Development of Conjugated Kefiran-Chondroitin Sulphate Cryogels with Enhanced Properties for Biomedical Applications
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
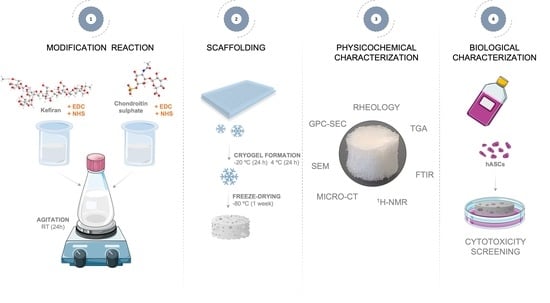
2.2. Synthesis and Characterization of Conjugated Kefiran-Chondroitin Sulfate
2.3. Preparation of Freeze-Dried Kefiran-Chondroitin Sulfate Cryogel
2.4. Methods
2.4.1. Physicochemical Characterization of Kefiran-Chondroitin Sulfate Material
- 1H-NMR
- FTIR
- GPC-SEC
- DSC
- STA
2.4.2. Characterization of Freeze-Dried Kefiran-CS Cryogel
- Micro-computed tomography (micro-CT) analysis
- Scanning electron microscopy (SEM) analysis
- Rheology of Kefiran-CS cryogels
2.4.3. Cytotoxicity Evaluation of Freeze-Dried Kefiran-CS Cryogel
- Human adipose-derived stem cells (hASCs) isolation and culture
- Cell viability assay
2.4.4. Statistical Analysis
3. Results and Discussion
3.1. 1H Nuclear Magnetic Resonance Spectroscopy (NMR)
3.2. Fourier Transform Infrared Spectroscopy (FTIR)
3.3. Gel Permeation Chromatography–Size Exclusion Chromatography (GPC-SEC)
3.4. Differential Scanning Calorimetry (DSC)
3.5. Simultaneous Thermal Analysis (STA)
3.6. Assessment of the Freeze-Dried Kefiran-CS Cryogels’ Morphological Properties
3.7. Assessment of the Kefiran-CS Cryogels’ Mechanical Properties
3.8. Cytotoxicity Screening
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Han, F.; Wang, J.; Ding, L.; Hu, Y.; Li, W.; Yuan, Z.; Guo, Q.; Zhu, C.; Yu, L.; Wang, H.; et al. Tissue Engineering and Regenerative Medicine: Achievements, Future, and Sustainability in Asia. Front. Bioeng. Biotechnol. 2020, 8, 83. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ahmad, Z.; Salman, S.; Khan, S.A.; Amin, A.; Rahman, Z.U.; Al-Ghamdi, Y.O.; Akhtar, K.; Bakhsh, E.M.; Khan, S.B.; Ahmad, Z.; et al. Versatility of Hydrogels: From Synthetic Strategies, Classification, and Properties to Biomedical Applications. Gels 2022, 8, 167. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Mooney, D.J. Designing Hydrogels for Controlled Drug Delivery. Nat. Rev. Mater. 2016, 1, 16071. [Google Scholar] [CrossRef] [PubMed]
- Reddy, M.S.B.; Ponnamma, D.; Choudhary, R.; Sadasivuni, K.K. A Comparative Review of Natural and Synthetic Biopolymer Composite Scaffolds. Polymers 2021, 13, 1105. [Google Scholar] [CrossRef] [PubMed]
- Mantha, S.; Pillai, S.; Khayambashi, P.; Upadhyay, A.; Zhang, Y.; Tao, O.; Pham, H.M.; Tran, S.D. Smart Hydrogels in Tissue Engineering and Regenerative Medicine. Materials 2019, 12, 3323. [Google Scholar] [CrossRef] [Green Version]
- Bao, W.; Li, M.; Yang, Y.; Wan, Y.; Wang, X.; Bi, N.; Li, C. Advancements and Frontiers in the High Performance of Natural Hydrogels for Cartilage Tissue Engineering. Front. Chem. 2020, 8, 53. [Google Scholar] [CrossRef] [Green Version]
- Chevalier, X.; Conrozier, T. Access to Highly Purified Chondroitin Sulfate for Appropriate Treatment of Osteoarthritis: A Review. Med. Access Point Care 2017, 1, e134–e144. [Google Scholar] [CrossRef] [Green Version]
- Sood, A.; Gupta, A.; Agrawal, G. Recent Advances in Polysaccharides Based Biomaterials for Drug Delivery and Tissue Engineering Applications. Carbohydr. Polym. Technol. Appl. 2021, 2, 100067. [Google Scholar] [CrossRef]
- Radhouani, H.; Bicho, D.; Gonçalves, C.; Maia, F.R.; Reis, R.L.; Oliveira, J.M. Kefiran Cryogels as Potential Scaffolds for Drug Delivery and Tissue Engineering Applications. Mater. Today Commun. 2019, 20, 100554. [Google Scholar]
- Song, R.; Murphy, M.; Li, C.; Ting, K.; Soo, C.; Zheng, Z. Current Development of Biodegradable Polymeric Materials for Biomedical Applications. Drug Des. Devel. Ther. 2018, 12, 3117–3145. [Google Scholar] [CrossRef] [Green Version]
- Tyshkunova, I.V.; Poshina, D.N.; Skorik, Y.A. Cellulose Cryogels as Promising Materials for Biomedical Applications. Int. J. Mol. Sci. 2022, 23, 2037. [Google Scholar] [CrossRef]
- Rogers, Z.J.; Bencherif, S.A. Cryogelation and Cryogels. Gels 2019, 5, 46. [Google Scholar] [CrossRef] [Green Version]
- Demirci, S.; Sahiner, M.; Ari, B.; Sunol, A.K.; Sahiner, N. Chondroitin Sulfate-Based Cryogels for Biomedical Applications. Gels 2021, 7, 127. [Google Scholar] [CrossRef]
- Exarhopoulos, S.; Goulas, A.; Dimitreli, G. Biodegradable Films from Kefiran-Based Cryogel Systems. Macromol 2022, 2, 324–345. [Google Scholar] [CrossRef]
- Radhouani, H.; Gonçalves, C.; Maia, F.R.; Oliveira, J.M.; Reis, R.L. Kefiran Biopolymer: Evaluation of Its Physicochemical and Biological Properties. J. Bioact. Compat. Polym. 2018, 33, 461–478. [Google Scholar] [CrossRef]
- Vashist, S.K. Comparison of 1-Ethyl-3-(3-Dimethylaminopropyl) Carbodiimide Based Strategies to Crosslink Antibodies on Amine-Functionalized Platforms for Immunodiagnostic Applications. Diagnostics 2012, 2, 23–33. [Google Scholar] [CrossRef] [Green Version]
- Correia, S.; Gonçalves, C.; Oliveira, J.M.; Radhouani, H.; Reis, R.L. Impact of Kefiran Exopolysaccharide Extraction on Its Applicability for Tissue Engineering and Regenerative Medicine. Pharmaceutics 2022, 14, 1713. [Google Scholar] [CrossRef]
- Welzel, P.B.; Prokoph, S.; Zieris, A.; Grimmer, M.; Zschoche, S.; Freudenberg, U.; Werner, C. Modulating Biofunctional StarPEG Heparin Hydrogels by Varying Size and Ratio of the Constituents. Polymers 2011, 3, 602–620. [Google Scholar] [CrossRef] [Green Version]
- Suriano, R.; Griffini, G.; Chiari, M.; Levi, M.; Turri, S. Rheological and Mechanical Behavior of Polyacrylamide Hydrogels Chemically Crosslinked with Allyl Agarose for Two-Dimensional Gel Electrophoresis. J. Mech. Behav. Biomed. Mater. 2014, 30, 339–346. [Google Scholar] [CrossRef]
- Hurle, K.; Maia, F.R.; Ribeiro, V.P.; Pina, S.; Oliveira, J.M.; Goetz-Neunhoeffer, F.; Reis, R.L. Osteogenic Lithium-Doped Brushite Cements for Bone Regeneration. Bioact. Mater. 2022, 16, 403–417. [Google Scholar] [CrossRef]
- Speciale, I.; Notaro, A.; Garcia-Vello, P.; Di Lorenzo, F.; Armiento, S.; Molinaro, A.; Marchetti, R.; Silipo, A.; De Castro, C. Liquid-State NMR Spectroscopy for Complex Carbohydrate Structural Analysis: A Hitchhiker’s Guide. Carbohydr. Polym. 2022, 277, 118885. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, I.M.; Gonçalves, C.; Oliveira, E.P.; Simón-Vázquez, R.; da Silva Morais, A.; González-Fernández, Á.; Reis, R.L.; Oliveira, J.M. PAMAM Dendrimers Functionalised with an Anti-TNF α Antibody and Chondroitin Sulphate for Treatment of Rheumatoid Arthritis. Mater. Sci. Eng. C Mater. Biol. Appl. 2021, 121, 111845. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.L.; Zhu, C.F.; Liu, H.C.; Zhu, J.M. Quantitative Analysis of Degree of Substitution/Molar Substitution of Etherified Polysaccharide Derivatives. Des. Monomers Polym. 2022, 25, 75–88. [Google Scholar] [CrossRef] [PubMed]
- Radhouani, H.; Correia, S.; Gonçalves, C.; Reis, R.L.; Oliveira, J.M. Synthesis and Characterization of Biocompatible Methacrylated Kefiran Hydrogels: Towards Tissue Engineering Applications. Polymers 2021, 13, 1342. [Google Scholar] [CrossRef]
- Gharaghani, M.; Mousavi, M.; Khodaiyan, F.; Yarmand, M.S.; Omar-Aziz, M.; Hosseini, S.S. Octenyl Succinylation of Kefiran: Preparation, Characterization and Functional Properties. Int. J. Biol. Macromol. 2021, 166, 1197–1209. [Google Scholar] [CrossRef]
- Gonçalves, C.; Radhouani, H.; Oliveira, J.M.; Reis, R.L. Sulfation of Microbial Polysaccharides. In Polysaccharides of Microbial Origin; Oliveira, J., Radhouani, H., Reis, R.L., Eds.; Springer International Publishing: Cham, Switzerland, 2021; pp. 1–18. ISBN 978-3-030-35734-4. [Google Scholar]
- Kowalczuk, D.; Pitucha, M. Application of FTIR Method for the Assessment of Immobilization of Active Substances in the Matrix of Biomedical Materials. Materials 2019, 12, 2972. [Google Scholar] [CrossRef] [Green Version]
- Nienhaus, K.; Nienhaus, G.U. Ligand Dynamics in Heme Proteins Observed by Fourier Transform Infrared Spectroscopy at Cryogenic Temperatures. In Methods in Enzymology; Academic Press: Cambridge, MA, USA, 2008; Volume 437, pp. 347–378. ISBN 9780123742780. [Google Scholar]
- Coates, J. Interpretation of Infrared Spectra, A Practical Approach. In Encyclopedia of Analytical Chemistry; Wiley: Hoboken, NJ, USA, 2006; pp. 10815–10837. [Google Scholar]
- Gómez-Ordóñez, E.; Rupérez, P. FTIR-ATR Spectroscopy as a Tool for Polysaccharide Identification in Edible Brown and Red Seaweeds. Food Hydrocoll. 2011, 25, 1514–1520. [Google Scholar] [CrossRef]
- Lin-Vien, D.; Colthup, N.B.; Fateley, W.G.; Grasselli, J.G. Organic Sulfur Compounds. In The Handbook of Infrared and Raman Characteristic Frequencies of Organic Molecules; Elsevier: Amsterdam, The Netherlands, 1991; pp. 225–250. [Google Scholar]
- Hong, T.; Yin, J.Y.; Nie, S.P.; Xie, M.Y. Applications of Infrared Spectroscopy in Polysaccharide Structural Analysis: Progress, Challenge and Perspective. Food Chem. 2021, 12, 100168. [Google Scholar] [CrossRef]
- Gunasekaran, S.; Govindan, S.; Ramani, P. Sulfated Modification, Characterization and Bioactivities of an Acidic Polysaccharide Fraction from an Edible Mushroom Pleurotus Eous (Berk.) Sacc. Heliyon 2021, 7, e05964. [Google Scholar] [CrossRef]
- Balani, K.; Verma, V.; Agarwal, A.; Narayan, R. Physical, Thermal, and Mechanical Properties of Polymers. In Biosurfaces; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2015; pp. 329–344. [Google Scholar]
- Ferreira, P.; Alves, P.; Coimbra, P.; Gil, M.H. Improving Polymeric Surfaces for Biomedical Applications: A Review. J. Coat. Technol. Res. 2015, 12, 463–475. [Google Scholar] [CrossRef]
- Dragostin, O.; Profire, L. Molecular Weight of polymers Used in Biomedical Applications. In Characterization of Polymeric Biomaterials; Elsevier: Amsterdam, The Netherlands, 2017; pp. 101–121. ISBN 978-0-08-100737-2 (print); 978-0-08-100743-3 (online). [Google Scholar]
- Samir, A.; Ashour, F.H.; Hakim, A.A.A.; Bassyouni, M. Recent Advances in Biodegradable Polymers for Sustainable Applications. npj Mater. Degrad. 2022, 6, 68. [Google Scholar] [CrossRef]
- Sohail, K.; Khan, I.U.; Shahzad, Y.; Hussain, T.; Ranjha, N.M. Ph-Sensitive Polyvinylpyrrolidone-Acrylic Acid Hydrogels: Impact of Material Parameters on Swelling and Drug Release. Braz. J. Pharm. Sci. 2014, 50, 173–184. [Google Scholar] [CrossRef]
- Elvina Tresia Butar-Butar, M.; Yohana Chaerunisaa, A.; Devianti Sagita Mayang Kusuma Dewi, N. Thermal Behavior of Polymers in Solid-State. Sci. Pharm. 2022, 1, 8–19. [Google Scholar]
- Necolau, M.I.; Damian, C.M.; Olaret, E.; Iovu, H.; Balanuca, B. Comparative Thermo-Mechanical Properties of Sustainable Epoxy Polymer Networks Derived from Linseed Oil. Polymers 2022, 14, 4212. [Google Scholar] [CrossRef]
- Olăreț, E.; Stancu, I.C.; Iovu, H.; Serafim, A. Computed Tomography as a Characterization Tool for Engineered Scaffolds with Biomedical Applications. Materials 2021, 14, 6763. [Google Scholar] [CrossRef]
- Ferreira-Gonçalves, T.; Constantin, C.; Neagu, M.; Reis, C.P.; Sabri, F.; Simón-Vázquez, R. Safety and Efficacy Assessment of Aerogels for Biomedical Applications. Biomed. Pharmacother. 2021, 144, 112356. [Google Scholar] [CrossRef]
- Bose, S.; Vahabzadeh, S.; Bandyopadhyay, A. Bone Tissue Engineering Using 3D Printing. Mater. Today 2013, 16, 496–504. [Google Scholar] [CrossRef]
- Lynch, C.R.; Kondiah, P.P.D.; Choonara, Y.E. Advanced Strategies for Tissue Engineering in Regenerative Medicine: A Biofabrication and Biopolymer Perspective. Molecules 2021, 26, 2518. [Google Scholar] [CrossRef]
- Suamte, L.; Tirkey, A.; Barman, J.; Jayasekhar Babu, P. Various Manufacturing Methods and Ideal Properties of Scaffolds for Tissue Engineering Applications. Smart Mater. Manuf. 2023, 1, 100011. [Google Scholar] [CrossRef]
- Kumar, A.; Jacob, A. Techniques in Scaffold Fabrication Process for Tissue Engineering Applications: A Review. J. Appl. Biol. Biotechnol. 2022, 10, 163–176. [Google Scholar] [CrossRef]
- Haider, A.; Haider, S.; Rao Kummara, M.; Kamal, T.; Alghyamah, A.A.A.; Jan Iftikhar, F.; Bano, B.; Khan, N.; Amjid Afridi, M.; Soo Han, S.; et al. Advances in the Scaffolds Fabrication Techniques Using Biocompatible Polymers and Their Biomedical Application: A Technical and Statistical Review. J. Saudi Chem. Soc. 2020, 24, 186–215. [Google Scholar] [CrossRef]
- Hedayati, R.; Janbaz, S.; Sadighi, M.; Mohammadi-Aghdam, M.; Zadpoor, A.A. How Does Tissue Regeneration Influence the Mechanical Behavior of Additively Manufactured Porous Biomaterials? J. Mech. Behav. Biomed. Mater. 2017, 65, 831–841. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- De Santis, R.; Gloria, A.; Russo, T.; Ronca, A.; D’Amora, U.; Negri, G.; Ronca, D.; Ambrosio, L. Viscoelastic Properties of Rapid Prototyped Magnetic Nanocomposite Scaffolds for Osteochondral Tissue Regeneration. Proc. Procedia CIRP 2016, 49, 76–82. [Google Scholar] [CrossRef] [Green Version]
- Cacopardo, L.; Guazzelli, N.; Nossa, R.; Mattei, G.; Ahluwalia, A. Engineering Hydrogel Viscoelasticity. J. Mech. Behav. Biomed. Mater. 2019, 89, 162–167. [Google Scholar] [CrossRef]
- Christ, H.A.; Bourgat, Y.; Menzel, H. Optimization of Critical Parameters for Carbodiimide Mediated Production of Highly Modified Chitosan. Polymers 2021, 13, 2702. [Google Scholar] [CrossRef]
- Prasadh, S.; Wong, R.C.W. Unraveling the Mechanical Strength of Biomaterials Used as a Bone Scaffold in Oral and Maxillofacial Defects. Oral Sci. Int. 2018, 15, 48–55. [Google Scholar] [CrossRef]
- Dai, R.; Wang, Z.; Samanipour, R.; Koo, K.-I.; Kim, K. Adipose-Derived Stem Cells for Tissue Engineering and Regenerative Medicine Applications. Stem Cells Int. 2016, 2016, 6737345. [Google Scholar] [CrossRef] [Green Version]
- Zhao, X.; Li, Q.; Guo, Z.; Li, Z. Constructing a Cell Microenvironment with Biomaterial Scaffolds for Stem Cell Therapy. Stem Cell Res. Ther. 2021, 12, 583. [Google Scholar] [CrossRef]







| Samples | Mw (kDa) | Mn (kDa) | PDI |
|---|---|---|---|
| Kefiran | 810.64 ± 2.38 | 748.84 ± 29.48 | 1.082 |
| CS | 45.713 ± 0.23 | 33.84 ± 0.12 | 1.352 |
| Kefiran-CS | 1649 ± 4.52 | 1449 ± 1.23 | 1.138 |
| Sample | Ge/Pa | ξ/nm | ne/(mol/m3) |
|---|---|---|---|
| Kefiran [17] | (99.6 ± 13.9) × 10−4 | 34.7 ± 1.7 | 0.040 ± 0.006 |
| Kefiran-CS | (24.6 ± 4.2) × 10−4 | 55.5 ± 3.2 | 0.010 ± 0.002 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Radhouani, H.; Gonçalves, C.; Maia, F.R.; Oliveira, E.P.; Reis, R.L.; Oliveira, J.M. Development of Conjugated Kefiran-Chondroitin Sulphate Cryogels with Enhanced Properties for Biomedical Applications. Pharmaceutics 2023, 15, 1662. https://doi.org/10.3390/pharmaceutics15061662
Radhouani H, Gonçalves C, Maia FR, Oliveira EP, Reis RL, Oliveira JM. Development of Conjugated Kefiran-Chondroitin Sulphate Cryogels with Enhanced Properties for Biomedical Applications. Pharmaceutics. 2023; 15(6):1662. https://doi.org/10.3390/pharmaceutics15061662
Chicago/Turabian StyleRadhouani, Hajer, Cristiana Gonçalves, F. Raquel Maia, Eduarda P. Oliveira, Rui L. Reis, and Joaquim M. Oliveira. 2023. "Development of Conjugated Kefiran-Chondroitin Sulphate Cryogels with Enhanced Properties for Biomedical Applications" Pharmaceutics 15, no. 6: 1662. https://doi.org/10.3390/pharmaceutics15061662
APA StyleRadhouani, H., Gonçalves, C., Maia, F. R., Oliveira, E. P., Reis, R. L., & Oliveira, J. M. (2023). Development of Conjugated Kefiran-Chondroitin Sulphate Cryogels with Enhanced Properties for Biomedical Applications. Pharmaceutics, 15(6), 1662. https://doi.org/10.3390/pharmaceutics15061662