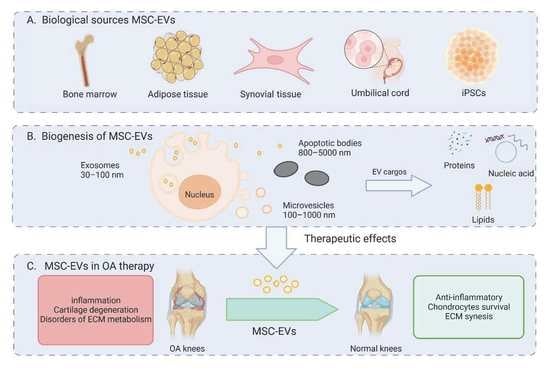
Mesenchymal Stromal Cells-Derived Extracellular Vesicles as Potential Treatments for Osteoarthritis
Abstract
:1. Introduction
2. Pathophysiology of OA
3. Biological Characteristics of EV
3.1. Production and Release of EVs
3.2. Characteristics of MSC-Derived EVs
3.3. Features of Various MSC-Derived Extracellular Vesicles
3.3.1. BMSC-EVs
3.3.2. ADSC-EVs
3.3.3. SMSC-EVs
3.3.4. EVs derived from other MSCs
4. Therapeutic Application of MSC-EV in OA
4.1. Immunomodulatory Effect
4.2. Promoting Chondrocyte Survival
4.3. Promoting ECM Synthesis
5. The Role of MSC-EVs as Drug-Delivery Vesicles
6. Treatment of OA with Engineered MSC-EVs
6.1. Modification of MSC
6.1.1. Genetic Modification
6.1.2. Cell Micro-Environment Modification
6.2. MSC-EV Modification
7. Summary and Prospect
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hunter, D.J.; March, L.; Chew, M. Osteoarthritis in 2020 and beyond: A Lancet Commission. Lancet 2020, 396, 1711–1712. [Google Scholar] [CrossRef]
- Katz, J.N.; Arant, K.R.; Loeser, R.F. Diagnosis and Treatment of Hip and Knee Osteoarthritis: A Review. JAMA 2021, 325, 568–578. [Google Scholar] [CrossRef]
- Zhao, X.; Shah, D.; Gandhi, K.; Wei, W.; Dwibedi, N.; Webster, L.; Sambamoorthi, U. Clinical, Humanistic, and Economic Burden of Osteoarthritis among Noninstitutionalized Adults in the United States. Osteoarthr. Cartil. 2019, 27, 1618–1626. [Google Scholar] [CrossRef]
- Hunter, D.J.; Bierma-Zeinstra, S. Osteoarthritis. Lancet 2019, 393, 1745–1759. [Google Scholar] [CrossRef]
- Kloppenburg, M.; Berenbaum, F. Osteoarthritis Year in Review 2019: Epidemiology and Therapy. Osteoarthr. Cartil. 2020, 28, 242–248. [Google Scholar] [CrossRef] [Green Version]
- Hart, D.A. Osteoarthritis as an Umbrella Term for Different Subsets of Humans Undergoing Joint Degeneration: The Need to Address the Differences to Develop Effective Conservative Treatments and Prevention Strategies. Int. J. Mol. Sci. 2022, 23, 15365. [Google Scholar] [CrossRef]
- Hu, Y.; Chen, X.; Wang, S.; Jing, Y.; Su, J. Subchondral Bone Microenvironment in Osteoarthritis and Pain. Bone Res. 2021, 9, 20. [Google Scholar] [CrossRef]
- Nguyen, T.H.; Duong, C.M.; Nguyen, X.-H.; Than, U.T.T. Mesenchymal Stem Cell-Derived Extracellular Vesicles for Osteoarthritis Treatment: Extracellular Matrix Protection, Chondrocyte and Osteocyte Physiology, Pain and Inflammation Management. Cells 2021, 10, 2887. [Google Scholar] [CrossRef]
- Musiał-Wysocka, A.; Kot, M.; Majka, M. The Pros and Cons of Mesenchymal Stem Cell-Based Therapies. Cell Transpl. 2019, 28, 801–812. [Google Scholar] [CrossRef] [Green Version]
- Zhuang, Y.; Jiang, S.; Yuan, C.; Lin, K. The Potential Therapeutic Role of Extracellular Vesicles in Osteoarthritis. Front. Bioeng. Biotechnol. 2022, 10, 1022368. [Google Scholar] [CrossRef]
- Zhang, X.; Lu, Y.; Wu, S.; Zhang, S.; Li, S.; Tan, J. An Overview of Current Research on Mesenchymal Stem Cell-Derived Extracellular Vesicles: A Bibliometric Analysis From 2009 to 2021. Front. Bioeng. Biotechnol. 2022, 10, 910812. [Google Scholar] [CrossRef] [PubMed]
- Liu, A.; Lin, D.; Zhao, H.; Chen, L.; Cai, B.; Lin, K.; Shen, S.G. Optimized BMSC-Derived Osteoinductive Exosomes Immobilized in Hierarchical Scaffold via Lyophilization for Bone Repair through Bmpr2/Acvr2b Competitive Receptor-Activated Smad Pathway. Biomaterials 2021, 272, 120718. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Yu, H.; Sun, M.; Yang, P.; Hu, X.; Ao, Y.; Cheng, J. The Tissue Origin Effect of Extracellular Vesicles on Cartilage and Bone Regeneration. Acta Biomater. 2021, 125, 253–266. [Google Scholar] [CrossRef] [PubMed]
- Ragni, E.; Papait, A.; Orfei, C.P.; Silini, A.R.; Colombini, A.; Viganò, M.; Libonati, F.; Parolini, O.; de Girolamo, L. Amniotic Membrane-Mesenchymal Stromal Cells Secreted Factors and Extracellular Vesicle-MiRNAs: Anti-Inflammatory and Regenerative Features for Musculoskeletal Tissues. Stem Cells Transl. Med. 2021, 10, 1044–1062. [Google Scholar] [CrossRef] [PubMed]
- Yue, L.; Berman, J. What Is Osteoarthritis? JAMA 2022, 327, 1300. [Google Scholar] [CrossRef]
- Sen, R.; Hurley, J.A. Osteoarthritis; StatPearls Publishing: St. Petersburg, FL, USA, 2023. [Google Scholar]
- Sulzbacher, I. Osteoarthritis: Histology and Pathogenesis. Wien. Med. Wochenschr. 2013, 163, 212–219. [Google Scholar] [CrossRef]
- An, S.; Hu, H.; Li, Y.; Hu, Y. Pyroptosis Plays a Role in Osteoarthritis. Aging Dis. 2020, 11, 1146–1157. [Google Scholar] [CrossRef]
- Boyde, A. The Bone Cartilage Interface and Osteoarthritis. Calcif. Tissue Int. 2021, 109, 303–328. [Google Scholar] [CrossRef]
- Qian, Z.; Gao, X.; Jin, X.; Kang, X.; Wu, S. Cartilage-Specific Deficiency of Clock Gene Bmal1 Accelerated Articular Cartilage Degeneration in Osteoarthritis by up-Regulation of MTORC1 Signaling. Int. Immunopharmacol. 2023, 115, 109692. [Google Scholar] [CrossRef]
- Goldring, M.B.; Otero, M. Inflammation in Osteoarthritis. Curr. Opin. Rheumatol. 2011, 23, 471–478. [Google Scholar] [CrossRef] [PubMed]
- Sandell, L.J.; Aigner, T. Articular Cartilage and Changes in Arthritis: Cell Biology of Osteoarthritis. Arthritis Res. Ther. 2001, 3, 107–113. [Google Scholar] [CrossRef] [Green Version]
- Karsdal, M.A.; Bay-Jensen, A.C.; Lories, R.J.; Abramson, S.; Spector, T.; Pastoureau, P.; Christiansen, C.; Attur, M.; Henriksen, K.; Goldring, S.R.; et al. The Coupling of Bone and Cartilage Turnover in Osteoarthritis: Opportunities for Bone Antiresorptives and Anabolics as Potential Treatments? Ann. Rheum. Dis. 2014, 73, 336–348. [Google Scholar] [CrossRef] [PubMed]
- Burr, D.B.; Gallant, M.A. Bone Remodelling in Osteoarthritis. Nat. Rev. Rheumatol. 2012, 8, 665–673. [Google Scholar] [CrossRef] [PubMed]
- Cucchiarini, M.; de Girolamo, L.; Filardo, G.; Oliveira, J.M.; Orth, P.; Pape, D.; Reboul, P. Basic Science of Osteoarthritis. J. Exp. Orthop. 2016, 3, 22. [Google Scholar] [CrossRef] [Green Version]
- Mathiessen, A.; Conaghan, P.G. Synovitis in Osteoarthritis: Current Understanding with Therapeutic Implications. Arthritis Res. Ther. 2017, 19, 18. [Google Scholar] [CrossRef] [Green Version]
- Baggio, C.; Boscaro, C.; Oliviero, F.; Trevisi, L.; Ramaschi, G.; Ramonda, R.; Bolego, C.; Cignarella, A. Gender Differences and Pharmacological Regulation of Angiogenesis Induced by Synovial Fluids in Inflammatory Arthritis. Biomed. Pharmacother. 2022, 152, 113181. [Google Scholar] [CrossRef] [PubMed]
- van Niel, G.; D’Angelo, G.; Raposo, G. Shedding Light on the Cell Biology of Extracellular Vesicles. Nat. Rev. Mol. Cell Biol. 2018, 19, 213–228. [Google Scholar] [CrossRef]
- Colombo, M.; Raposo, G.; Théry, C. Biogenesis, Secretion, and Intercellular Interactions of Exosomes and Other Extracellular Vesicles. Annu. Rev. Cell Dev. Biol. 2014, 30, 255–289. [Google Scholar] [CrossRef]
- Meng, F.; Xue, X.; Yin, Z.; Gao, F.; Wang, X.; Geng, Z. Research Progress of Exosomes in Bone Diseases: Mechanism, Diagnosis and Therapy. Front. Bioeng. Biotechnol. 2022, 10, 866627. [Google Scholar] [CrossRef]
- Gurunathan, S.; Kang, M.-H.; Song, H.; Kim, N.H.; Kim, J.-H. The Role of Extracellular Vesicles in Animal Reproduction and Diseases. J. Anim. Sci. Biotechnol. 2022, 13, 62. [Google Scholar] [CrossRef]
- Kong, L.; Zheng, L.-Z.; Qin, L.; Ho, K.K.W. Role of Mesenchymal Stem Cells in Osteoarthritis Treatment. J. Orthop. Transl. 2017, 9, 89–103. [Google Scholar] [CrossRef] [PubMed]
- Chen, Q.; Shou, P.; Zheng, C.; Jiang, M.; Cao, G.; Yang, Q.; Cao, J.; Xie, N.; Velletri, T.; Zhang, X.; et al. Fate Decision of Mesenchymal Stem Cells: Adipocytes or Osteoblasts? Cell Death Differ. 2016, 23, 1128–1139. [Google Scholar] [CrossRef] [Green Version]
- Zha, K.; Li, X.; Yang, Z.; Tian, G.; Sun, Z.; Sui, X.; Dai, Y.; Liu, S.; Guo, Q. Heterogeneity of Mesenchymal Stem Cells in Cartilage Regeneration: From Characterization to Application. NPJ Regen. Med. 2021, 6, 14. [Google Scholar] [CrossRef] [PubMed]
- Mancuso, P.; Raman, S.; Glynn, A.; Barry, F.; Murphy, J.M. Mesenchymal Stem Cell Therapy for Osteoarthritis: The Critical Role of the Cell Secretome. Front. Bioeng. Biotechnol. 2019, 7, 9. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jin, H.J.; Bae, Y.K.; Kim, M.; Kwon, S.-J.; Jeon, H.B.; Choi, S.J.; Kim, S.W.; Yang, Y.S.; Oh, W.; Chang, J.W. Comparative Analysis of Human Mesenchymal Stem Cells from Bone Marrow, Adipose Tissue, and Umbilical Cord Blood as Sources of Cell Therapy. Int. J. Mol. Sci. 2013, 14, 17986–18001. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rider, D.A.; Dombrowski, C.; Sawyer, A.A.; Ng, G.H.B.; Leong, D.; Hutmacher, D.W.; Nurcombe, V.; Cool, S.M. Autocrine Fibroblast Growth Factor 2 Increases the Multipotentiality of Human Adipose-Derived Mesenchymal Stem Cells. Stem Cells 2008, 26, 1598–1608. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.-J.; Im, G.-I. Chondrogenic Differentiation of Adipose Tissue-Derived Mesenchymal Stem Cells: Greater Doses of Growth Factor Are Necessary. J. Orthop. Res. 2009, 27, 612–619. [Google Scholar] [CrossRef]
- Chijimatsu, R.; Miwa, S.; Okamura, G.; Miyahara, J.; Tachibana, N.; Ishikura, H.; Higuchi, J.; Maenohara, Y.; Tsuji, S.; Sameshima, S.; et al. Divergence in Chondrogenic Potential between in Vitro and in Vivo of Adipose- and Synovial-Stem Cells from Mouse and Human. Stem Cell Res. Ther. 2021, 12, 405. [Google Scholar] [CrossRef]
- Gu, Y.; Li, T.; Ding, Y.; Sun, L.; Tu, T.; Zhu, W.; Hu, J.; Sun, X. Changes in Mesenchymal Stem Cells Following Long-Term Culture in Vitro. Mol. Med. Rep. 2016, 13, 5207–5215. [Google Scholar] [CrossRef] [Green Version]
- Kean, T.J.; Lin, P.; Caplan, A.I.; Dennis, J.E. MSCs: Delivery Routes and Engraftment, Cell-Targeting Strategies, and Immune Modulation. Stem Cells Int. 2013, 2013, e732742. [Google Scholar] [CrossRef] [Green Version]
- Barkholt, L.; Flory, E.; Jekerle, V.; Lucas-Samuel, S.; Ahnert, P.; Bisset, L.; Büscher, D.; Fibbe, W.; Foussat, A.; Kwa, M.; et al. Risk of Tumorigenicity in Mesenchymal Stromal Cell–Based Therapies—Bridging Scientific Observations and Regulatory Viewpoints. Cytotherapy 2013, 15, 753–759. [Google Scholar] [CrossRef] [PubMed]
- Tao, S.-C.; Guo, S.-C.; Zhang, C.-Q. Modularized Extracellular Vesicles: The Dawn of Prospective Personalized and Precision Medicine. Adv. Sci. 2018, 5, 1700449. [Google Scholar] [CrossRef]
- Ma, M.; Cui, G.; Liu, Y.; Tang, Y.; Lu, X.; Yue, C.; Zhang, X. Mesenchymal Stem Cell-Derived Extracellular Vesicles, Osteoimmunology and Orthopedic Diseases. PeerJ 2023, 11, e14677. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Xia, J.; Huang, R.; Hu, Y.; Fan, J.; Shu, Q.; Xu, J. Mesenchymal Stem Cell-Derived Extracellular Vesicles Alter Disease Outcomes via Endorsement of Macrophage Polarization. Stem Cell Res. Ther. 2020, 11, 424. [Google Scholar] [CrossRef]
- Kou, M.; Huang, L.; Yang, J.; Chiang, Z.; Chen, S.; Liu, J.; Guo, L.; Zhang, X.; Zhou, X.; Xu, X.; et al. Mesenchymal Stem Cell-Derived Extracellular Vesicles for Immunomodulation and Regeneration: A next Generation Therapeutic Tool? Cell Death Dis. 2022, 13, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.; Lee, M.; Bae, E.; Ryu, J.; Kim, H.; Kim, J.; Barreda, H.; Shigemoto-Kuroda, T.; Oh, J.; Lee, R. Molecular Profiles of Functionally Effective MSC-Derived Extracellular Vesicles in Immunomodulation. Cytotherapy 2019, 21, e3. [Google Scholar] [CrossRef]
- Lai, P.; Weng, J.; Guo, L.; Chen, X.; Du, X. Novel Insights into MSC-EVs Therapy for Immune Diseases. Biomark. Res. 2019, 7, 6. [Google Scholar] [CrossRef] [Green Version]
- Vonk, L.A.; van Dooremalen, S.F.J.; Liv, N.; Klumperman, J.; Coffer, P.J.; Saris, D.B.F.; Lorenowicz, M.J. Mesenchymal Stromal/Stem Cell-Derived Extracellular Vesicles Promote Human Cartilage Regeneration In Vitro. Theranostics 2018, 8, 906–920. [Google Scholar] [CrossRef]
- Li, S.; Liu, J.; Liu, S.; Jiao, W.; Wang, X. Mesenchymal Stem Cell-Derived Extracellular Vesicles Prevent the Development of Osteoarthritis via the CircHIPK3/MiR-124-3p/MYH9 Axis. J. Nanobiotechnol. 2021, 19, 194. [Google Scholar] [CrossRef]
- Zhang, J.; Rong, Y.; Luo, C.; Cui, W. Bone Marrow Mesenchymal Stem Cell-Derived Exosomes Prevent Osteoarthritis by Regulating Synovial Macrophage Polarization. Aging 2020, 12, 25138–25152. [Google Scholar] [CrossRef]
- Rong, Y.; Zhang, J.; Jiang, D.; Ji, C.; Liu, W.; Wang, J.; Ge, X.; Tang, P.; Yu, S.; Cui, W.; et al. Hypoxic Pretreatment of Small Extracellular Vesicles Mediates Cartilage Repair in Osteoarthritis by Delivering MiR-216a-5p. Acta Biomater. 2021, 122, 325–342. [Google Scholar] [CrossRef]
- Li, S.; Stöckl, S.; Lukas, C.; Götz, J.; Herrmann, M.; Federlin, M.; Grässel, S. HBMSC-Derived Extracellular Vesicles Attenuate IL-1β-Induced Catabolic Effects on OA-Chondrocytes by Regulating Pro-Inflammatory Signaling Pathways. Front. Bioeng. Biotechnol. 2020, 8, 603598. [Google Scholar] [CrossRef] [PubMed]
- Tao, S.-C.; Yuan, T.; Zhang, Y.-L.; Yin, W.-J.; Guo, S.-C.; Zhang, C.-Q. Exosomes Derived from MiR-140-5p-Overexpressing Human Synovial Mesenchymal Stem Cells Enhance Cartilage Tissue Regeneration and Prevent Osteoarthritis of the Knee in a Rat Model. Theranostics 2017, 7, 180–195. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.; Li, F.; Yuan, Y.; Shan, L.; Cui, Y.; Qu, J.; Lian, F. Synovial Mesenchymal Stem Cell-Derived EV-Packaged MiR-31 Downregulates Histone Demethylase KDM2A to Prevent Knee Osteoarthritis. Mol. Ther. Nucleic Acids 2020, 22, 1078–1091. [Google Scholar] [CrossRef]
- Yan, L.; Liu, G.; Wu, X. The Umbilical Cord Mesenchymal Stem Cell-derived Exosomal LncRNA H19 Improves Osteochondral Activity through MiR-29b-3p/FoxO3 Axis. Clin. Transl. Med. 2021, 11, e255. [Google Scholar] [CrossRef]
- Wu, J.; Kuang, L.; Chen, C.; Yang, J.; Zeng, W.-N.; Li, T.; Chen, H.; Huang, S.; Fu, Z.; Li, J.; et al. MiR-100-5p-Abundant Exosomes Derived from Infrapatellar Fat Pad MSCs Protect Articular Cartilage and Ameliorate Gait Abnormalities via Inhibition of MTOR in Osteoarthritis. Biomaterials 2019, 206, 87–100. [Google Scholar] [CrossRef] [PubMed]
- He, L.; He, T.; Xing, J.; Zhou, Q.; Fan, L.; Liu, C.; Chen, Y.; Wu, D.; Tian, Z.; Liu, B.; et al. Bone Marrow Mesenchymal Stem Cell-Derived Exosomes Protect Cartilage Damage and Relieve Knee Osteoarthritis Pain in a Rat Model of Osteoarthritis. Stem Cell Res. Ther. 2020, 11, 276. [Google Scholar] [CrossRef]
- Ye, P.; Mi, Z.; Wei, D.; Gao, P.; Ma, M.; Yang, H. MiR-3960 from Mesenchymal Stem Cell-Derived Extracellular Vesicles Inactivates SDC1/Wnt/β-Catenin Axis to Relieve Chondrocyte Injury in Osteoarthritis by Targeting PHLDA2. Stem Cells Int. 2022, 2022, 9455152. [Google Scholar] [CrossRef]
- Woo, C.H.; Kim, H.K.; Jung, G.Y.; Jung, Y.J.; Lee, K.S.; Yun, Y.E.; Han, J.; Lee, J.; Kim, W.S.; Choi, J.S.; et al. Small Extracellular Vesicles from Human Adipose-Derived Stem Cells Attenuate Cartilage Degeneration. J. Extracell. Vesicles 2020, 9, 1735249. [Google Scholar] [CrossRef] [Green Version]
- Molnar, V.; Pavelić, E.; Vrdoljak, K.; Čemerin, M.; Klarić, E.; Matišić, V.; Bjelica, R.; Brlek, P.; Kovačić, I.; Tremolada, C.; et al. Mesenchymal Stem Cell Mechanisms of Action and Clinical Effects in Osteoarthritis: A Narrative Review. Genes 2022, 13, 949. [Google Scholar] [CrossRef]
- Tofiño-Vian, M.; Guillén, M.I.; Pérez Del Caz, M.D.; Silvestre, A.; Alcaraz, M.J. Microvesicles from Human Adipose Tissue-Derived Mesenchymal Stem Cells as a New Protective Strategy in Osteoarthritic Chondrocytes. Cell Physiol. Biochem. 2018, 47, 11–25. [Google Scholar] [CrossRef] [PubMed]
- Lu, L.; Wang, J.; Fan, A.; Wang, P.; Chen, R.; Lu, L.; Yin, F. Synovial Mesenchymal Stem Cell-Derived Extracellular Vesicles Containing MicroRN555A-26a-5p Ameliorate Cartilage Damage of Osteoarthritis. J. Gene Med. 2021, 23, e3379. [Google Scholar] [CrossRef]
- El Omar, R.; Beroud, J.; Stoltz, J.-F.; Menu, P.; Velot, E.; Decot, V. Umbilical Cord Mesenchymal Stem Cells: The New Gold Standard for Mesenchymal Stem Cell-Based Therapies? Tissue Eng. Part B Rev. 2014, 20, 523–544. [Google Scholar] [CrossRef] [PubMed]
- Hu, H.; Dong, L.; Bu, Z.; Shen, Y.; Luo, J.; Zhang, H.; Zhao, S.; Lv, F.; Liu, Z. MiR-23a-3p-Abundant Small Extracellular Vesicles Released from Gelma/Nanoclay Hydrogel for Cartilage Regeneration. J. Extracell. Vesicles 2020, 9, 1778883. [Google Scholar] [CrossRef] [PubMed]
- Hsueh, Y.-H.; Buddhakosai, W.; Le, P.N.; Tu, Y.-Y.; Huang, H.-C.; Lu, H.-E.; Chen, W.-L.; Tu, Y.-K. Therapeutic Effect of Induced Pluripotent Stem Cell -Derived Extracellular Vesicles in an in Vitro and in Vivo Osteoarthritis Model. J. Orthop. Translat. 2022, 38, 141–155. [Google Scholar] [CrossRef] [PubMed]
- Han, P.-F.; Wei, L.; Duan, Z.-Q.; Zhang, Z.-L.; Chen, T.-Y.; Lu, J.-G.; Zhao, R.-P.; Cao, X.-M.; Li, P.-C.; Lv, Z.; et al. Contribution of IL-1β, 6 and TNF-α to the Form of Post-Traumatic Osteoarthritis Induced by “Idealized” Anterior Cruciate Ligament Reconstruction in a Porcine Model. Int. Immunopharmacol. 2018, 65, 212–220. [Google Scholar] [CrossRef]
- Heard, B.J.; Solbak, N.M.; Achari, Y.; Chung, M.; Hart, D.A.; Shrive, N.G.; Frank, C.B. Changes of Early Post-Traumatic Osteoarthritis in an Ovine Model of Simulated ACL Reconstruction Are Associated with Transient Acute Post-Injury Synovial Inflammation and Tissue Catabolism. Osteoarthr. Cartil. 2013, 21, 1942–1949. [Google Scholar] [CrossRef] [Green Version]
- Yunna, C.; Mengru, H.; Lei, W.; Weidong, C. Macrophage M1/M2 Polarization. Eur. J. Pharmacol. 2020, 877, 173090. [Google Scholar] [CrossRef]
- Curtale, G.; Rubino, M.; Locati, M. MicroRNAs as Molecular Switches in Macrophage Activation. Front. Immunol. 2019, 10, 799. [Google Scholar] [CrossRef] [Green Version]
- Li, Y.-S.; Luo, W.; Zhu, S.-A.; Lei, G.-H. T Cells in Osteoarthritis: Alterations and Beyond. Front. Immunol. 2017, 8, 356. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shan, Y.; Qi, C.; Liu, Y.; Gao, H.; Zhao, D.; Jiang, Y. Increased Frequency of Peripheral Blood Follicular Helper T Cells and Elevated Serum IL-21 Levels in Patients with Knee Osteoarthritis. Mol. Med. Rep. 2017, 15, 1095–1102. [Google Scholar] [CrossRef] [Green Version]
- Lopes, E.B.P.; Filiberti, A.; Husain, S.A.; Humphrey, M.B. Immune Contributions to Osteoarthritis. Curr. Osteoporos. Rep. 2017, 15, 593–600. [Google Scholar] [CrossRef]
- Ye, X.; Lu, Q.; Yang, A.; Rao, J.; Xie, W.; He, C.; Wang, W.; Li, H.; Zhang, Z. MiR-206 Regulates the Th17/Treg Ratio during Osteoarthritis. Mol. Med. 2021, 27, 64. [Google Scholar] [CrossRef]
- Zhu, W.; Zhang, X.; Jiang, Y.; Liu, X.; Huang, L.; Wei, Q.; Huang, Y.; Wu, W.; Gu, J. Alterations in Peripheral T Cell and B Cell Subsets in Patients with Osteoarthritis. Clin. Rheumatol. 2020, 39, 523–532. [Google Scholar] [CrossRef]
- Gaudino, S.J.; Kumar, P. Cross-Talk Between Antigen Presenting Cells and T Cells Impacts Intestinal Homeostasis, Bacterial Infections, and Tumorigenesis. Front. Immunol. 2019, 10, 360. [Google Scholar] [CrossRef] [Green Version]
- Zhang, S.; Chuah, S.J.; Lai, R.C.; Hui, J.H.P.; Lim, S.K.; Toh, W.S. MSC Exosomes Mediate Cartilage Repair by Enhancing Proliferation, Attenuating Apoptosis and Modulating Immune Reactivity. Biomaterials 2018, 156, 16–27. [Google Scholar] [CrossRef]
- Lai, P.; Chen, X.; Guo, L.; Wang, Y.; Liu, X.; Liu, Y.; Zhou, T.; Huang, T.; Geng, S.; Luo, C.; et al. A Potent Immunomodulatory Role of Exosomes Derived from Mesenchymal Stromal Cells in Preventing CGVHD. J. Hematol. Oncol. 2018, 11, 135. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Monguió-Tortajada, M.; Roura, S.; Gálvez-Montón, C.; Pujal, J.M.; Aran, G.; Sanjurjo, L.; Franquesa, M.; Sarrias, M.-R.; Bayes-Genis, A.; Borràs, F.E. Nanosized UCMSC-Derived Extracellular Vesicles but Not Conditioned Medium Exclusively Inhibit the Inflammatory Response of Stimulated T Cells: Implications for Nanomedicine. Theranostics 2017, 7, 270–284. [Google Scholar] [CrossRef] [PubMed]
- Khare, D.; Or, R.; Resnick, I.; Barkatz, C.; Almogi-Hazan, O.; Avni, B. Mesenchymal Stromal Cell-Derived Exosomes Affect MRNA Expression and Function of B-Lymphocytes. Front. Immunol. 2018, 9, 3053. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guo, L.; Lai, P.; Wang, Y.; Huang, T.; Chen, X.; Geng, S.; Huang, X.; Luo, C.; Wu, S.; Ling, W.; et al. Extracellular Vesicles Derived from Mesenchymal Stem Cells Prevent Skin Fibrosis in the CGVHD Mouse Model by Suppressing the Activation of Macrophages and B Cells Immune Response. Int. Immunopharmacol. 2020, 84, 106541. [Google Scholar] [CrossRef] [PubMed]
- Carreras-Planella, L.; Monguió-Tortajada, M.; Borràs, F.E.; Franquesa, M. Immunomodulatory Effect of MSC on B Cells Is Independent of Secreted Extracellular Vesicles. Front. Immunol. 2019, 10, 1288. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, S.; Stöckl, S.; Lukas, C.; Herrmann, M.; Brochhausen, C.; König, M.A.; Johnstone, B.; Grässel, S. Curcumin-Primed Human BMSC-Derived Extracellular Vesicles Reverse IL-1β-Induced Catabolic Responses of OA Chondrocytes by Upregulating MiR-126-3p. Stem Cell Res. Ther. 2021, 12, 252. [Google Scholar] [CrossRef] [PubMed]
- Ragni, E.; Perucca Orfei, C.; De Luca, P.; Colombini, A.; Viganò, M.; de Girolamo, L. Secreted Factors and EV-MiRNAs Orchestrate the Healing Capacity of Adipose Mesenchymal Stem Cells for the Treatment of Knee Osteoarthritis. Int. J. Mol. Sci. 2020, 21, 1582. [Google Scholar] [CrossRef] [Green Version]
- Chen, P.; Zheng, L.; Wang, Y.; Tao, M.; Xie, Z.; Xia, C.; Gu, C.; Chen, J.; Qiu, P.; Mei, S.; et al. Desktop-Stereolithography 3D Printing of a Radially Oriented Extracellular Matrix/Mesenchymal Stem Cell Exosome Bioink for Osteochondral Defect Regeneration. Theranostics 2019, 9, 2439–2459. [Google Scholar] [CrossRef]
- Zhao, C.; Chen, J.-Y.; Peng, W.-M.; Yuan, B.; Bi, Q.; Xu, Y.-J. Exosomes from Adipose-derived Stem Cells Promote Chondrogenesis and Suppress Inflammation by Upregulating MiR-145 and MiR-221. Mol. Med. Rep. 2020, 21, 1881–1889. [Google Scholar] [CrossRef] [Green Version]
- Zhang, S.; Jin, Z. Bone Mesenchymal Stem Cell-Derived Extracellular Vesicles Containing Long Noncoding RNA NEAT1 Relieve Osteoarthritis. Oxid. Med. Cell Longev. 2022, 2022, 5517648. [Google Scholar] [CrossRef]
- Zhou, Y.; Ming, J.; Li, Y.; Li, B.; Deng, M.; Ma, Y.; Chen, Z.; Zhang, Y.; Li, J.; Liu, S. Exosomes Derived from MiR-126-3p-Overexpressing Synovial Fibroblasts Suppress Chondrocyte Inflammation and Cartilage Degradation in a Rat Model of Osteoarthritis. Cell Death Discov. 2021, 7, 37. [Google Scholar] [CrossRef] [PubMed]
- Malemud, C.J. Inhibition of MMPs and ADAM/ADAMTS. Biochem. Pharmacol. 2019, 165, 33–40. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Shi, Y.; Xue, P.; Ma, X.; Li, J.; Zhang, J. Mesenchymal Stem Cell-Derived Exosomal MicroRNA-136-5p Inhibits Chondrocyte Degeneration in Traumatic Osteoarthritis by Targeting ELF3. Arthritis Res. Ther. 2020, 22, 256. [Google Scholar] [CrossRef]
- Zhang, S.; Teo, K.Y.W.; Chuah, S.J.; Lai, R.C.; Lim, S.K.; Toh, W.S. MSC Exosomes Alleviate Temporomandibular Joint Osteoarthritis by Attenuating Inflammation and Restoring Matrix Homeostasis. Biomaterials 2019, 200, 35–47. [Google Scholar] [CrossRef] [PubMed]
- Wang, R.; Xu, B. TGF-Β1-Modified MSC-Derived Exosomal MiR-135b Attenuates Cartilage Injury via Promoting M2 Synovial Macrophage Polarization by Targeting MAPK6. Cell Tissue Res. 2021, 384, 113–127. [Google Scholar] [CrossRef] [PubMed]
- Mao, G.; Zhang, Z.; Hu, S.; Zhang, Z.; Chang, Z.; Huang, Z.; Liao, W.; Kang, Y. Exosomes Derived from MiR-92a-3p-Overexpressing Human Mesenchymal Stem Cells Enhance Chondrogenesis and Suppress Cartilage Degradation via Targeting WNT5A. Stem Cell Res. Ther. 2018, 9, 247. [Google Scholar] [CrossRef] [Green Version]
- Liu, C.; Li, Y.; Yang, Z.; Zhou, Z.; Lou, Z.; Zhang, Q. Kartogenin Enhances the Therapeutic Effect of Bone Marrow Mesenchymal Stem Cells Derived Exosomes in Cartilage Repair. Nanomed 2020, 15, 273–288. [Google Scholar] [CrossRef]
- Wang, Y.; Yu, D.; Liu, Z.; Zhou, F.; Dai, J.; Wu, B.; Zhou, J.; Heng, B.C.; Zou, X.H.; Ouyang, H.; et al. Exosomes from Embryonic Mesenchymal Stem Cells Alleviate Osteoarthritis through Balancing Synthesis and Degradation of Cartilage Extracellular Matrix. Stem Cell Res. Ther. 2017, 8, 189. [Google Scholar] [CrossRef] [Green Version]
- Zhou, D.; Zhou, F.; Sheng, S.; Wei, Y.; Chen, X.; Su, J. Intra-Articular Nanodrug Delivery Strategies for Treating Osteoarthritis. Drug Discov. Today 2023, 28, 103482. [Google Scholar] [CrossRef] [PubMed]
- Ren, X.; Liu, H.; Wu, X.; Weng, W.; Wang, X.; Su, J. Reactive Oxygen Species (ROS)-Responsive Biomaterials for the Treatment of Bone-Related Diseases. Front. Bioeng. Biotechnol. 2021, 9, 820468. [Google Scholar] [CrossRef]
- Yajun, W.; Jin, C.; Zhengrong, G.; Chao, F.; Yan, H.; Weizong, W.; Xiaoqun, L.; Qirong, Z.; Huiwen, C.; Hao, Z.; et al. Betaine Attenuates Osteoarthritis by Inhibiting Osteoclastogenesis and Angiogenesis in Subchondral Bone. Front. Pharmacol. 2021, 12, 723988. [Google Scholar] [CrossRef]
- Yu, X.; Dong, M.; Wang, L.; Yang, Q.; Wang, L.; Han, W.; Dong, J.; Liu, T.; Kong, Y.; Niu, W. Nanotherapy for Bone Repair: Milk-Derived Small Extracellular Vesicles Delivery of Icariin. Drug Deliv. 2023, 30, 2169414. [Google Scholar] [CrossRef] [PubMed]
- Liang, Y.; Xu, X.; Li, X.; Xiong, J.; Li, B.; Duan, L.; Wang, D.; Xia, J. Chondrocyte-Targeted MicroRNA Delivery by Engineered Exosomes toward a Cell-Free Osteoarthritis Therapy. ACS Appl. Mater. Interfaces 2020, 12, 36938–36947. [Google Scholar] [CrossRef] [PubMed]
- Ohno, S.; Takanashi, M.; Sudo, K.; Ueda, S.; Ishikawa, A.; Matsuyama, N.; Fujita, K.; Mizutani, T.; Ohgi, T.; Ochiya, T.; et al. Systemically Injected Exosomes Targeted to EGFR Deliver Antitumor MicroRNA to Breast Cancer Cells. Mol. Ther. 2013, 21, 185–191. [Google Scholar] [CrossRef] [Green Version]
- Li, C.; Li, W.; Pu, G.; Wu, J.; Qin, F. Exosomes Derived from MiR-338-3p-Modified Adipose Stem Cells Inhibited Inflammation Injury of Chondrocytes via Targeting RUNX2 in Osteoarthritis. J. Orthop. Surg. Res. 2022, 17, 567. [Google Scholar] [CrossRef]
- Cha, J.M.; Shin, E.K.; Sung, J.H.; Moon, G.J.; Kim, E.H.; Cho, Y.H.; Park, H.D.; Bae, H.; Kim, J.; Bang, O.Y. Efficient Scalable Production of Therapeutic Microvesicles Derived from Human Mesenchymal Stem Cells. Sci. Rep. 2018, 8, 1171. [Google Scholar] [CrossRef] [Green Version]
- Esmaeili, A.; Hosseini, S.; Kamali, A.; Hosseinzadeh, M.; Shekari, F.; Baghaban Eslaminejad, M. Co-Aggregation of MSC/Chondrocyte in a Dynamic 3D Culture Elevates the Therapeutic Effect of Secreted Extracellular Vesicles on Osteoarthritis in a Rat Model. Sci. Rep. 2022, 12, 19827. [Google Scholar] [CrossRef]
- Deszcz, I.; Lis-Nawara, A.; Grelewski, P.; Dragan, S.; Bar, J. Utility of Direct 3D Co-Culture Model for Chondrogenic Differentiation of Mesenchymal Stem Cells on Hyaluronan Scaffold (Hyaff-11). Regen. Biomater. 2020, 7, 543–552. [Google Scholar] [CrossRef]
- Mohyeldin, A.; Garzón-Muvdi, T.; Quiñones-Hinojosa, A. Oxygen in Stem Cell Biology: A Critical Component of the Stem Cell Niche. Cell Stem Cell 2010, 7, 150–161. [Google Scholar] [CrossRef] [Green Version]
- Arslan, F.; Lai, R.C.; Smeets, M.B.; Akeroyd, L.; Choo, A.; Aguor, E.N.E.; Timmers, L.; van Rijen, H.V.; Doevendans, P.A.; Pasterkamp, G.; et al. Mesenchymal Stem Cell-Derived Exosomes Increase ATP Levels, Decrease Oxidative Stress and Activate PI3K/Akt Pathway to Enhance Myocardial Viability and Prevent Adverse Remodeling after Myocardial Ischemia/Reperfusion Injury. Stem Cell Res. 2013, 10, 301–312. [Google Scholar] [CrossRef] [Green Version]
- Guan, P.; Liu, C.; Xie, D.; Mao, S.; Ji, Y.; Lin, Y.; Chen, Z.; Wang, Q.; Fan, L.; Sun, Y. Exosome-Loaded Extracellular Matrix-Mimic Hydrogel with Anti-Inflammatory Property Facilitates/Promotes Growth Plate Injury Repair. Bioact. Mater. 2022, 10, 145–158. [Google Scholar] [CrossRef]
- Tao, S.-C.; Huang, J.-Y.; Gao, Y.; Li, Z.-X.; Wei, Z.-Y.; Dawes, H.; Guo, S.-C. Small Extracellular Vesicles in Combination with Sleep-Related CircRNA3503: A Targeted Therapeutic Agent with Injectable Thermosensitive Hydrogel to Prevent Osteoarthritis. Bioact. Mater. 2021, 6, 4455–4469. [Google Scholar] [CrossRef]
- Fuhrmann, G.; Serio, A.; Mazo, M.; Nair, R.; Stevens, M.M. Active Loading into Extracellular Vesicles Significantly Improves the Cellular Uptake and Photodynamic Effect of Porphyrins. J. Control. Release 2015, 205, 35–44. [Google Scholar] [CrossRef]
- Zhou, W.; Zhou, Y.; Chen, X.; Ning, T.; Chen, H.; Guo, Q.; Zhang, Y.; Liu, P.; Zhang, Y.; Li, C.; et al. Pancreatic Cancer-Targeting Exosomes for Enhancing Immunotherapy and Reprogramming Tumor Microenvironment. Biomaterials 2021, 268, 120546. [Google Scholar] [CrossRef]
- Li, H.; Zhang, Z.; Li, Y.; Su, L.; Duan, Y.; Zhang, H.; An, J.; Ni, T.; Li, X.; Zhang, X. Therapeutic Effect of Rapamycin-Loaded Small Extracellular Vesicles Derived from Mesenchymal Stem Cells on Experimental Autoimmune Uveitis. Front. Immunol. 2022, 13, 864956. [Google Scholar] [CrossRef]
- Fan, J.; Lee, C.-S.; Kim, S.; Chen, C.; Aghaloo, T.; Lee, M. Generation of Small RNA-Modulated Exosome Mimetics for Bone Regeneration. ACS Nano 2020, 14, 11973–11984. [Google Scholar] [CrossRef]
- Ko, K.-W.; Yoo, Y.-I.; Kim, J.Y.; Choi, B.; Park, S.-B.; Park, W.; Rhim, W.-K.; Han, D.K. Attenuation of Tumor Necrosis Factor-α Induced Inflammation by Umbilical Cord-Mesenchymal Stem Cell Derived Exosome-Mimetic Nanovesicles in Endothelial Cells. Tissue Eng. Regen. Med. 2020, 17, 155–163. [Google Scholar] [CrossRef]
- Xu, X.; Liang, Y.; Li, X.; Ouyang, K.; Wang, M.; Cao, T.; Li, W.; Liu, J.; Xiong, J.; Li, B.; et al. Exosome-Mediated Delivery of Kartogenin for Chondrogenesis of Synovial Fluid-Derived Mesenchymal Stem Cells and Cartilage Regeneration. Biomaterials 2021, 269, 120539. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Liao, L.; Wang, S.; Tan, J. Cell Therapy with Autologous Mesenchymal Stem Cells-How the Disease Process Impacts Clinical Considerations. Cytotherapy 2013, 15, 893–904. [Google Scholar] [CrossRef]
- Li, C.; Zhao, H.; Cheng, L.; Wang, B. Allogeneic vs. Autologous Mesenchymal Stem/Stromal Cells in Their Medication Practice. Cell Biosci. 2021, 11, 187. [Google Scholar] [CrossRef]
- Harrell, C.R.; Jovicic, N.; Djonov, V.; Arsenijevic, N.; Volarevic, V. Mesenchymal Stem Cell-Derived Exosomes and Other Extracellular Vesicles as New Remedies in the Therapy of Inflammatory Diseases. Cells 2019, 8, 1605. [Google Scholar] [CrossRef] [Green Version]




| EVs Source | Affected Tissue | Study Type | Disease Model | EVs Dose | Molecular Mechanism | Action Effect | Ref. |
|---|---|---|---|---|---|---|---|
| hBMSC | Chondrocytes | In vitro | - | 1.14 g/mL | Reduce the expression of COX2, ILs and collagenase activity induced by TNF-α while increasing the expression of proteoglycans and type II collagen | Prevent the harmful impact of inflammatory mediators on cartilage homeostasis and facilitate its regeneration | [49] |
| BMSC | Chondrocytes | In vitro and in vivo | CIOA | - | The overexpression of circHIPK3 resulted in the improvement of IL-1β-induced chondrocyte injury by binding to miR-124-3p | Promote chondrocyte proliferation and migration induction while inhibit chondrocyte apoptosis | [50] |
| BMSC | Synovial macrophages | In vitro and in vivo | The modified Hulth method | 1 μg/mL | Induce synovial macrophages to transform from M1 to M2, decrease the expression of IL-1β, TNF-α, and IL-6 while increasing the expression of IL-10, as well as improving the expression of chondrogenic genes, collagen II, and sox9 | Inhibit OA progression | [51] |
| BMSC | Chondrocyte | In vitro and in vivo | ACLT | 200 μg of total protein of sEVs precipitated in 200 μL PBS | The expression of miR-216a-3p was upregulated under hypoxic conditions, leading to the downregulation of JAK2 | Enhance cell proliferation and migration, while decreasing apoptosis | [52] |
| hBMSC | Chondrocytes | In Vitro | - | 10 ug/mL | Suppress the pro-inflammatory Erk1/2, PI3K/Akt, p38, TAK1, and NF-κB signaling pathways that are activated by IL-1β | Stimulate the proliferation and migration of chondrocytes affected by osteoarthritis while decreasing their apoptosis rate | [53] |
| SMSC | Articular chondrocytes | In vitro and in vivo | ACLT | 1011 exosomes particles/mL | The overexpression of miR-140-5p effectively inhibited the reduction of extracellular matrix secretion by targeting RalA | Enhance proliferation, migration of chondrocytes, and prevent OA | [54] |
| SMSC | Articular chondrocytes | In vitro and in vivo | ACLT | 5 μL SMSC-EV particles per mL | The KDM2A/E2F1/PTTG1 pathway is involved in the beneficial effects of miR-31 on osteoarthritis | Relieve inflammation and cartilage damage in the knee joints | [55] |
| UMSC | Chondrocyte | In vitro and in vivo | Unilateral cartilage defect | 1 mg/mL | Exosomal H19 against miR-29b-3p to upregulate FoxO3 | Enhance chondrocyte migration, secretion of matrix, suppression of apoptosis, as well as reduction of senescence | [56] |
| Infrapatellar fat pad MSCs | Chondrocyte | In vitro and in vivo | DMM surgery | 1010 particles/mL | MiR100-5p is involved in inhibiting the mTOR-autophagy pathway regulation | The maintenance of cartilage homeostasis can protect the articular cartilage from damage and alleviate gait abnormalities in OA mice | [57] |
| EVs Source | Related Cargo | Effect | Reference | |
|---|---|---|---|---|
| 1 | BMSCs | MiR-135b | MiR-135b promoted M2 polarization of synovial macrophages through targeting MAPK6, thus improving cartilage damage | [92] |
| 2 | hMSCs | - | Activation of AKT/ERK phosphorylation through AMP hydrolysis by MSC exosomes can effectively reduce inflammation and sustain mediate matrix homeostasis. | [91] |
| 3 | ADMSCs | - | Reduce inflammatory mediators and restore the ECM by upregulation of annexin A1. | [62] |
| 4 | hMSC-miR-92a-3p | - | Exosomal miR-92a-3p regulates cartilage development and homeostasis by directly targeting WNT5A. | [93] |
| 5 | hUMSCs | MiR-23a-3p | Improved the migration, proliferation and chondrogenic differentiation of the cells, resulting in the formation of glycosaminoglycan, extracellular matrix and collagen II. | [65] |
| 6 | BMSC with kartogenin preconditioning | - | KGN preconditioning endowed BMSC-Exos with stronger chondral matrix formation and less degradation | [94] |
| 7 | infrapatellar fat pad (IPFP) MSCs | miR-100-5p | Protect articular cartilage from damage and ameliorate gait abnormality in OA mice via inhibition of mTOR-autophagy pathway. | [57] |
| 8 | umbilical cord mesenchymal stem cells (UMSCs) | lncRNA H19 | Promote chondrocyte migration, matrix secretion via against miR-29b-3p to upregulate FoxO3 in chondrocytes. | [56] |
| 9 | embryonic stem cell-induced mesenchymal stem cells (ESC-MSCs) | - | balance of synthesis and degradation about chondrocyte extracellular matrix (ECM) | [95] |
| NCT Number | Title | Conditions | Status | Study Results | Information Provided by (Responsible Party) | |
|---|---|---|---|---|---|---|
| 1 | NCT04173650 | MSC EVs in Dystrophic Epidermolysis Bullosa | Dystrophic Epidermolysis Bullosa | Not yet recruiting | No Results Available | Third Affiliated Hospital, Sun Yat-Sen University |
| 2 | NCT05078385 | Safety of Mesenchymal Stem Cell Extracellular Vesicles (BM-MSC-EVs) for the Treatment of Burn Wounds | Not yet recruiting | Not yet recruiting | No Results Available | Aegle Therapeutics |
| 3 | NCT05881668 | MSC-EV in Acute-on-Chronic Liver Failure After Liver Transplantation | Liver Failure, Acute on Chronic | Not yet recruiting | No Results Available | Aegle Therapeutics |
| 4 | NCT05078385 | Safety of Mesenchymal Stem Cell Extracellular Vesicles (BM-MSC-EVs) for the Treatment of Burn Wounds | Burns | Not yet recruiting | No Results Available | Maimónides Biomedical Research Institute of Córdoba |
| 5 | NCT05523011 | Safety and Tolerability Study of MSC Exosome Ointment | Completed | Not yet recruiting | No Results Available | Paracrine Therapeutics Dermatology Pte. Ltd. |
| 6 | NCT05060107 | Intra-articular Injection of MSC-derived Exosomes in Knee Osteoarthritis (ExoOA-1) | Osteoarthritis, Knee | Not yet recruiting | No Results Available | Francisco Espinoza, Universidad de los Andes, Chile |
| 7 | NCT05216562 | Efficacy and Safety of EXOSOME-MSC Therapy to Reduce Hyper-inflammation In Moderate COVID-19 Patients | SARS-CoV2 Infection | Recruiting | No Results Available | Dermama Bioteknologi Laboratorium|Kementerian Riset dan Teknologi/Badan Riset dan Inovasi Nasional, Indonesia |
| 8 | NCT05738629 | Safety and Efficacy of Pluripotent Stem Cell-derived Mesenchymal Stem Cell Exosome (PSC-MSC-Exo) Eye Drops Treatment for Dry Eye Diseases Post | Dry Eye Disease | Not yet recruiting | No Results Available | Second Affiliated Hospital, School of Medicine, Zhejiang University|Zhejiang University|Hangzhou yuansheng biotechnology Co., Ltd., China. |
| 9 | NCT03437759 | MSC-Exos Promote Healing of MHs | Macular Holes | Unknown status | No Results Available | Tianjin Medical University |
| 10 | NCT04850469 | Study of MSC-Exo on the Therapy for Intensively Ill Children | Sepsis|Critical Illness | Withdrawn | No Results Available | Children’s Hospital of Fudan University |
| 11 | NCT04388982 | The Safety and the Efficacy Evaluation of Allogenic Adipose MSC-Exos in Patients with Alzheimer’s Disease | Alzheimer Disease | Unknown status | No Results Available | Ruijin Hospital|Cellular Biomedicine Group Ltd. |
| 12 | NCT05243368 | Evaluation of Personalized Nutritional Intervention on Wound Healing of Cutaneous Ulcers in Diabetics | Foot, Diabetic | Not yet recruiting | No Results Available | Maimónides Biomedical Research Institute of Córdoba |
| 13 | NCT04798716 | The Use of Exosomes for the Treatment of Acute Respiratory Distress Syndrome or Novel Coronavirus Pneumonia Caused by COVID-19 | COVID19|Novel Coronavirus Pneumonia|Acute Respiratory Distress Syndrome | Not yet recruiting | No Results Available | AVEM HealthCare |
| 14 | NCT04602442 | Safety and Efficiency of Method of Exosome Inhalation in COVID-19 Associated Pneumonia | COVID19|SARS-CoV-2 PNEUMONIA|COVID-19 | Unknown status | No Results Available | Olga Tyumina|Clinics of the Federal State Budgetary Educational Institution SSMU|Samara Regional Clinical Hospital V.D. Seredavin|State-Financed Health |
| 15 | NCT04491240 | Evaluation of Safety and Efficiency of Method of Exosome Inhalation in SARS-CoV-2 Associated Pneumonia | COVID19|SARS-CoV-2 PNEUMONIA|COVID-19 | Completed | Has Results | State-Financed Health Facility “Samara Regional Medical Center Dinasty”|Clinics of the Federal State Budgetary Educational Institution SSMU|Samara Regional |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yuan, S.; Li, G.; Zhang, J.; Chen, X.; Su, J.; Zhou, F. Mesenchymal Stromal Cells-Derived Extracellular Vesicles as Potential Treatments for Osteoarthritis. Pharmaceutics 2023, 15, 1814. https://doi.org/10.3390/pharmaceutics15071814
Yuan S, Li G, Zhang J, Chen X, Su J, Zhou F. Mesenchymal Stromal Cells-Derived Extracellular Vesicles as Potential Treatments for Osteoarthritis. Pharmaceutics. 2023; 15(7):1814. https://doi.org/10.3390/pharmaceutics15071814
Chicago/Turabian StyleYuan, Shunling, Guangfeng Li, Jinbo Zhang, Xiao Chen, Jiacan Su, and Fengjin Zhou. 2023. "Mesenchymal Stromal Cells-Derived Extracellular Vesicles as Potential Treatments for Osteoarthritis" Pharmaceutics 15, no. 7: 1814. https://doi.org/10.3390/pharmaceutics15071814
APA StyleYuan, S., Li, G., Zhang, J., Chen, X., Su, J., & Zhou, F. (2023). Mesenchymal Stromal Cells-Derived Extracellular Vesicles as Potential Treatments for Osteoarthritis. Pharmaceutics, 15(7), 1814. https://doi.org/10.3390/pharmaceutics15071814