SLNTM). Eur. J. Pharm. Biopharm. 1998, 46, 145–151. [Google Scholar] [CrossRef]
- Zhang, X.; Pan, W.; Gan, L.; Zhu, C.; Gan, Y.; Nie, S. Preparation of a Dispersible PEGylate Nanostructured Lipid Carriers (NLC) Loaded with 10-Hydroxycamptothecin by Spray-Drying. Chem. Pharm. Bull. 2008, 56, 1645–1650. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xia, D.; Shrestha, N.; van de Streek, J.; Mu, H.; Yang, M. Spray drying of fenofibrate loaded nanostructured lipid carriers. Asian J. Pharm. Sci. 2016, 11, 507–515. [Google Scholar] [CrossRef] [Green Version]
- Zhong, Q.; Zhang, L. Nanoparticles fabricated from bulk solid lipids: Preparation, properties, and potential food applications. Adv. Colloid Interface Sci. 2019, 273, 102033. [Google Scholar] [CrossRef] [PubMed]
- Marante, T.; Viegas, C.; Duarte, I.; Macedo, A.S.; Fonte, P. An Overview on Spray-Drying of Protein-Loaded Polymeric Nanoparticles for Dry Powder Inhalation. Pharmaceutics 2020, 12, 1032. [Google Scholar] [CrossRef]
- Lababidi, N.; Sigal, V.; Koenneke, A.; Schwarzkopf, K.; Manz, A.; Schneider, M. Microfluidics as tool to prepare size-tunable PLGA nanoparticles with high curcumin encapsulation for efficient mucus penetration. Beilstein J. Nanotechnol. 2019, 10, 2280–2293. [Google Scholar] [CrossRef]
- Lopes, C.; Cristóvão, J.; Silvério, V.; Lino, P.R.; Fonte, P. Microfluidic production of mRNA-loaded lipid nanoparticles for vaccine applications. Expert Opin. Drug Deliv. 2022, 19, 1381–1395. [Google Scholar] [CrossRef]
- Garg, S.; Heuck, G.; Ip, S.; Ramsay, E. Microfluidics: A transformational tool for nanomedicine development and production. J. Drug Target. 2016, 24, 821–835. [Google Scholar] [CrossRef]
- Chen, S.; Liu, W.; Wan, J.; Cheng, X.; Gu, C.; Zhou, H.; Chen, S.; Zhao, X.; Tang, Y.; Yang, X. Preparation of Coenzyme Q10 nanostructured lipid carriers for epidermal targeting with high-pressure microfluidics technique. Drug Dev. Ind. Pharm. 2013, 39, 20–28. [Google Scholar] [CrossRef]
- Yuan, H.; Miao, J.; Du, Y.-Z.; You, J.; Hu, F.-Q.; Zeng, S. Cellular uptake of solid lipid nanoparticles and cytotoxicity of encapsulated paclitaxel in A549 cancer cells. Int. J. Pharm. 2008, 348, 137–145. [Google Scholar] [CrossRef]
- Uner, B.; Ozdemir, S.; Yildirim, E.; Yaba, A.; Tas, C.; Uner, M.; Ozsoy, Y. Loteprednol loaded nanoformulations for corneal delivery: Ex-vivo permeation study, ocular safety assessment and stability studies. J. Drug Deliv. Sci. Technol. 2023, 81, 104252. [Google Scholar] [CrossRef]
- Soares, S.; Fonte, P.; Costa, A.; Andrade, J.; Seabra, V.; Ferreira, D.; Reis, S.; Sarmento, B. Effect of freeze-drying, cryoprotectants and storage conditions on the stability of secondary structure of insulin-loaded solid lipid nanoparticles. Int. J. Pharm. 2013, 456, 370–381. [Google Scholar] [CrossRef]
- Liu, J.; Gong, T.; Wang, C.; Zhong, Z.; Zhang, Z. Solid lipid nanoparticles loaded with insulin by sodium cholate-phosphatidylcholine-based mixed micelles: Preparation and characterization. Int. J. Pharm. 2007, 340, 153–162. [Google Scholar] [CrossRef] [PubMed]
- Sarmento, B.; Martins, S.; Ferreira, D.; Souto, E.B. Oral insulin delivery by means of solid lipid nanoparticles. Int. J. Nanomed. 2007, 2, 743–749. [Google Scholar]
- Kumar, R.; Singh, A.; Garg, N. Acoustic cavitation assisted hot melt mixing technique for solid lipid nanoparticles formulation, characterization, and controlled delivery of poorly water soluble drugs. J. Drug Deliv. Sci. Technol. 2019, 54, 101277. [Google Scholar] [CrossRef]
- Ban, C.; Jo, M.; Park, Y.H.; Kim, J.H.; Han, J.Y.; Lee, K.W.; Kweon, D.-H.; Choi, Y.J. Enhancing the oral bioavailability of curcumin using solid lipid nanoparticles. Food Chem. 2020, 302, 125328. [Google Scholar] [CrossRef]
- Ji, H.; Tang, J.; Li, M.; Ren, J.; Zheng, N.; Wu, L. Curcumin-loaded solid lipid nanoparticles with Brij78 and TPGS improved in vivo oral bioavailability and in situ intestinal absorption of curcumin. Drug Deliv. 2016, 23, 459–470. [Google Scholar] [CrossRef] [PubMed]
- Fathi, M.; Mozafari, M.R.; Mohebbi, M. Nanoencapsulation of food ingredients using lipid based delivery systems. Trends Food Sci. Technol. 2012, 23, 13–27. [Google Scholar] [CrossRef]
- Esfanjani, A.F.; Assadpour, E.; Jafari, S.M. Improving the bioavailability of phenolic compounds by loading them within lipid-based nanocarriers. Trends Food Sci. Technol. 2018, 76, 56–66. [Google Scholar] [CrossRef]
- Aditya, N.P.; Macedo, A.S.; Doktorovova, S.; Souto, E.B.; Kim, S.; Chang, P.-S.; Ko, S. Development and evaluation of lipid nanocarriers for quercetin delivery: A comparative study of solid lipid nanoparticles (SLN), nanostructured lipid carriers (NLC), and lipid nanoemulsions (LNE). LWT 2014, 59, 115–121. [Google Scholar] [CrossRef]
- Gonçalves, R.F.; Vicente, A.A.; Pinheiro, A.C. Incorporation of curcumin-loaded lipid-based nano delivery systems into food: Release behavior in food simulants and a case study of application in a beverage. Food Chem. 2023, 405, 134740. [Google Scholar] [CrossRef]
- Babazadeh, A.; Ghanbarzadeh, B.; Hamishehkar, H. Formulation of food grade nanostructured lipid carrier (NLC) for potential applications in medicinal-functional foods. J. Drug Deliv. Sci. Technol. 2017, 39, 50–58. [Google Scholar] [CrossRef]
- Madureira, A.R.; Campos, D.A.; Fonte, P.; Nunes, S.; Reis, F.; Gomes, A.M.; Sarmento, B.; Pintado, M.M. Characterization of solid lipid nanoparticles produced with carnauba wax for rosmarinic acid oral delivery. RSC Adv. 2015, 5, 22665–22673. [Google Scholar] [CrossRef]
- Park, S.J.; Garcia, C.V.; Shin, G.H.; Kim, J.T. Development of nanostructured lipid carriers for the encapsulation and controlled release of vitamin D3. Food Chem. 2017, 225, 213–219. [Google Scholar] [CrossRef]
- He, J.; Huang, S.; Sun, X.; Han, L.; Chang, C.; Zhang, W.; Zhong, Q. Carvacrol Loaded Solid Lipid Nanoparticles of Propylene Glycol Monopalmitate and Glyceryl Monostearate: Preparation, Characterization, and Synergistic Antimicrobial Activity. Nanomaterials 2019, 9, 1162. [Google Scholar] [CrossRef] [Green Version]
- Li, Y.; Wu, Z.-F.; Kuang, W.-W.; Li, Y.-H.; Chen, B.-Y.; Yang, M. Effect of nanostructured lipid carriers (NLCs) in improving stability of essential oils and its application. China J. Chin. Mater. Med. 2020, 45, 523–530. [Google Scholar]
- Shtay, R.; Tan, C.P.; Schwarz, K. Development and characterization of solid lipid nanoparticles (SLNs) made of cocoa butter: A factorial design study. J. Food Eng. 2018, 231, 30–41. [Google Scholar] [CrossRef]
- Hashemi, F.S.; Farzadnia, F.; Aghajani, A.; Ahmadzadeh NobariAzar, F.; Pezeshki, A. Conjugated linoleic acid loaded nanostructured lipid carrier as a potential antioxidant nanocarrier for food applications. Food Sci. Nutr. 2020, 8, 4185–4195. [Google Scholar] [CrossRef]
- Gonçalves, R.F.S.; Martins, J.T.; Abrunhosa, L.; Baixinho, J.; Matias, A.A.; Vicente, A.A.; Pinheiro, A.C. Lipid-based nanostructures as a strategy to enhance curcumin bioaccessibility: Behavior under digestion and cytotoxicity assessment. Food Res. Int. 2021, 143, 110278. [Google Scholar] [CrossRef] [PubMed]
- Salvi, V.R.; Pawar, P. Nanostructured lipid carriers (NLC) system: A novel drug targeting carrier. J. Drug Deliv. Sci. Technol. 2019, 51, 255–267. [Google Scholar] [CrossRef]
- Keck, C.M.; Baisaeng, N.; Durand, P.; Prost, M.; Meinke, M.C.; Müller, R.H. Oil-enriched, ultra-small nanostructured lipid carriers (usNLC): A novel delivery system based on flip–flop structure. Int. J. Pharm. 2014, 477, 227–235. [Google Scholar] [CrossRef] [PubMed]
- Gratieri, T.; Krawczyk-Santos, A.P.; Da Rocha, P.B.R.; Cunha-Filho, M.; Gelfuso, G.M.; Marreto, R.N.; Taveira, S.F. SLN- and NLC-Encapsulating Antifungal Agents: Skin Drug Delivery and their Unexplored Potential for Treating Onychomycosis. Curr. Pharm. Des. 2017, 23, 6684–6695. [Google Scholar] [CrossRef] [PubMed]
- Agrawal, M.; Saraf, S.; Saraf, S.; Dubey, S.K.; Puri, A.; Patel, R.J.; Ajazuddin; Ravichandiran, V.; Murty, U.S.; Alexander, A. Recent strategies and advances in the fabrication of nano lipid carriers and their application towards brain targeting. J. Control Release 2020, 321, 372–415. [Google Scholar] [CrossRef]
- Hsu, S.-H.; Wen, C.-J.; Al-Suwayeh, S.A.; Chang, H.-W.; Yen, T.-C.; Fang, J.-Y. Physicochemical characterization and in vivo bioluminescence imaging of nanostructured lipid carriers for targeting the brain: Apomorphine as a model drug. Nanotechnology 2010, 21, 405101. [Google Scholar] [CrossRef]
- Yan, J.; Kang, D.D.; Dong, Y. Harnessing lipid nanoparticles for efficient CRISPR delivery. Biomater. Sci. 2021, 9, 6001–6011. [Google Scholar] [CrossRef]
- Fonte, P.; Andrade, J.C.; Seabra, V.; Sarmento, B. Chitosan-Based Nanoparticles as Delivery Systems of Therapeutic Proteins. Methods Mol. Biol. 2012, 899, 471–487. [Google Scholar] [CrossRef]
- Tan, S.L.; Billa, N. Improved Bioavailability of Poorly Soluble Drugs through Gastrointestinal Muco-Adhesion of Lipid Nanoparticles. Pharmaceutics 2021, 13, 1817. [Google Scholar] [CrossRef]
- da Silva, S.B.; Amorim, M.; Fonte, P.; Madureira, R.; Ferreira, D.; Pintado, M.; Sarmento, B. Natural extracts into chitosan nanocarriers for rosmarinic acid drug delivery. Pharm. Biol. 2015, 53, 642–652. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fonte, P.; Nogueira, T.; Gehm, C.; Ferreira, D.; Sarmento, B. Chitosan-coated solid lipid nanoparticles enhance the oral absorption of insulin. Drug Deliv. Transl. Res. 2011, 1, 299–308. [Google Scholar] [CrossRef] [PubMed]
- Sarmento, B.; Mazzaglia, D.; Bonferoni, M.C.; Neto, A.P.; do Céu Monteiro, M.; Seabra, V. Effect of chitosan coating in overcoming the phagocytosis of insulin loaded solid lipid nanoparticles by mononuclear phagocyte system. Carbohydr. Polym. 2011, 84, 919–925. [Google Scholar] [CrossRef]
- Akbari, J.; Saeedi, M.; Ahmadi, F.; Hashemi, S.M.H.; Babaei, A.; Yaddollahi, S.; Rostamkalaei, S.S.; Asare-Addo, K.; Nokhodchi, A. Solid lipid nanoparticles and nanostructured lipid carriers: A review of the methods of manufacture and routes of administration. Pharm. Dev. Technol. 2022, 27, 525–544. [Google Scholar] [CrossRef]
- Algarni, A.; Pilkington, E.H.; Suys, E.J.A.; Al-Wassiti, H.; Pouton, C.W.; Truong, N.P. In vivo delivery of plasmid DNA by lipid nanoparticles: The influence of ionizable cationic lipids on organ-selective gene expression. Biomater. Sci. 2022, 10, 2940–2952. [Google Scholar] [CrossRef] [PubMed]
- Melamed, J.R.; Yerneni, S.S.; Arral, M.L.; LoPresti, S.T.; Chaudhary, N.; Sehrawat, A.; Muramatsu, H.; Alameh, M.-G.; Pardi, N.; Weissman, D.; et al. Ionizable lipid nanoparticles deliver mRNA to pancreatic β cells via macrophage-mediated gene transfer. Sci. Adv. 2023, 9, eade1444. [Google Scholar] [CrossRef]
- Cheng, Q.; Wei, T.; Farbiak, L.; Johnson, L.T.; Dilliard, S.A.; Siegwart, D.J. Selective organ targeting (SORT) nanoparticles for tissue-specific mRNA delivery and CRISPR–Cas gene editing. Nat. Nanotechnol. 2020, 15, 313–320. [Google Scholar] [CrossRef]
- Wang, X.; Liu, S.; Sun, Y.; Yu, X.; Lee, S.M.; Cheng, Q.; Wei, T.; Gong, J.; Robinson, J.; Zhang, D.; et al. Preparation of selective organ-targeting (SORT) lipid nanoparticles (LNPs) using multiple technical methods for tissue-specific mRNA delivery. Nat. Protoc. 2023, 18, 265–291. [Google Scholar] [CrossRef]
- Liu, D.; Liu, F.; Liu, Z.; Wang, L.; Zhang, N. Tumor Specific Delivery and Therapy by Double-Targeted Nanostructured Lipid Carriers with Anti-VEGFR-2 Antibody. Mol. Pharm. 2011, 8, 2291–2301. [Google Scholar] [CrossRef]
- Nawaz, M.; Heydarkhan-Hagvall, S.; Tangruksa, B.; González-King Garibotti, H.; Jing, Y.; Maugeri, M.; Kohl, F.; Hultin, L.; Reyahi, A.; Camponeschi, A.; et al. Lipid Nanoparticles Deliver the Therapeutic VEGFA mRNA In Vitro and In Vivo and Transform Extracellular Vesicles for Their Functional Extensions. Adv. Sci. 2023, 10, 2206187. [Google Scholar] [CrossRef] [PubMed]
- Zha, W.; Wang, J.; Guo, Z.; Zhang, Y.; Wang, Y.; Dong, S.; Liu, C.; Xing, H.; Li, X. Efficient delivery of VEGF-A mRNA for promoting diabetic wound healing via ionizable lipid nanoparticles. Int. J. Pharm. 2023, 632, 122565. [Google Scholar] [CrossRef] [PubMed]
- Marques, A.C.; Costa, P.C.; Velho, S.; Amaral, M.H. Lipid Nanoparticles Functionalized with Antibodies for Anticancer Drug Therapy. Pharmaceutics 2023, 15, 216. [Google Scholar] [CrossRef]
- Di Filippo, L.D.; Lobato Duarte, J.; Hofstätter Azambuja, J.; Isler Mancuso, R.; Tavares Luiz, M.; Hugo Sousa Araújo, V.; Delbone Figueiredo, I.; Barretto-De-Souza, L.; Miguel Sábio, R.; Sasso-Cerri, E.; et al. Glioblastoma multiforme targeted delivery of docetaxel using bevacizumab-modified nanostructured lipid carriers impair in vitro cell growth and in vivo tumor progression. Int. J. Pharm. 2022, 618, 121682. [Google Scholar] [CrossRef]
- Zwicke, G.L.; Mansoori, G.A.; Jeffery, C.J. Utilizing the folate receptor for active targeting of cancer nanotherapeutics. Nano Rev. 2012, 3, 18496. [Google Scholar] [CrossRef] [PubMed]
- Narmani, A.; Rezvani, M.; Farhood, B.; Darkhor, P.; Mohammadnejad, J.; Amini, B.; Refahi, S.; Abdi Goushbolagh, N. Folic acid functionalized nanoparticles as pharmaceutical carriers in drug delivery systems. Drug Dev. Res. 2019, 80, 404–424. [Google Scholar] [CrossRef]
- Li, M.; Pei, J.; Ma, Z.; Fu, J.; Chen, F.; Du, S. Docetaxel-loaded ultrasmall nanostructured lipid carriers for cancer therapy: In vitro and in vivo evaluation. Cancer Chemother. Pharmacol. 2020, 85, 731–739. [Google Scholar] [CrossRef]
- Han, C.; Li, Y.; Sun, M.; Liu, C.; Ma, X.; Yang, X.; Yuan, Y.; Pan, W. Small peptide-modified nanostructured lipid carriers distribution and targeting to EGFR-overexpressing tumor in vivo. Artif. Cells Nanomed. Biotechnol. 2014, 42, 161–166. [Google Scholar] [CrossRef]
- Huang, R.; Li, J.; Kebebe, D.; Wu, Y.; Zhang, B.; Liu, Z. Cell penetrating peptides functionalized gambogic acid-nanostructured lipid carrier for cancer treatment. Drug Deliv. 2018, 25, 757–765. [Google Scholar] [CrossRef]
- Kebebe, D.; Wu, Y.; Zhang, B.; Yang, J.; Liu, Y.; Li, X.; Ma, Z.; Lu, P.; Liu, Z.; Li, J. Dimeric c(RGD) peptide conjugated nanostructured lipid carriers for efficient delivery of Gambogic acid to breast cancer. Int. J. Nanomed. 2019, 14, 6179–6195. [Google Scholar] [CrossRef] [Green Version]
- Olerile, L.D.; Liu, Y.; Zhang, B.; Wang, T.; Mu, S.; Zhang, J.; Selotlegeng, L.; Zhang, N. Near-infrared mediated quantum dots and paclitaxel co-loaded nanostructured lipid carriers for cancer theragnostic. Colloids Surf. B 2017, 150, 121–130. [Google Scholar] [CrossRef] [PubMed]
- Garbuzenko, O.B.; Kuzmov, A.; Taratula, O.; Pine, S.R.; Minko, T. Strategy to enhance lung cancer treatment by five essential elements: Inhalation delivery, nanotechnology, tumor-receptor targeting, chemo- and gene therapy. Theranostics 2019, 9, 8362–8376. [Google Scholar] [CrossRef] [PubMed]
- Desmet, E.; Van Gele, M.; Lambert, J. Topically applied lipid- and surfactant-based nanoparticles in the treatment of skin disorders. Expert Opin. Drug Deliv. 2017, 14, 109–122. [Google Scholar] [CrossRef] [PubMed]
- Garcês, A.; Amaral, M.H.; Sousa Lobo, J.; Silva, A.C. Formulations based on solid lipid nanoparticles (SLN) and nanostructured lipid carriers (NLC) for cutaneous use: A review. Eur. J. Pharm. Sci. 2018, 112, 159–167. [Google Scholar] [CrossRef]
- Sütő, B.; Berkó, S.; Kozma, G.; Kukovecz, Á.; Budai-Szűcs, M.; Erős, G.; Kemény, L.; Sztojkov-Ivanov, A.; Gáspár, R.; Csányi, E. Development of ibuprofen-loaded nanostructured lipid carrier-based gels: Characterization and investigation of in vitro and in vivo penetration through the skin. Int. J. Nanomed. 2016, 11, 1201–1212. [Google Scholar] [CrossRef] [Green Version]
- Puglia, C.; Sarpietro, M.G.; Bonina, F.; Castelli, F.; Zammataro, M.; Chiechio, S. Development, Characterization, and In Vitro and In Vivo Evaluation of Benzocaine- and Lidocaine-Loaded Nanostructrured Lipid Carriers. J. Pharm. Sci. 2011, 100, 1892–1899. [Google Scholar] [CrossRef]
- Zhao, X.; Sun, Y.; Li, Z. Topical anesthesia therapy using lidocaine-loaded nanostructured lipid carriers: Tocopheryl polyethylene glycol 1000 succinate-modified transdermal delivery system. Drug Des. Dev. Ther. 2018, 12, 4231–4240. [Google Scholar] [CrossRef] [Green Version]
- Viegas, J.S.R.; Praça, F.G.; Caron, A.L.; Suzuki, I.; Silvestrini, A.V.P.; Medina, W.S.G.; Del Ciampo, J.O.; Kravicz, M.; Bentley, M.V.L.B. Nanostructured lipid carrier co-delivering tacrolimus and TNF-α siRNA as an innovate approach to psoriasis. Drug Deliv. Transl. Res. 2020, 10, 646–660. [Google Scholar] [CrossRef]
- Esposito, E.; Sguizzato, M.; Drechsler, M.; Mariani, P.; Carducci, F.; Nastruzzi, C.; Cortesi, R. Progesterone lipid nanoparticles: Scaling up and in vivo human study. Eur. J. Pharm. Biopharm. 2017, 119, 437–446. [Google Scholar] [CrossRef]
- Muller, R.H.; Shegokar, R.; M Keck, C. 20 years of lipid nanoparticles (SLN & NLC): Present state of development & industrial applications. Curr. Drug Discov. Technol. 2011, 8, 207–227. [Google Scholar]
- Tichota, D.M.; Silva, A.C.; Lobo, J.M.S.; Amara, M.H. Design, characterization, and clinical evaluation of argan oil nanostructured lipid carriers to improve skin hydration. Int. J. Nanomed. 2014, 9, 3855–3864. [Google Scholar]
- Štecová, J.; Mehnert, W.; Blaschke, T.; Kleuser, B.; Sivaramakrishnan, R.; Zouboulis, C.C.; Seltmann, H.; Korting, H.C.; Kramer, K.D.; Schäfer-Korting, M. Cyproterone Acetate Loading to Lipid Nanoparticles for Topical Acne Treatment: Particle Characterisation and Skin Uptake. Pharm. Res. 2007, 24, 991–1000. [Google Scholar] [CrossRef]
- Hassett, K.J.; Benenato, K.E.; Jacquinet, E.; Lee, A.; Woods, A.; Yuzhakov, O.; Himansu, S.; Deterling, J.; Geilich, B.M.; Ketova, T.; et al. Optimization of Lipid Nanoparticles for Intramuscular Administration of mRNA Vaccines. Mol. Ther. Nucleic Acids 2019, 15, 1–11. [Google Scholar] [CrossRef] [Green Version]
- Dhiman, N.; Awasthi, R.; Sharma, B.; Kharkwal, H.; Kulkarni, G.T. Lipid Nanoparticles as Carriers for Bioactive Delivery. Front. Chem. 2021, 9, 580118. [Google Scholar] [CrossRef]
- Doktorovova, S.; Kovacevic, A.B.; Garcia, M.L.; Souto, E.B. Preclinical safety of solid lipid nanoparticles and nanostructured lipid carriers: Current evidence from in vitro and in vivo evaluation. Eur. J. Pharm. Biopharm. 2016, 108, 235–252. [Google Scholar] [CrossRef] [PubMed]
- Doktorovova, S.; Souto, E.B.; Silva, A.M. Nanotoxicology applied to solid lipid nanoparticles and nanostructured lipid carriers—A systematic review of in vitro data. Eur. J. Pharm. Biopharm. 2014, 87, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Sacchetti, F.; Marraccini, C.; D’arca, D.; Pelà, M.; Pinetti, D.; Maretti, E.; Hanuskova, M.; Iannuccelli, V.; Costi, M.P.; Leo, E. Enhanced anti-hyperproliferative activity of human thymidylate synthase inhibitor peptide by solid lipid nanoparticle delivery. Colloids Surf. B 2015, 136, 346–354. [Google Scholar] [CrossRef]
- Maretti, E.; Rossi, T.; Bondi, M.; Croce, M.A.; Hanuskova, M.; Leo, E.; Sacchetti, F.; Iannuccelli, V. Inhaled Solid Lipid Microparticles to target alveolar macrophages for tuberculosis. Int. J. Pharm. 2014, 462, 74–82. [Google Scholar] [CrossRef]
- Almeida, H.; Lobão, P.; Frigerio, C.; Fonseca, J.; Silva, R.; Sousa Lobo, J.M.; Amaral, M.H. Preparation, characterization and biocompatibility studies of thermoresponsive eyedrops based on the combination of nanostructured lipid carriers (NLC) and the polymer Pluronic F-127 for controlled delivery of ibuprofen. Pharm. Dev. Technol. 2017, 22, 336–349. [Google Scholar] [CrossRef]
- Schöler, N.; Hahn, H.; Müller, R.H.; Liesenfeld, O. Effect of lipid matrix and size of solid lipid nanoparticles (SLN) on the viability and cytokine production of macrophages. Int. J. Pharm. 2002, 231, 167–176. [Google Scholar] [CrossRef]
- Miglietta, A.; Cavalli, R.; Bocca, C.; Gabriel, L.; Rosa Gasco, M. Cellular uptake and cytotoxicity of solid lipid nanospheres (SLN) incorporating doxorubicin or paclitaxel. Int. J. Pharm. 2000, 210, 61–67. [Google Scholar] [CrossRef]
- Souto, E.B.; Baldim, I.; Oliveira, W.P.; Rao, R.; Yadav, N.; Gama, F.M.; Mahant, S. SLN and NLC for topical, dermal, and transdermal drug delivery. Expert Opin. Drug Deliv. 2020, 17, 357–377. [Google Scholar] [CrossRef] [PubMed]
- Patidar, A.; Thakur, D.S.; Kumar, P.; Verma, J. A review on novel lipid based nanocarriers. Int. J. Pharm. Pharm. Sci. 2010, 2, 30–35. [Google Scholar]
- Tabatt, K.; Sameti, M.; Olbrich, C.; Müller, R.H.; Lehr, C.-M. Effect of cationic lipid and matrix lipid composition on solid lipid nanoparticle-mediated gene transfer. Eur. J. Pharm. Biopharm. 2004, 57, 155–162. [Google Scholar] [CrossRef] [PubMed]
- Hwang, T.-L.; Aljuffali, I.A.; Lin, C.-F.; Chang, C.-C.; Fang, J.-Y. Cationic additives in nanosystems activate cytotoxicity and inflammatory response of human neutrophils: Lipid nanoparticles versus polymeric nanoparticles. Int. J. Nanomed. 2015, 10, 371–385. [Google Scholar] [CrossRef] [Green Version]
- Hwang, T.-L.; Aljuffali, I.A.; Hung, C.-F.; Chen, C.-H.; Fang, J.-Y. The impact of cationic solid lipid nanoparticles on human neutrophil activation and formation of neutrophil extracellular traps (NETs). Biol. Chem. Interactions 2015, 235, 106–114. [Google Scholar] [CrossRef] [PubMed]
- Müller, R.H.; Rühl, D.; Runge, S.; Schulze-Forster, K.; Mehnert, W. Cytotoxicity of Solid Lipid Nanoparticles as a Function of the Lipid Matrix and the Surfactant. Pharm. Res. 1997, 14, 458–462. [Google Scholar] [CrossRef]
- Han, F.; Li, S.; Yin, R.; Liu, H.; Xu, L. Effect of surfactants on the formation and characterization of a new type of colloidal drug delivery system: Nanostructured lipid carriers. Colloids Surf. A Physicochem. Eng. Asp. 2008, 315, 210–216. [Google Scholar] [CrossRef]
- Schwarz, J.C.; Weixelbaum, A.; Pagitsch, E.; Löw, M.; Resch, G.P.; Valenta, C. Nanocarriers for dermal drug delivery: Influence of preparation method, carrier type and rheological properties. Int. J. Pharm. 2012, 437, 83–88. [Google Scholar] [CrossRef]
- Jores, K.; Mehnert, W.; Drechsler, M.; Bunjes, H.; Johann, C.; Mäder, K. Investigations on the structure of solid lipid nanoparticles (SLN) and oil-loaded solid lipid nanoparticles by photon correlation spectroscopy, field-flow fractionation and transmission electron microscopy. J. Control Release 2004, 95, 217–227. [Google Scholar] [CrossRef]
- Brugè, F.; Damiani, E.; Puglia, C.; Offerta, A.; Armeni, T.; Littarru, G.P.; Tiano, L. Nanostructured lipid carriers loaded with CoQ10: Effect on human dermal fibroblasts under normal and UVA-mediated oxidative conditions. Int. J. Pharm. 2013, 455, 348–356. [Google Scholar] [CrossRef] [PubMed]
- Brugè, F.; Damiani, E.; Marcheggiani, F.; Offerta, A.; Puglia, C.; Tiano, L. A comparative study on the possible cytotoxic effects of different nanostructured lipid carrier (NLC) compositions in human dermal fibroblasts. Int. J. Pharm. 2015, 495, 879–885. [Google Scholar] [CrossRef] [PubMed]
- Ezzati Nazhad Dolatabadi, J.; Hamishehkar, H.; Eskandani, M.; Valizadeh, H. Formulation, characterization and cytotoxicity studies of alendronate sodium-loaded solid lipid nanoparticles. Colloids Surf. B 2014, 117, 21–28. [Google Scholar] [CrossRef] [PubMed]
- Bhushan, S.; Kakkar, V.; Pal, H.C.; Mondhe, D.; Kaur, I.P. The augmented anticancer potential of AP9-cd loaded solid lipid nanoparticles in human leukemia Molt-4 cells and experimental tumor. Biol. Chem. Interact. 2016, 244, 84–93. [Google Scholar] [CrossRef] [PubMed]








| Compound | Classification | Source | Function |
|---|---|---|---|
| 1-Tetradecanol (myristyl alcohol) | Straight chain saturated fatty alcohol | Myristica fragrans | Solid lipid |
| Beeswax | Wax ester | Honey bees (Apis mellifera) | Solid lipid |
| Caprylic/capric triglyceride | Triglyceride | Coconut oil | Liquid lipid |
| Castor oil | Fatty acid composed | Castor beans | Liquid lipid |
| Cetyl palmitate | Wax ester | Stony corals, Psidium guajava | Solid lipid |
| Cholesteryl myristate | Cholesterol ester | Trachyrhamphus serratus | Solid lipid |
| Cholesterol | Modified steroid | Animal, vegetable fat | Solid lipid |
| Compritol® 888 ATO | Mixture of mono-, di- and triglycerides of behenic acid (C22) | - | Surfactant |
| 1,2-dioleoyl-3-dimethylammonium propane (DODAP) | Ionizable cationic lipid | - | Solid lipid |
| Dipalmitoylphosphatidylcholine (DPPC) | Phospholipid | Pulmonary surfactant | Solid lipid |
| 1,2-distearoyl-sn-glycero-3-phosphoethanolamine (DSPE) | Amine phospholipid | Escherichia coli | Solid lipid |
| Gelucire® 50/13 | Mixture of fatty acids (C16 and C18), esters of glycerol, PEG esters and free PEG | - | Surfactant |
| Glyceryl monostearate | Glycerol ester of a saturated fatty acid | Aristolochia cucurbitifolia, Lobelia longisepala | Surfactant |
| Labrafac™ CC | Mixture of medium chain triglycerides, mainly from caprylic (C8) and capric (C10) acids | - | Liquid lipid |
| Lecithin | Mixture of phospholipids in oil | Soybean, egg | Surfactant |
| Miglyol® 812 N | Glycerol triester of caprylic and capric acid (triglyceride esters) | Coconut, palm kernel oil | Liquid lipid |
| Myristylmyristate | Tetradecanoate ester | Coconut, palm kernel oil | Solid lipid |
| Oleic acid | Middle chain triglyceride | Olive oil | Liquid lipid |
| Palmitic acid | Saturated fatty acid | Palm oil | Solid lipid |
| Phosphatidylcholine | Phospholipid | Soybeans, eggs | Solid lipid |
| Poloxamer 407/Pluronic® F-127 | Triblock copolymer | - | Surfactant |
| Precirol® ATO-5 | Mixtures of diesters of glycerin and stearic acid | - | Solid lipid |
| Polyvinylalcohol (PVA) | Synthetic polymer of vinyl alcohol | - | Surfactant |
| Sodium lauryl sulfate (SLS) | Ethoxylated lauryl alcohol | Coconut, palm kernel oil | Surfactant |
| Squalene | Triterpenoid | Olive, wheat germ, and rice bran oils | Liquid lipid |
| Steric acid | Saturated fatty acid | Animal, vegetable fat | Solid lipid |
| Tricaprin | Triglyceride | Milkfat, palm kernel oil, and coconut oil | Solid lipid |
| Tripalmitin | Triglyceride | Lysiphlebia japonica, Tagetes erecta | Solid lipid |
| Tristearin | Triglyceride | Lysiphlebia japonica, Sciadopitys verticillata | Solid lipid |
| Tween® | Mixture of sorbitol, ethylene oxide, and oleic acid | - | Surfactant |
| Solid–Lipid | Surfactant | Drug | Production Method | Therapeutic Purpose | Delivery Route | Characteristics | Ref |
|---|---|---|---|---|---|---|---|
| Gelucire® 50/13 | Tween® 85 | Grapeseed-derived proanthocyanidins | Melt Emulsification Technique | Chronic Respiratory Diseases | Spray Instillation | Size: 243 ± 24 nm PdI: 0.41 Zeta: −14.5 ± 1.0 mV EE: NA | [36] |
| Palmitic Acid/Cholesteryl Myristate (68,5/31,5%) (w/w) | Sodium Lauryl Sulfate (SLS) | Rifampicin | Melt Emulsification Technique | Tuberculosis | NA | Size: 400 ± 20 nm PdI: 0.43 ± 0.09 Zeta: −35.3 ± 0.29 mV EE: 56.48% (w/w) | [37] |
| Compritol 888 ATO, cholesterol, and Tf-PEG-OA | 1% Polyvinylalcohol (PVA) | Paclitaxel (PTX) | Solvent Evaporation Method | Leukemia | NA | Size: 176 nm PdI: NA Zeta: −22.5 ± 1.56 mV EE: 92.5 ± 1.35% | [38] |
| Tripalmitin/Hydrogenated soybean phosphatidylcholine (HSPC) (80/20%) (w/w) | Polyethylene glycol monostearate (PGM) | Apomorphine | NA | Parkinson’s Disease | Oral | Size: 63.20 ± 0.98 nm PdI: 0.31 ± 0.02 Zeta: 7.3 ± 0.25 mV EE: NA | [39] |
| Compritol® 888 ATO | Tween® 80 | Quercetin | NA | Alzheimer’s Disease | Oral | Size: 0.42 to 4.62 µm PdI: NA Zeta: −23.6 to −5.13 mV EE: 85.7% | [40] |
| Beeswax | Tween® 80 Poloxamer 407 | NA | Hot melt microemulsion | Skin Hydration | Topical | Size: 95.72 ± 9.63 nm PdI: 0.323 ± 0.03 Zeta: −9.85 ± 0.57 mV EE: NA | [41] |
| Stearic Acid | Poloxamer 407 Soybean Phosphatidylcholine | Resveratrol | Sonication | Anti-tumoral | Topical | Size:155.50 ± 0.26 nm PdI: 0.140 ± 0.02 Zeta: −2.60 ± 1.27 mV EE: NA | [42] |
| Poly Lactic-co-Glycolic Acid (PLGA) | 1% polyoxyethylenepolyoxypropylene | Apigenin | Nanoprecipitation | Cosmetic | Topical | Size: 102.19 ± 0.002 nm PdI: 0.258 Zeta: 12.1 ± 0.0 mV EE: 87.2 ± 0.005 | [43] |
| Tricaprin | Cetyl Palmitate, Tween® 60 Tego Care 450 Amphisol K, 1-Tetradecanol | Resveratrol | Hot melt homogenization | Cosmetic | Topical | Size: 102.190 ± 0.002 nm PdI: 0.258 Zeta: 12.1 ± 0.0 mV EE: 52.45% | [44] |
| Solid Lipid | Liquid Lipid | Surfactant | Drug | Production Method | Therapeutic Purpose | Delivery Route | Characteristics | Ref |
|---|---|---|---|---|---|---|---|---|
| Stearic acid | Oleic acid | Soya Lecithin Glyceryl Monostearate | Docetaxel (DTX) | Modified film ultrasonication–dispersion method | Murine Malignant Melanoma | Parenteral | Size: 203.67 ± 4.15 nm PdI: NA Zeta: −31.17 ± 2.20 mV EE: 89.39 ± 0.99% | [49] |
| Precirol® ATO-5 | Squalene | Myverol | Lovastatin | Hot melt homogenization | Cholesterol | Oral | Size: 278.8 ± 0.6 nm PdI: ≤0.25 Zeta: −32.4 ± 0.4 mV EE: 83.8 ± 2.5 | [50] |
| Comprito® 888 ATO | Miglyol 812N | Lecithin | Vinpocetin (VIN) | High-pressure homogenization | Brain Disorders | Oral | Size: 177 ± 5.4 nm PdI: NA Zeta: −24.7 ± 1.4 mV EE: 95.3 ± 1.4 | [51] |
| Precirol® ATO-5 | Oleic Acid | Tween® 80 | 1-carbaldehyde-3,4-dimethoxyxanthone (LEM2) | Ultrasonication | Melanoma | Topical | Size: 219.67 ± 5.26 nm PdI: ≤0.3 Zeta: −24.88 ± 1.78 mV EE: 72% | [52] |
| Cetyl Palmitate | Miglyol 812N | Tween® 60 | Curcumin | Modified hot homogenization | Brain Disorders | Oral/Intravenous | Size: 183 ± 12 nm PdI: 0.13 ± 0.01 Zeta: −21 ± 2 mV EE: 82 ± 15% | [53] |
| Glyceryl Tribehenate | Oleic acid | P 407 | Raloxifene hydrochloride (RLX) | Hot homogenization | Osteoporosis | Oral | Size: 120 ± 3 nm PdI: 0.293 Zeta: 14.4 ± 0.5 mV EE: 91.71 | [54] |
| Precirol ATO-5 | Miglyol 812N | Tween® 80 | Rifapentine (RPT) | Hot ultra-sonication | Tuberculosis | Oral/Pulmonary | Size: 242 ± 9 nm PdI: 0.17 ± 0.01 Zeta: −22 ± 2 mV EE: | [55] |
| Glycerol monostearate (GMS) | Medium chain triglyceride (MCT) | Poloxamer 188 Soybean lecithin | Amoitone B | Emulsion-evaporation and low temperature-solidification | Tumor Therapy | NA | Size: 241.2 ± 4.4 nm PDI: NA Zeta: 18.4 ± 0.2 mV EE: 71.5 ± 1.1% | [56] |
| Myristyl Myristate | Crodamolt GTCC-LQ | Pluronic F128 | Metvan | Sonication | Bone Cancer | NA | Size: 230.8 ± 3.1 nm PdI: 0.235 ± 0.010 Zeta: −7.9 ± 0.8 mV EE: 77.6 ± 4.8% | [57] |
| Stearic acid or beeswax | Carvacrol | Kolliphor188® | Carvacrol | Warm microemulsion oil in water (o/w) | Leishmaniasis | Parenteral | Size: 98 ± 0.80 nm PdI: 0.166 ± 0.04 Zeta: −25 ± 5 mV | [58] |
| SLN | NLC | |
|---|---|---|
| Lipids | Use of physiological lipids; however, there is a lower stability comparatively with other materials | |
| Solvents | Absence of organic solvents | |
| Application | Application in different industries (food, cosmetic, pharmaceutical) | |
| Bioavailability | Improved bioavailability of drugs | |
| Drugs loaded | Loads both lipophilic and hydrophilic drugs; however, has difficulty in loading therapeutic proteins | |
| Drug delivery | Targeted drug delivery and enhanced drug permeation | |
| Scale-up | Cheaper and easier to scale up than polymeric nanoparticles | |
| Protection | Protection of drug molecules from enzymatic activity, harsh pH, and moisture | |
| Cytotoxicity | Cytotoxicity concerns due to the nature and concentration of matrix lipids | |
| Drug loading capacity | Limited drug loading capacity | Improved drug loading capacity |
| Controlled drug release profile | Difficulty in adjusting the drug release profile | Better controlled drug release profile |
| Polymorphic transitions | Prone to polymorphic transitions | No polymorphic transition takes place |
| Release during storage | Unwanted drug release during storage | Minimal drug release during storage |
| Physical stability | Possible particle aggregation or fusion during storage | Better physical stability during storage |
| Water content | High water content | Low water content |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Viegas, C.; Patrício, A.B.; Prata, J.M.; Nadhman, A.; Chintamaneni, P.K.; Fonte, P. Solid Lipid Nanoparticles vs. Nanostructured Lipid Carriers: A Comparative Review. Pharmaceutics 2023, 15, 1593. https://doi.org/10.3390/pharmaceutics15061593
Viegas C, Patrício AB, Prata JM, Nadhman A, Chintamaneni PK, Fonte P. Solid Lipid Nanoparticles vs. Nanostructured Lipid Carriers: A Comparative Review. Pharmaceutics. 2023; 15(6):1593. https://doi.org/10.3390/pharmaceutics15061593
Chicago/Turabian StyleViegas, Cláudia, Ana B. Patrício, João M. Prata, Akhtar Nadhman, Pavan Kumar Chintamaneni, and Pedro Fonte. 2023. "Solid Lipid Nanoparticles vs. Nanostructured Lipid Carriers: A Comparative Review" Pharmaceutics 15, no. 6: 1593. https://doi.org/10.3390/pharmaceutics15061593
APA StyleViegas, C., Patrício, A. B., Prata, J. M., Nadhman, A., Chintamaneni, P. K., & Fonte, P. (2023). Solid Lipid Nanoparticles vs. Nanostructured Lipid Carriers: A Comparative Review. Pharmaceutics, 15(6), 1593. https://doi.org/10.3390/pharmaceutics15061593