More Than Pigments: The Potential of Astaxanthin and Bacterioruberin-Based Nanomedicines
Abstract
:1. Introduction
2. Materials and Methods
3. Carotenoids: Structure and Source, Dietary Effects in Animals, and Human Consumption
4. Antioxidant and Anti-Inflammatory Activity of Xanthophylls
5. The Highly Marketable Astaxanthin
6. Bacterioruberin, a Xanthophyll Hidden in the Salt
7. AST and BR Production and Extraction
8. Functional Foods vs. Nanomedicines?
8.1. Few Carotenoids Are Regarded as Drugs
8.2. Most Carotenoids Are Regarded as Food
8.3. Protection of Carotenoids’ Labile Structure in Foods
8.4. Characteristic Features of Nanomedicines
9. AST and BR-Based Nanomedicines
9.1. Nanomedicines for Oral Delivery of AST and BR
9.1.1. Nanomedicines to Treat Inflammatory Bowel Diseases (IBDs)
9.1.2. Nanomedicines to Treat Liver Damage
9.1.3. Nanomedicines to Treat Inherited Retinal Degeneration
9.2. Nanomedicines for Topical Delivery of AST and BR
9.2.1. Nanomedicines to Treat Atopic Dermatitis (AD) and Psoriasis (PS)
9.2.2. Nanomedicines to Treat UV-Induced Skin Damage
9.2.3. Nanomedicines to Treat Dry Eye Disease (DED)
9.2.4. Nanomedicines for Otoprotection
9.3. Nanomedicines for Intra-Articular Delivery of AST
Nanomedicines to Treat Osteoarthritis (OA)
9.4. Nanomedicines for Endovenous Delivery of AST
9.4.1. Nanomedicines to Treat Liver Injury
9.4.2. Nanomedicines to Treat Diabetic Nephropathy
9.5. Nanomedicines for Nose to Brain Delivery of AST
10. Discussion
11. Conclusions
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| 8-OHdG | 8-Hydroxy-2’-deoxyguanosine |
| AD | atopic dermatitis |
| ADME | absorption, distribution, metabolism, excretion |
| ARE | Antioxidant Response Element |
| AST | astaxanthin |
| BD | biodistribution |
| BR | bacterioruberin |
| BTRL | biotechnological readiness levels |
| BUN | blood urea nitrogen |
| CAGR | compound annual growth rate |
| CAT | catalase |
| CAP | capsaicin |
| cdb | conjugated double bonds |
| Chol | cholesterol |
| COPD | chronic obstructive pulmonary disease |
| DED | dry eye disease |
| DMPC | 1,2-Dimyristoyl-sn-glycero-3-phosphocholine |
| DMSO | dimethylsufoxide |
| DN | diabetic nephropathy |
| DOTAP | dioleoyl-3-trimethylammonium propane |
| DPPH | 1,1-diphenyl-2-picrylhydrazyl |
| DSS | dextran sodium sulfate |
| EE | encapsulation efficiency |
| EPC | egg phosphatidylcholine |
| FDA | Food and Drug Administration |
| GIT | gastrointestinal tract |
| GPx | glutathione peroxidases |
| GSH | glutathione |
| GST | glutathione S-transferases |
| HA | hyaluronic acid |
| HEI-OC1 | house ear institute organ of corti 1 cells |
| HO-1 | heme oxygenase-1 |
| HRMCs | Human Renal Mesangial Cells |
| IBD | inflammatory bowel disease |
| IC50 | half inhibition concentration |
| IL | interleukin |
| JNK | c-Jun N-terminal kinase |
| Keap1 | Kelch-like ECH- associated protein 1 |
| LA | lactobionic acid |
| LC | loading capacity |
| LD50 | lethal dose 50 |
| LPS | lipopolysaccharide |
| MAPKs | mitogen-activated protein kinases |
| MDA | malondialdehyde |
| MMP | matrix metalloproteinases |
| mPEG-PLA | methoxy (polyethylene glycol) |
| MPO | myeloperoxidase |
| MRP | multidrug resistance-associated protein |
| NAC | nanostructured archaeolipid carriers |
| NF-κβ | Nuclear factor kappa-light-chain-enhancer of activated B cells |
| Np | nanoparticle |
| NQO1 | dehydrogenase quinone 1 |
| Nrf2 | Nuclear factor erythroid-related factor 2 |
| NSAID | nonsteroidal anti-inflammatory drug |
| OA | osteoarthritis |
| OS | oxidative stress |
| PA | phthalic anhydride |
| PA | polar archaeolipids |
| PD | pharmacodynamics |
| PEG | poly (ethylene glycol) |
| PGPMe | 3′-sn-glycerol-1′-methylphosphate |
| PK | pharmacokinetics |
| PPS | poly (propylene sulphide) |
| Prxs | peroxiredoxins |
| PS | psoriasis |
| PTK | polythioketal |
| RD | Rhodamine 123 |
| RNS | reactive nitrogen species |
| ROS | reactive oxygen species |
| SC | stratum corneum |
| SLN | solid lipid nanoparticles |
| SOD | superoxide dismutase |
| SPC | soybean phosphatidylcholine |
| SR-A1 | Scavenger receptors class A |
| TNF-α | Tumor Necrosis Factor-alpha |
| TPP | triphenylphosphonium bromide |
| Trx | thioredoxin |
| TrxR | thioredoxin reductase |
References
- Aziz, E.; Batool, R.; Akhtar, W.; Rehman, S.; Shahzad, T.; Malik, A.; Shariati, M.A.; Laishevtcev, A.; Plygun, S.; Heydari, M.; et al. Xanthophyll: Health Benefits and Therapeutic Insights. Life Sci. 2020, 240, 117104. [Google Scholar] [CrossRef] [PubMed]
- Britton, G. Functions of Intact Carotenoids. In Carotenoids; Springer: Berlin/Heidelberg, Germany, 2008; pp. 189–212. [Google Scholar] [CrossRef]
- Lawler, T.; Liu, Y.; Christensen, K.; Vajaranant, T.S.; Mares, J. Dietary Antioxidants, Macular Pigment, and Glaucomatous Neurodegeneration: A Review of the Evidence. Nutrients 2019, 11, 1002. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wingerath, T.; Sies, H.; Stahl, W. Xanthophyll Esters in Human Skin. Arch. Biochem. Biophys. 1998, 355, 271–274. [Google Scholar] [CrossRef] [PubMed]
- Dorey, C.K. Carotenoid, Tocopherol, and Retinol Concentrations in Elderly Human Brain Vitamin A Assessment View Project Impact of Zeaxanthin Supplementation on Tissues of Adult Quail View Project Neal Craft Craft Nutrition Consulting. J. Nutr. Health Aging 2004, 8, 156–162. [Google Scholar]
- Cannavale, C.N.; Hassevoort, K.M.; Edwards, C.G.; Thompson, S.V.; Burd, N.A.; Holscher, H.D.; Erdman, J.W.; Cohen, N.J.; Khan, N.A. Serum Lutein Is Related to Relational Memory Performance. Nutrients 2019, 11, 768. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Britton, G.; Khachik, F. Carotenoids in Food BT-Carotenoids: Volume 5: Nutrition and Health; Springer: Berlin/Heidelberg, Germany, 2009; pp. 45–66. [Google Scholar]
- Baldwin, H.; Webster, G.; Stein Gold, L.; Callender, V.; Cook-Bolden, F.E.; Guenin, E. 50 Years of Topical Retinoids for Acne: Evolution of Treatment. Am. J. Clin. Dermatol. 2021, 22, 315–327. [Google Scholar] [CrossRef] [PubMed]
- Kumar Saini, R.; Hariram Nile, S.; Won Park, S. Carotenoids from fruits and vegetables: Chemistry, Analysis, Occurrence, Bioavailability and Biological activities. Carotenoids Fruits Veg. Chem. Anal. Occurr. Bioavailab. Biol. Activ. 2015, 76, 735–750. [Google Scholar] [CrossRef]
- Meléndez-Martínez, A.J.; Britton, G.; Vicario, I.M.; Heredia, F.J. Relationship between the Colour and the Chemical Structure of Carotenoid Pigments. Food Chem. 2007, 101, 1145–1150. [Google Scholar] [CrossRef]
- Carotenoid Database. Available online: http://carotenoiddb.jp/ (accessed on 18 May 2023).
- Yabuzaki, J. Carotenoids Database: Structures, Chemical Fingerprints and Distribution among Organisms. Database 2017, 2017, bax004. [Google Scholar] [CrossRef] [Green Version]
- Fernandes, A.S.; Nascimento, T.C.D.; Jacob-Lopes, E.; De Rosso, V.V.; Zepka, L.Q. Introductory Chapter: Carotenoids—A Brief Overview on Its Structure, Biosynthesis, Synthesis, and Applications. Prog. Carotenoid Res. 2018, 1, 1–17. [Google Scholar] [CrossRef] [Green Version]
- Maoka, T. Carotenoids as Natural Functional Pigments. J. Nat. Med. 2020, 74, 1–16. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dhar, M.K.; Mishra, S.; Bhat, A.; Chib, S.; Kaul, S. Plant Carotenoid Cleavage Oxygenases: Structure-Function Relationships and Role in Development and Metabolism. Brief. Funct. Genomics 2020, 19, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Sun, T.; Li, L. Toward the ‘Golden’ Era: The Status in Uncovering the Regulatory Control of Carotenoid Accumulation in Plants. Plant Sci. 2020, 290, 110331. [Google Scholar] [CrossRef] [PubMed]
- Muzzopappa, F.; Kirilovsky, D. Changing Color for Photoprotection: The Orange Carotenoid Protein. Trends Plant Sci. 2020, 25, 92–104. [Google Scholar] [CrossRef]
- Rivera, S.M.; Canela-Garayoa, R. Analytical Tools for the Analysis of Carotenoids in Diverse Materials. J. Chromatogr. A 2012, 1224, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Grivard, A.; Goubet, I.; de Duarte Filho, L.M.S.; Thiéry, V.; Chevalier, S.; de Oliveira-Junior, R.G.; El Aouad, N.; Guedes da Silva Almeida, J.R.; Sitarek, P.; Quintans-Junior, L.J.; et al. Archaea Carotenoids: Natural Pigments with Unexplored Innovative Potential. Mar. Drugs 2022, 20, 524. [Google Scholar] [CrossRef]
- Britton, G. Carotenoids: Handbook; Springer Science & Business Media: Berlin/Heidelberg, Germany, 2004. [Google Scholar]
- Britton, G.; Liaaen-Jensen, S.; Pfander, H.; Schmidt-Dannert, C. Carotenoids. Volume 3: Biosynthesis and Metabolism. Q. Rev. Biol. 2001, 76, 227–228. [Google Scholar] [CrossRef]
- Blaner, W.S. Vitamin A and Provitamin A Carotenoids. In Present Knowledge in Nutrition; Academic Press: Cambridge, MA, USA, 2020; pp. 73–91. [Google Scholar] [CrossRef]
- Lafountain, A.M.; Prum, R.O.; Frank, H.A. Diversity, Physiology, and Evolution of Avian Plumage Carotenoids and the Role of Carotenoid–Protein Interactions in Plumage Color Appearance. Arch. Biochem. Biophys. 2015, 572, 201–212. [Google Scholar] [CrossRef]
- Duarte, R.C.; Flores, A.A.V.; Stevens, M. Camouflage through Colour Change: Mechanisms, Adaptive Value and Ecological Significance. Philos. Trans. Royal Soc. B Biol. Sci. 2017, 372, 342. [Google Scholar] [CrossRef] [Green Version]
- Carotenoids Market Size, Share, Trends and Industry Analysis. Available online: https://www.marketsandmarkets.com/Market-Reports/carotenoid-market-158421566.html (accessed on 18 May 2023).
- Carotenoids Market Size & Share Analysis—Industry Research Report—Growth Trends. Available online: https://www.mordorintelligence.com/industry-reports/carotenoids-market-industry (accessed on 18 May 2023).
- Carotenoids Market Size to Hit USD 1.84 Billion by 2027. Available online: https://www.fortunebusinessinsights.com/industry-reports/carotenoids-market-100180 (accessed on 18 May 2023).
- Torregrosa-Crespo, J.; Montero, Z.; Fuentes, J.L.; García-Galbis, M.R.; Garbayo, I.; Vílchez, C.; Martínez-Espinosa, R.M. Exploring the Valuable Carotenoids for the Large-Scale Production by Marine Microorganisms. Mar. Drugs 2018, 16, 203. [Google Scholar] [CrossRef] [Green Version]
- Ottinger, M.; Clauss, K.; Kuenzer, C. Aquaculture: Relevance, Distribution, Impacts and Spatial Assessments—A Review. Ocean Coast. Manag. 2016, 119, 244–266. [Google Scholar] [CrossRef]
- Feed Carotenoids Market Size & Share Analysis—Industry Research Report—Growth Trends. Available online: https://www.mordorintelligence.com/industry-reports/global-feed-carotenoids-market-industry (accessed on 18 May 2023).
- Novoveská, L.; Ross, M.E.; Stanley, M.S.; Pradelles, R.; Wasiolek, V.; Sassi, J.F. Microalgal Carotenoids: A Review of Production, Current Markets, Regulations, and Future Direction. Mar. Drugs 2019, 17, 640. [Google Scholar] [CrossRef] [Green Version]
- Amengual, J. Bioactive Properties of Carotenoids in Human Health. Nutrients 2019, 11, 2388. [Google Scholar] [CrossRef] [Green Version]
- Mussagy, C.U.; Dufossé, L. A Review of Natural Astaxanthin Production in a Circular Bioeconomy Context Using Paracoccus Carotinifaciens. Bioresour. Technol. 2023, 369, 128499. [Google Scholar] [CrossRef] [PubMed]
- Sies, H.; Berndt, C.; Jones, D.P. Oxidative Stress. Annu. Rev. Biochem. 2017, 86, 715–748. [Google Scholar] [CrossRef]
- Forman, H.J.; Zhang, H. Targeting Oxidative Stress in Disease: Promise and Limitations of Antioxidant Therapy. Nat. Rev. Drug Discov. 2021, 20, 689–709. [Google Scholar] [CrossRef] [PubMed]
- Hajam, Y.A.; Rani, R.; Ganie, S.Y.; Sheikh, T.A.; Javaid, D.; Qadri, S.S.; Pramodh, S.; Alsulimani, A.; Alkhanani, M.F.; Harakeh, S.; et al. Oxidative Stress in Human Pathology and Aging: Molecular Mechanisms and Perspectives. Cells 2022, 11, 552. [Google Scholar] [CrossRef]
- Ursini, F.; Maiorino, M.; Forman, H.J. Redox Homeostasis: The Golden Mean of Healthy Living. Redox. Biol. 2016, 8, 205–215. [Google Scholar] [CrossRef]
- Anderson, M.T.; Staal, F.J.T.; Gitler, C.; Herzenberg, L.A.; Herzenberg, L.A. Separation of Oxidant-Initiated and Redox-Regulated Steps in the NF-Kappa B Signal Transduction Pathway. Proc. Natl. Acad. Sci. USA 1994, 91, 11527–11531. [Google Scholar] [CrossRef] [Green Version]
- Flohé, L.; Brigelius-Flohé, R.; Saliou, C.; Traber, M.G.; Packer, L. Redox Regulation of NF-Kappa B Activation. Free Radic. Biol. Med. 1997, 22, 1115–1126. [Google Scholar] [CrossRef]
- Wardyn, J.D.; Ponsford, A.H.; Sanderson, C.M. Dissecting Molecular Cross-Talk between Nrf2 and NF-ΚB Response Pathways. Biochem. Soc. Trans. 2015, 43, 621. [Google Scholar] [CrossRef] [Green Version]
- Ahmed, S.M.U.; Luo, L.; Namani, A.; Wang, X.J.; Tang, X. Nrf2 Signaling Pathway: Pivotal Roles in Inflammation. Biochim. Biophys. Acta Mol. Basis Dis. 2017, 1863, 585–597. [Google Scholar] [CrossRef]
- Cuadrado, A.; Manda, G.; Hassan, A.; Alcaraz, M.J.; Barbas, C.; Daiber, A.; Ghezzi, P.; León, R.; López, M.G.; Oliva, B.; et al. Transcription Factor NRF2 as a Therapeutic Target for Chronic Diseases: A Systems Medicine Approach. Pharmacol. Rev. 2018, 70, 348–383. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gao, W.; Guo, L.; Yang, Y.; Wang, Y.; Xia, S.; Gong, H.; Zhang, B.K.; Yan, M. Dissecting the Crosstalk Between Nrf2 and NF-ΚB Response Pathways in Drug-Induced Toxicity. Front. Cell Dev. Biol. 2022, 9, 3940. [Google Scholar] [CrossRef] [PubMed]
- Moore, A.L.; Joy, A.; Tom, R.; Gust, D.; Moore, T.A.; Bensasson, R.V.; Land, E.J. Photoprotection by Carotenoids During Photosynthesis: Motional Dependence of Intramolecular Energy Transfer. Science 1982, 216, 982–984. [Google Scholar] [CrossRef] [PubMed]
- Ramel, F.; Birtic, S.; Cuiné, S.; Triantaphylidès, C.; Ravanat, J.L.; Havaux, M. Chemical Quenching of Singlet Oxygen by Carotenoids in Plants. Plant Physiol. 2012, 158, 1267–1278. [Google Scholar] [CrossRef] [Green Version]
- Fiedor, J.; Burda, K. Potential Role of Carotenoids as Antioxidants in Human Health and Disease. Nutrients 2014, 6, 466. [Google Scholar] [CrossRef] [Green Version]
- Jones, D.L.; Baxter, B.K. DNA Repair and Photoprotection: Mechanisms of Overcoming Environmental Ultraviolet Radiation Exposure in Halophilic Archaea. Front. Microbiol. 2017, 8, 1882. [Google Scholar] [CrossRef] [Green Version]
- Yoshimura, K.; Kouyama, T. Structural Role of Bacterioruberin in the Trimeric Structure of Archaerhodopsin-2. J. Mol. Biol. 2008, 375, 1267–1281. [Google Scholar] [CrossRef]
- Fassett, R.G.; Coombes, J.S. Astaxanthin in Cardiovascular Health and Disease. Molecules 2012, 17, 2030. [Google Scholar] [CrossRef]
- Ekpe, L.; Inaku, K.; Ekpe, V.; Lawson Ekpe, C. Antioxidant Effects of Astaxanthin in Various Diseases—A Review. J. Mol. Pathophysiol. 2018, 7, 1–6. [Google Scholar] [CrossRef]
- Capelli, B.; Bagchi, D.; Cysewski, G.R. Synthetic Astaxanthin Is Significantly Inferior to Algal-Based Astaxanthin as an Antioxidant and May Not Be Suitable as a Human Nutraceutical Supplement. Nutrafoods 2014, 12, 145–152. [Google Scholar] [CrossRef]
- Squillaci, G.; Parrella, R.; Carbone, V.; Minasi, P.; La Cara, F.; Morana, A. Carotenoids from the Extreme Halophilic Archaeon Haloterrigena Turkmenica: Identification and Antioxidant Activity. Extremophiles 2017, 21, 933–945. [Google Scholar] [CrossRef]
- Higa, L.H.; Schilrreff, P.; Briski, A.M.; Jerez, H.E.; de Farias, M.A.; Villares Portugal, R.; Romero, E.L.; Morilla, M.J. Bacterioruberin from Haloarchaea plus Dexamethasone in Ultra-Small Macrophage-Targeted Nanoparticles as Potential Intestinal Repairing Agent. Colloids Surf. B Biointerfaces 2020, 19, 110961. [Google Scholar] [CrossRef] [PubMed]
- Sahli, K.; Gomri, M.A.; Esclapez, J.; Gómez-Villegas, P.; Ghennai, O.; Bonete, M.J.; León, R.; Kharroub, K. Bioprospecting and Characterization of Pigmented Halophilic Archaeal Strains from Algerian Hypersaline Environments with Analysis of Carotenoids Produced by Halorubrum Sp. BS2. J. Basic Microbiol. 2020, 60, 624–638. [Google Scholar] [CrossRef]
- Caimi, A.T.; Yasynska, O.; Rivas Rojas, P.C.; Romero, E.L.; Morilla, M.J. Improved Stability and Biological Activity of Bacterioruberin in Nanovesicles. J. Drug. Deliv. Sci. Technol. 2022, 77, 103896. [Google Scholar] [CrossRef]
- Niu, T.; Zhou, J.; Wang, F.; Xuan, R.; Chen, J.; Wu, W.; Chen, H. Safety Assessment of Astaxanthin from Haematococcus Pluvialis: Acute Toxicity, Genotoxicity, Distribution and Repeat-Dose Toxicity Studies in Gestation Mice. Regul. Toxicol. Pharmacol. 2020, 115, 104695. [Google Scholar] [CrossRef] [PubMed]
- Wu, Q.; Zhang, X.S.; Wang, H.D.; Zhang, X.; Yu, Q.; Li, W.; Zhou, M.L.; Wang, X.L. Astaxanthin Activates Nuclear Factor Erythroid-Related Factor 2 and the Antioxidant Responsive Element (Nrf2-ARE) Pathway in the Brain after Subarachnoid Hemorrhage in Rats and Attenuates Early Brain Injury. Mar. Drugs 2014, 12, 6125–6141. [Google Scholar] [CrossRef]
- Ma, H.; Chen, S.; Xiong, H.; Wang, M.; Hang, W.; Zhu, X.; Zheng, Y.; Ge, B.; Li, R.; Cui, H. Astaxanthin from Haematococcus Pluvialis Ameliorates the Chemotherapeutic Drug (Doxorubicin) Induced Liver Injury through the Keap1/Nrf2/HO-1 Pathway in Mice. Food Funct. 2020, 11, 4659–4671. [Google Scholar] [CrossRef]
- Cui, G.; Li, L.; Xu, W.; Wang, M.; Jiao, D.; Yao, B.; Xu, K.; Chen, Y.; Yang, S.; Long, M.; et al. Astaxanthin Protects Ochratoxin A-Induced Oxidative Stress and Apoptosis in the Heart via the Nrf2 Pathway. Oxid. Med. Cell Longev. 2020, 2020, 7639109. [Google Scholar] [CrossRef] [Green Version]
- Cheng, J.; Eroglu, A. The Promising Effects of Astaxanthin on Lung Diseases. Adv. Nutr. 2021, 12, 850–864. [Google Scholar] [CrossRef]
- Hussein, G.; Nakagawa, T.; Goto, H.; Shimada, Y.; Matsumoto, K.; Sankawa, U.; Watanabe, H. Astaxanthin Ameliorates Features of Metabolic Syndrome in SHR/NDmcr-Cp. Life Sci. 2007, 80, 522–529. [Google Scholar] [CrossRef] [PubMed]
- Zhuge, F.; Ni, Y.; Wan, C.; Liu, F.; Fu, Z. Anti-Diabetic Effects of Astaxanthin on an STZ-Induced Diabetic Model in Rats. Endocr. J. 2021, 68, 451–459. [Google Scholar] [CrossRef] [PubMed]
- Fang, Q.; Guo, S.; Zhou, H.; Han, R.; Wu, P.; Han, C. Astaxanthin Protects against Early Burn-Wound Progression in Rats by Attenuating Oxidative Stress-Induced Inflammation and Mitochondria-Related Apoptosis. Sci. Rep. 2017, 7, 41440. [Google Scholar] [CrossRef] [Green Version]
- Fan, Q.; Chen, Z.; Wu, Y.; Zhu, J.; Yu, Z. Study on the Enhancement of Immune Function of Astaxanthin from Haematococcus Pluvialis. Foods 2021, 10, 1847. [Google Scholar] [CrossRef]
- Hou, J.; Cui, H.L. In Vitro Antioxidant, Antihemolytic, and Anticancer Activity of the Carotenoids from Halophilic Archaea. Curr. Microbiol. 2018, 75, 266–271. [Google Scholar] [CrossRef] [PubMed]
- Lizama, C.; Romero-Parra, J.; Andrade, D.; Riveros, F.; Bórquez, J.; Ahmed, S.; Venegas-Salas, L.; Cabalín, C.; Simirgiotis, M.J. Analysis of Carotenoids in Haloarchaea Species from Atacama Saline Lakes by High Resolution Uhplc-q-Orbitrap-Mass Spectrometry: Antioxidant Potential and Biological Effect on Cell Viability. Antioxidants 2021, 10, 1230. [Google Scholar] [CrossRef] [PubMed]
- Gómez-villegas, P.; Vigara, J.; Vila, M.; Varela, J.; Barreira, L.; Léon, R. Antioxidant, Antimicrobial, and Bioactive Potential of Two New Haloarchaeal Strains Isolated from Odiel Salterns (Southwest Spain). Biology 2020, 9, 298. [Google Scholar] [CrossRef] [PubMed]
- Giani, M.; Gervasi, L.; Loizzo, M.R.; Martínez-Espinosa, R.M. Carbon Source Influences Antioxidant, Antiglycemic, and Antilipidemic Activities of Haloferax Mediterranei Carotenoid Extracts. Mar. Drugs 2022, 20, 659. [Google Scholar] [CrossRef] [PubMed]
- Zalazar, L.; Pagola, P.; Miró, M.V.; Churio, M.S.; Cerletti, M.; Martínez, C.; Iniesta-Cuerda, M.; Soler, A.J.; Cesari, A.; De Castro, R. Bacterioruberin Extracts from a Genetically Modified Hyperpigmented Haloferax Volcanii Strain: Antioxidant Activity and Bioactive Properties on Sperm Cells. J. Appl. Microbiol. 2019, 126, 796–810. [Google Scholar] [CrossRef]
- Fariq, A.; Yasmin, A.; Jamil, M. Production, Characterization and Antimicrobial Activities of Bio-Pigments by Aquisalibacillus Elongatus MB592, Salinicoccus Sesuvii MB597, and Halomonas Aquamarina MB598 Isolated from Khewra Salt Range, Pakistan. Extremophiles 2019, 23, 435–449. [Google Scholar] [CrossRef] [PubMed]
- Simioni, Y.R.; Perez, N.S.; Barbosa, L.R.S.; Perez, A.P.; Schilrreff, P.; Romero, E.L.; Morilla, M.J. Enhancing the Anti-Psoriatic Activity of Vitamin D3 Employing Nanostructured Archaeolipid Carriers. J. Drug Deliv. Sci. Technol. 2022, 73, 103455. [Google Scholar] [CrossRef]
- Raza, S.H.A.; Naqvi, S.R.Z.; Abdelnour, S.A.; Schreurs, N.; Mohammedsaleh, Z.M.; Khan, I.; Shater, A.F.; Abd El-Hack, M.E.; Khafaga, A.F.; Quan, G.; et al. Beneficial Effects and Health Benefits of Astaxanthin Molecules on Animal Production: A Review. Res. Vet. Sci. 2021, 138, 69–78. [Google Scholar] [CrossRef]
- Ursoniu, S.; Sahebkar, A.; Serban, M.C.; Banach, M. Lipid Profile and Glucose Changes after Supplementation with Astaxanthin: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Arch. Med. Sci. 2015, 11, 253–266. [Google Scholar] [CrossRef]
- Hormozi, M.; Ghoreishi, S.; Baharvand, P. Astaxanthin Induces Apoptosis and Increases Activity of Antioxidant Enzymes in LS-180 Cells. Artif. Cells Nanomed. Biotechnol. 2019, 47, 891–895. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lai, T.T.; Yang, C.M.; Yang, C.H. Astaxanthin Protects Retinal Photoreceptor Cells against High Glucose-Induced Oxidative Stress by Induction of Antioxidant Enzymes via the PI3K/Akt/Nrf2 Pathway. Antioxidants 2020, 9, 729. [Google Scholar] [CrossRef]
- Davinelli, S.; Saso, L.; D’angeli, F.; Calabrese, V.; Intrieri, M.; Scapagnini, G. Astaxanthin as a Modulator of Nrf2, NF-ΚB, and Their Crosstalk: Molecular Mechanisms and Possible Clinical Applications. Molecules 2022, 27, 502. [Google Scholar] [CrossRef]
- Zarneshan, S.N.; Fakhri, S.; Farzaei, M.H.; Khan, H.; Saso, L. Astaxanthin Targets PI3K/Akt Signaling Pathway toward Potential Therapeutic Applications. Food Chem. Toxicol. 2020, 145, 111714. [Google Scholar] [CrossRef]
- Kishimoto, Y.; Yoshida, H.; Kondo, K. Potential Anti-Atherosclerotic Properties of Astaxanthin. Mar. Drugs 2016, 14, 35. [Google Scholar] [CrossRef]
- Kohandel, Z.; Farkhondeh, T.; Aschner, M.; Pourbagher-Shahri, A.M.; Samarghandian, S. Anti-Inflammatory Action of Astaxanthin and Its Use in the Treatment of Various Diseases. Biomed. Pharmacother. 2022, 145, 112179. [Google Scholar] [CrossRef]
- Hegazy, G.E.; Abu-Serie, M.M.; Abo-Elela, G.M.; Ghozlan, H.; Sabry, S.A.; Soliman, N.A.; Abdel-Fattah, Y.R. In Vitro Dual (Anticancer and Antiviral) Activity of the Carotenoids Produced by Haloalkaliphilic Archaeon Natrialba sp. M6. Sci. Rep. 2020, 10, 5986. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Katagiri, M.; Satoh, A.; Tsuji, S.; Shirasawa, T. Effects of Astaxanthin-Rich Haematococcus Pluvialis Extract on Cognitive Function: A Randomised, Double-Blind, Placebo-Controlled Study. J. Clin. Biochem. Nutr. 2012, 51, 102–107. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Giannaccare, G.; Pellegrini, M.; Senni, C.; Bernabei, F.; Scorcia, V.; Cicero, A.F.G. Clinical Applications of Astaxanthin in the Treatment of Ocular Diseases: Emerging Insights. Mar. Drugs 2020, 18, 239. [Google Scholar] [CrossRef]
- Chalyk, N.E.; Klochkov, V.A.; Bandaletova, T.Y.; Kyle, N.H.; Petyaev, I.M. Continuous Astaxanthin Intake Reduces Oxidative Stress and Reverses Age-Related Morphological Changes of Residual Skin Surface Components in Middle-Aged Volunteers. Nutr. Res. 2017, 48, 40–48. [Google Scholar] [CrossRef]
- Del Río, E.; Acién, F.G.; García-Malea, M.C.; Rivas, J.; Molina-Grima, E.; Guerrero, M.G. Efficiency Assessment of the One-Step Production of Astaxanthin by the Microalga Haematococcus Pluvialis. Biotechnol. Bioeng. 2008, 100, 397–402. [Google Scholar] [CrossRef] [Green Version]
- Molino, A.; Iovine, A.; Casella, P.; Mehariya, S.; Chianese, S.; Cerbone, A.; Rimauro, J.; Musmarra, D. Microalgae Characterization for Consolidated and New Application in Human Food, Animal Feed and Nutraceuticals. Int. J. Environ. Res. Public Health 2018, 15, 2436. [Google Scholar] [CrossRef] [Green Version]
- Ambati, R.R.; Moi, P.S.; Ravi, S.; Aswathanarayana, R.G. Astaxanthin: Sources, Extraction, Stability, Biological Activities and Its Commercial Applications—A Review. Mar. Drugs 2014, 12, 128–152. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stachowiak, B.; Szulc, P. Astaxanthin for the Food Industry. Molecules 2021, 26, 2666. [Google Scholar] [CrossRef]
- Yang, H.E.; Yu, B.S.; Sim, S.J. Enhanced Astaxanthin Production of Haematococcus Pluvialis Strains Induced Salt and High Light Resistance with Gamma Irradiation. Bioresour. Technol. 2023, 372, 128651. [Google Scholar] [CrossRef]
- Higuera-Ciapara, I.; Félix-Valenzuela, L.; Goycoolea, F.M. Astaxanthin: A Review of Its Chemistry and Applications. Crit. Rev. Food Sci. Nutr. 2006, 46, 185–196. [Google Scholar] [CrossRef]
- Hwang, S.W.; Choi, H.I.; Sim, S.J. Acidic Cultivation of Haematococcus Pluvialis for Improved Astaxanthin Production in the Presence of a Lethal Fungus. Bioresour. Technol. 2019, 278, 138–144. [Google Scholar] [CrossRef]
- Jannel, S.; Caro, Y.; Bermudes, M.; Petit, T. Novel Insights into the Biotechnological Production of Haematococcus Pluvialis-Derived Astaxanthin: Advances and Key Challenges to Allow Its Industrial Use as Novel Food Ingredient. J. Mar. Sci. Eng. 2020, 8, 789. [Google Scholar] [CrossRef]
- Riccioni, G.; D’Orazio, N.; Franceschelli, S.; Speranza, L. Marine Carotenoids and Cardiovascular Risk Markers. Mar. Drugs 2011, 9, 1166. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Le Goff, M.; Le Ferrec, E.; Mayer, C.; Mimouni, V.; Lagadic-Gossmann, D.; Schoefs, B.; Ulmann, L. Microalgal Carotenoids and Phytosterols Regulate Biochemical Mechanisms Involved in Human Health and Disease Prevention. Biochimie 2019, 167, 106–118. [Google Scholar] [CrossRef] [PubMed]
- Oslan, S.N.H.; Tan, J.S.; Oslan, S.N.; Matanjun, P.; Mokhtar, R.A.M.; Shapawi, R.; Huda, N. Haematococcus Pluvialis as a Potential Source of Astaxanthin with Diverse Applications in Industrial Sectors: Current Research and Future Directions. Molecules 2021, 26, 6470. [Google Scholar] [CrossRef]
- Faraone, I.; Sinisgalli, C.; Ostuni, A.; Armentano, M.F.; Carmosino, M.; Milella, L.; Russo, D.; Labanca, F.; Khan, H. Astaxanthin Anticancer Effects Are Mediated through Multiple Molecular Mechanisms: A Systematic Review. Pharmacol. Res. 2020, 155, 104689. [Google Scholar] [CrossRef]
- Yamamoto, M.; Kensler, T.W.; Motohashi, H. The KEAP1-NRF2 System: A Thiol-Based Sensor-Effector Apparatus for Maintaining Redox Homeostasis. Physiol. Rev. 2018, 98, 1169–1203. [Google Scholar] [CrossRef] [Green Version]
- Sedlak, T.W.; Saleh, M.; Higginson, D.S.; Paul, B.D.; Juluri, K.R.; Snyder, S.H. Bilirubin and Glutathione Have Complementary Antioxidant and Cytoprotective Roles. Proc. Natl. Acad. Sci. USA 2009, 106, 5171–5176. [Google Scholar] [CrossRef] [Green Version]
- Pedruzzi, L.M.; Stockler-Pinto, M.B.; Leite, M.; Mafra, D. Nrf2-Keap1 System versus NF-ΚB: The Good and the Evil in Chronic Kidney Disease? Biochimie 2012, 94, 2461–2466. [Google Scholar] [CrossRef]
- Kobayashi, E.H.; Suzuki, T.; Funayama, R.; Nagashima, T.; Hayashi, M.; Sekine, H.; Tanaka, N.; Moriguchi, T.; Motohashi, H.; Nakayama, K.; et al. Nrf2 Suppresses Macrophage Inflammatory Response by Blocking Proinflammatory Cytokine Transcription. Nat. Commun. 2016, 7, 11624. [Google Scholar] [CrossRef] [Green Version]
- Li, M.Y.; Sun, L.; Niu, X.T.; Chen, X.M.; Tian, J.X.; Kong, Y.D.; Wang, G.Q. Astaxanthin Protects Lipopolysaccharide-Induced Inflammatory Response in Channa Argus through Inhibiting NF-ΚB and MAPKs Signaling Pathways. Fish Shellfish Immunol. 2019, 86, 280–286. [Google Scholar] [CrossRef] [PubMed]
- Song, X.; Wang, B.; Lin, S.; Jing, L.; Mao, C.; Xu, P.; Lv, C.; Liu, W.; Zuo, J. Astaxanthin Inhibits Apoptosis in Alveolar Epithelial Cells Type II in Vivo and in Vitro through the ROS-Dependent Mitochondrial Signalling Pathway. J. Cell Mol. Med. 2014, 18, 2198–2212. [Google Scholar] [CrossRef]
- Honda, M.; Kageyama, H.; Hibino, T.; Ichihashi, K.; Takada, W.; Goto, M. Isomerization of Commercially Important Carotenoids (Lycopene, β-Carotene, and Astaxanthin) by Natural Catalysts: Isothiocyanates and Polysulfides. J. Agric. Food Chem. 2020, 68, 3228–3237. [Google Scholar] [CrossRef] [PubMed]
- Milon, A.; Wolff, G.; Ourisson, G.; Nakatani, Y. Organization of Carotenoid-Phospholipid Bilayer Systems. Incorporation of Zeaxanthin, Astaxanthin, and Their C50 Homologues into Dimyristoylphosphatidylcholine Vesicles. Helv. Chim. Acta 1986, 69, 12–24. [Google Scholar] [CrossRef]
- Wisniewska, A.; Subczynski, W.K. Effects of Polar Carotenoids on the Shape of the Hydrophobic Barrier of Phospholipid Bilayers. Biochim. Biophys. Acta 1998, 1368, 235–246. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Woodall, A.A.; Britton, G.; Jackson, M.J. Carotenoids and Protection of Phospholipids in Solution or in Liposomes against Oxidation by Peroxyl Radicals: Relationship between Carotenoid Structure and Protective Ability. Biochim. Biophys. Acta Gen. Subj. 1997, 1336, 575–586. [Google Scholar] [CrossRef]
- Goto, S.; Kogure, K.; Abe, K.; Kimata, Y.; Kitahama, K.; Yamashita, E.; Terada, H. Efficient Radical Trapping at the Surface and inside the Phospholipid Membrane Is Responsible for Highly Potent Antiperoxidative Activity of the Carotenoid Astaxanthin. Biochim. Biophys. Acta Biomembr. 2001, 1512, 251–258. [Google Scholar] [CrossRef] [Green Version]
- Snell, T.W.; Carberry, J. Astaxanthin Bioactivity Is Determined by Stereoisomer Composition and Extraction Method. Nutrients 2022, 14, 1522. [Google Scholar] [CrossRef]
- Kuroki, T.; Ikeda, S.; Okada, T.; Maoka, T.; Kitamura, A.; Sugimoto, M.; Kume, S. Astaxanthin Ameliorates Heat Stress-Induced Impairment of Blastocyst Development In Vitro: –Astaxanthin Colocalization with and Action on Mitochondria. J. Assist. Reprod. Genet. 2013, 30, 623. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Z.W.; Xu, X.C.; Liu, T.; Yuan, S. Mitochondrion-Permeable Antioxidants to Treat ROS-Burst-Mediated Acute Diseases. Oxid. Med. Cell Longev. 2016, 2016, 6859523. [Google Scholar] [CrossRef] [Green Version]
- Liu, P.H.; Aoi, W.; Takami, M.; Terajima, H.; Tanimura, Y.; Naito, Y.; Itoh, Y.; Yoshikawa, T. The Astaxanthin-Induced Improvement in Lipid Metabolism during Exercise Is Mediated by a PGC-1α Increase in Skeletal Muscle. J. Clin. Biochem. Nutr. 2014, 54, 86. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, S.H.; Kim, H. Inhibitory Effect of Astaxanthin on Oxidative Stress-Induced Mitochondrial Dysfunction-A Mini-Review. Nutrients 2018, 10, 1137. [Google Scholar] [CrossRef] [Green Version]
- Landon, R.; Gueguen, V.; Petite, H.; Letourneur, D.; Pavon-Djavid, G.; Anagnostou, F. Impact of Astaxanthin on Diabetes Pathogenesis and Chronic Complications. Mar. Drugs 2020, 18, 357. [Google Scholar] [CrossRef] [PubMed]
- Ding, L.; Yang, J.; Yin, K.; Cheng, H.; Li, J.; Xue, C. The Spatial Arrangement of Astaxanthin in Bilayers Greatly Influenced the Structural Stability of DPPC Liposomes. Colloids Surf. B Biointerfaces 2022, 212, 112383. [Google Scholar] [CrossRef] [PubMed]
- Lin, Z.; Li, F.; Zhang, Y.; Tan, X.; Luo, P.; Liu, H. Analysis of Astaxanthin Molecular Targets Based on Network Pharmacological Strategies. J. Food Biochem. 2021, 45, e13717. [Google Scholar] [CrossRef] [PubMed]
- Régnier, P.; Bastias, J.; Rodriguez-Ruiz, V.; Caballero-Casero, N.; Caballo, C.; Sicilia, D.; Fuentes, A.; Maire, M.; Crepin, M.; Letourneur, D.; et al. Astaxanthin from Haematococcus Pluvialis Prevents Oxidative Stress on Human Endothelial Cells without Toxicity. Mar. Drugs 2015, 13, 2857. [Google Scholar] [CrossRef] [Green Version]
- Nishino, A.; Maoka, T.; Yasui, H. Analysis of Reaction Products of Astaxanthin and Its Acetate with Reactive Oxygen Species Using LC/PDA ESI-MS and ESR Spectrometry. Tetrahedron Lett. 2016, 57, 1967–1970. [Google Scholar] [CrossRef]
- Aoi, W.; Maoka, T.; Abe, R.; Fujishita, M.; Tominaga, K. Comparison of the Effect of Non-Esterified and Esterified Astaxanthins on Endurance Performance in Mice. J. Clin. Biochem. Nutr. 2018, 62, 161. [Google Scholar] [CrossRef] [Green Version]
- Hempel, J.; Schädle, C.N.; Leptihn, S.; Carle, R.; Schweiggert, R.M. Structure Related Aggregation Behavior of Carotenoids and Carotenoid Esters. J. Photochem. Photobiol. A Chem. 2016, 317, 161–174. [Google Scholar] [CrossRef]
- Billsten, H.H.; Sundström, V.; Polívka, T. Self-Assembled Aggregates of the Carotenoid Zeaxanthin: Time-Resolved Study of Excited States. J. Phys. Chem. A 2005, 109, 1521–1529. [Google Scholar] [CrossRef]
- Dai, M.; Li, C.; Yang, Z.; Sui, Z.; Li, J.; Dong, P.; Liang, X. The Astaxanthin Aggregation Pattern Greatly Influences Its Antioxidant Activity: A Comparative Study in Caco-2 Cells. Antioxidants 2020, 9, 126. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Britton, G. Carotenoid Research: History and New Perspectives for Chemistry in Biological Systems. Biochim. Biophys. Acta Mol. Cell Biol. Lipids 2020, 1865, 158699. [Google Scholar] [CrossRef] [PubMed]
- McNulty, H.P.; Byun, J.; Lockwood, S.F.; Jacob, R.F.; Mason, R.P. Differential Effects of Carotenoids on Lipid Peroxidation Due to Membrane Interactions: X-Ray Diffraction Analysis. Biochim. Biophys. Acta 2007, 1768, 167–174. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Böhm, F.; Edge, R.; Truscott, G. Interactions of Dietary Carotenoids with Activated (Singlet) Oxygen and Free Radicals: Potential Effects for Human Health. Mol. Nutr. Food Res. 2012, 56, 205–216. [Google Scholar] [CrossRef]
- Hama, S.; Uenishi, S.; Yamada, A.; Ohgita, T.; Tsuchiya, H.; Yamashita, E.; Kogure, K. Scavenging of Hydroxyl Radicals in Aqueous Solution by Astaxanthin Encapsulated in Liposomes. Biol. Pharm. Bull. 2012, 35, 2238–2242. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kamezaki, C.; Nakashima, A.; Yamada, A.; Uenishi, S.; Ishibashi, H.; Shibuya, N.; Hama, S.; Hosoi, S.; Yamashita, E.; Kogure, K. Synergistic Antioxidative Effect of Astaxanthin and Tocotrienol by Co-Encapsulated in Liposomes. J. Clin. Biochem. Nutr. 2016, 59, 100–106. [Google Scholar] [CrossRef] [Green Version]
- Martin, H.D.; Ruck, C.; Schmidt, M.; Sell, S.; Beutner, S.; Mayer, B.; Walsh, R. Chemistry of Carotenoid Oxidation and Free Radical Reactions. Pure Appl. Chem. 1999, 71, 2253–2262. [Google Scholar] [CrossRef]
- Kim, S.H.; Kim, H. Astaxanthin Modulation of Signaling Pathways That Regulate Autophagy. Mar. Drugs 2019, 17, 546. [Google Scholar] [CrossRef] [Green Version]
- Woese, C.R.; Fox, G.E. Phylogenetic Structure of the Prokaryotic Domain: The Primary Kingdoms. Proc. Natl. Acad. Sci. USA 1977, 74, 5088–5090. [Google Scholar] [CrossRef] [Green Version]
- Oren, A. Life at High Salt Concentrations. Prokaryotes 2006, 3, 263–282. [Google Scholar] [CrossRef]
- Oren, A. Diversity of Halophilic Microorganisms: Environments, Phylogeny, Physiology, and Applications. J. Ind. Microbiol. Biotechnol. 2002, 28, 56–63. [Google Scholar] [CrossRef] [PubMed]
- Gramain, A.; Díaz, G.C.; Demergasso, C.; Lowenstein, T.K.; Mcgenity, T.J. Archaeal Diversity along a Subterranean Salt Core from the Salar Grande (Chile). Environ. Microbiol. 2011, 13, 2105–2121. [Google Scholar] [CrossRef] [PubMed]
- Fang, C.J.; Ku, K.L.; Lee, M.H.; Su, N.W. Influence of Nutritive Factors on C50 Carotenoids Production by Haloferax Mediterranei ATCC 33500 with Two-Stage Cultivation. Bioresour. Technol. 2010, 101, 6487–6493. [Google Scholar] [CrossRef]
- Flores, N.; Hoyos, S.; Venegas, M.; Galetović, A.; Zúñiga, L.M.; Fábrega, F.; Paredes, B.; Salazar-Ardiles, C.; Vilo, C.; Ascaso, C.; et al. Haloterrigena sp. Strain SGH1, a Bacterioruberin-Rich, Perchlorate-Tolerant Halophilic Archaeon Isolated from Halite Microbial Communities, Atacama Desert, Chile. Front. Microbiol. 2020, 11, 324. [Google Scholar] [CrossRef]
- Flegler, A.; Runzheimer, K.; Kombeitz, V.; Mänz, A.T.; von Heilborn, D.H.; Etzbach, L.; Schieber, A.; Hölzl, G.; Hüttel, B.; Woehle, C.; et al. Arthrobacter Bussei sp. nov., a Pink-Coloured Organism Isolated from Cheese Made of Cow’s Milk. Int. J. Syst. Evol. Microbiol. 2020, 70, 3027–3036. [Google Scholar] [CrossRef] [PubMed]
- Flegler, A.; Lipski, A. The C50 Carotenoid Bacterioruberin Regulates Membrane Fluidity in Pink-Pigmented Arthrobacter Species. Arch. Microbiol. 2022, 204, 70. [Google Scholar] [CrossRef] [PubMed]
- Mandelli, F.; Miranda, V.S.; Rodrigues, E.; Mercadante, A.Z. Identification of Carotenoids with High Antioxidant Capacity Produced by Extremophile Microorganisms. World J. Microbiol. Biotechnol. 2012, 28, 1781–1790. [Google Scholar] [CrossRef]
- Lazrak, T.; Wolff, G.; Albrecht, A.M.; Nakatani, Y.; Ourisson, G.; Kates, M. Bacterioruberins Reinforce Reconstituted Halobacterium Lipid Membranes. Biochim. Biophys. Acta (BBA)—Biomembr. 1988, 939, 160–162. [Google Scholar] [CrossRef]
- Shahmohammadi, H.R.; Asgarani, E.; Terato, H.; Saito, T.; Ohyama, Y.; Gekko, K.; Yamamoto, O.; Ide, H. Protective Roles of Bacterioruberin and Intracellular KCl in the Resistance of Halobacterium Salinarium against DNA-Damaging Agents. J. Radiat. Res. 1998, 39, 251–262. [Google Scholar] [CrossRef] [Green Version]
- Will Chen, C.; Hsu, S.H.; Lin, M.T.; Hsu, Y.H. Mass Production of C50 Carotenoids by Haloferax Mediterranei in Using Extruded Rice Bran and Starch under Optimal Conductivity of Brined Medium. Bioprocess. Biosyst. Eng. 2015, 38, 2361–2367. [Google Scholar] [CrossRef]
- Yim, K.J.; Kwon, J.; Cha, I.T.; Oh, K.S.; Song, H.S.; Lee, H.W.; Rhee, J.K.; Song, E.J.; Rho, J.R.; Seo, M.L.; et al. Occurrence of Viable, Red-Pigmented Haloarchaea in the Plumage of Captive Flamingoes. Sci. Rep. 2015, 5, 16425. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Martínez-Espinosa, R.M.; Torregrosa-Crespo, J.; Martínez-Espinosa, R.M.; Torregrosa-Crespo, J. Haloarchaea May Contribute to the Colour of Avian Plumage in Marine Ecosystems. In Birds—Challenges and Opportunities for Business, Conservation and Research; IntechOpen: London, UK, 2021. [Google Scholar] [CrossRef]
- Lopes-Dos-Santos, R.M.A.; De Troch, M.; Bossier, P.; Van Stappen, G. Archivory in Hypersaline Aquatic Environments: Haloarchaea as a Dietary Source for the Brine Shrimp Artemia. FEMS Microbiol. Ecol. 2019, 95, fiz178. [Google Scholar] [CrossRef]
- Sui, L.; Ren, B.; Wang, S.; Gao, M.; Van Stappen, G. Archaea Haloferax Supplementation Improves Artemia Biomass Production in Hypersaline Conditions. Aquaculture 2020, 528, 735540. [Google Scholar] [CrossRef]
- Xie, W.; Ma, Y.; Ren, B.; Gao, M.; Sui, L. Artemia Nauplii Enriched with Archaea Halorubrum Increased Survival and Challenge Tolerance of Litopenaeus Vannamei Postlarvae. Aquaculture 2021, 533, 736087. [Google Scholar] [CrossRef]
- Geesink, P.; Ettema, T.J.G. The Human Archaeome in Focus. Nat. Microbiol. 2021, 7, 10–11. [Google Scholar] [CrossRef]
- Ourisson, G.; Nakatani, Y. Bacterial Carotenoids as Membrane Reinforcers: A General Role for Polyterpenoids: Membrane Stabilization. In Carotenoids; Springer: New York, NY, USA, 1989; pp. 237–245. [Google Scholar] [CrossRef]
- Burton, G.W.; Ingold, K.U. Beta-Carotene: An Unusual Type of Lipid Antioxidant. Science 1984, 224, 569–573. [Google Scholar] [CrossRef]
- Koga, Y.; Morii, H. Recent Advances in Structural Research on Ether Lipids from Archaea Including Comparative and Physiological Aspects. Biosci. Biotechnol. Biochem. 2005, 69, 2019–2034. [Google Scholar] [CrossRef] [Green Version]
- Hu, I.C. Production of Potential Coproducts from Microalgae. In Biomass, Biofuels, Biochemicals: Biofuels from Algae, 2nd ed.; Elsevier: Amsterdam, The Netherlands, 2019; pp. 345–358. [Google Scholar] [CrossRef]
- Khoo, K.S.; Lee, S.Y.; Ooi, C.W.; Fu, X.; Miao, X.; Ling, T.C.; Show, P.L. Recent Advances in Biorefinery of Astaxanthin from Haematococcus Pluvialis. Bioresour. Technol. 2019, 288, 121606. [Google Scholar] [CrossRef]
- Oren, A. The Microbiology of Red Brines. Adv. Appl. Microbiol. 2020, 113, 57–110. [Google Scholar] [CrossRef]
- Rodrigo-Baños, M.; Garbayo, I.; Vílchez, C.; Bonete, M.J.; Martínez-Espinosa, R.M. Carotenoids from Haloarchaea and Their Potential in Biotechnology. Mar. Drugs 2015, 13, 5508–5532. [Google Scholar] [CrossRef] [Green Version]
- Markou, G.; Nerantzis, E. Microalgae for High-Value Compounds and Biofuels Production: A Review with Focus on Cultivation under Stress Conditions. Biotechnol. Adv. 2013, 31, 1532–1542. [Google Scholar] [CrossRef] [PubMed]
- Calegari-Santos, R.; Diogo, R.A.; Fontana, J.D.; Bonfim, T.M.B. Carotenoid Production by Halophilic Archaea under Different Culture Conditions. Curr. Microbiol. 2016, 72, 641–651. [Google Scholar] [CrossRef]
- Pfeifer, K.; Ergal, İ.; Koller, M.; Basen, M.; Schuster, B.; Rittmann, S.K.M.R. Archaea Biotechnology. Biotechnol. Adv. 2021, 47, 107668. [Google Scholar] [CrossRef] [PubMed]
- Fernandez-Castillo, R.; Rodriguez-Valera, F.; Gonzalez-Ramos, J.; Ruiz-Berraquero, F. Accumulation of Poly (β-Hydroxybutyrate) by Halobacteria. Appl. Environ. Microbiol. 1986, 51, 214. [Google Scholar] [CrossRef] [Green Version]
- Schiraldi, C.; Giuliano, M.; De Rosa, M. Perspectives on Biotechnological Applications of Archaea. Archaea 2002, 1, 75. [Google Scholar] [CrossRef] [Green Version]
- Koller, M. Recycling of Waste Streams of the Biotechnological Poly(Hydroxyalkanoate) Production by Haloferax Mediterranei on Whey. Int. J. Polym. Sci. 2015, 2015, 370164. [Google Scholar] [CrossRef] [Green Version]
- Bhattacharyya, A.; Saha, J.; Haldar, S.; Bhowmic, A.; Mukhopadhyay, U.K.; Mukherjee, J. Production of Poly-3-(Hydroxybutyrate-Co-Hydroxyvalerate) by Haloferax Mediterranei Using Rice-Based Ethanol Stillage with Simultaneous Recovery and Re-Use of Medium Salts. Extremophiles 2014, 18, 463–470. [Google Scholar] [CrossRef]
- Becker, J.; Wittmann, C. Microbial Production of Extremolytes—High-Value Active Ingredients for Nutrition, Health Care, and Well-Being. Curr. Opin. Biotechnol. 2020, 65, 118–128. [Google Scholar] [CrossRef] [PubMed]
- Romero, E.L.; Morilla, M.J. Ether Lipids from Archaeas in Nano-Drug Delivery and Vaccination. Int. J. Pharm. 2023, 634, 122632. [Google Scholar] [CrossRef]
- Drugs@FDA Glossary of Terms—FDA. Available online: https://www.fda.gov/drugs/drug-approvals-and-databases/drugsfda-glossary-terms (accessed on 18 May 2023).
- Metibemu, D.S.; Ogungbe, I.V. Carotenoids in Drug Discovery and Medicine: Pathways and Molecular Targets Implicated in Human Diseases. Molecules 2022, 27, 6005. [Google Scholar] [CrossRef]
- Davies, J.; Ryan, K.S. Introducing the Parvome: Bioactive Compounds in the Microbial World. ACS Chem. Biol. 2012, 7, 252–259. [Google Scholar] [CrossRef] [PubMed]
- Brower, V. Nutraceuticals: Poised for a Healthy Slice of the Healthcare Market? Nat. Biotechnol. 1998, 16, 728–731. [Google Scholar] [CrossRef]
- Trottier, G.; Boström, P.J.; Lawrentschuk, N.; Fleshner, N.E. Nutraceuticals and Prostate Cancer Prevention: A Current Review. Nat. Rev. Urol. 2010, 7, 21–30. [Google Scholar] [CrossRef] [PubMed]
- Sohaimy, S. Functional Foods and Nutraceuticals-Modern Approach to Food Science; Citeseer: Princeton, NJ, USA, 2012. [Google Scholar]
- Our Food, Our Health-Healthy Diet and Safe Food in The Netherlands. Available online: https://www.researchgate.net/publication/27451618_Our_food_our_health-Healthy_diet_and_safe_food_in_the_Netherlands (accessed on 19 May 2023).
- FAO. Report On Functional Foods; FAO: Rome, Italy, 2007. [Google Scholar]
- How the FDA Regulates Nutraceuticals—FDA Reader. Available online: https://www.fdareader.com/blog/how-the-fda-regulates-nutraceuticals (accessed on 18 May 2023).
- Enzing, C.; Ploeg, M.; Barbosa, M.; Sijtsma, L.; Vigani, M.; Parisi, C.; Rodríguez Cerezo, E.; Institute for Prospective Technological Studies. Microalgae-Based Products for the Food and Feed Sector: An Outlook for Europe; JRC Scientific and Policy Reports; Joint Research Centre: Seville, Spain, 2014; pp. 19–37. [Google Scholar] [CrossRef]
- Compliance in Pharma Industry: Challenges & Solutions—Within3. Available online: https://within3.com/blog/compliance-in-pharma-industry (accessed on 18 May 2023).
- Eussen, S.R.B.M.; Verhagen, H.; Klungel, O.H.; Garssen, J.; Van Loveren, H.; Van Kranen, H.J.; Rompelberg, C.J.M. Functional Foods and Dietary Supplements: Products at the Interface between Pharma and Nutrition. Eur. J. Pharmacol. 2011, 668 (Suppl. S1), S2–S9. [Google Scholar] [CrossRef] [PubMed]
- Xiao, Y.D.; Huang, W.Y.; Li, D.J.; Song, J.F.; Liu, C.Q.; Wei, Q.Y.; Zhang, M.; Yang, Q.M. Thermal Degradation Kinetics of All-Trans and Cis-Carotenoids in a Light-Induced Model System. Food Chem. 2018, 239, 360–368. [Google Scholar] [CrossRef] [PubMed]
- Semitsoglou-Tsiapou, S.; Meador, T.B.; Peng, B.; Aluwihare, L. Photochemical (UV–Vis/H2O2) Degradation of Carotenoids: Kinetics and Molecular End Products. Chemosphere 2022, 286, 131697. [Google Scholar] [CrossRef]
- Bohn, T. Metabolic Fate of Bioaccessible and Non-Bioaccessible Carotenoids. Food Chem. Funct. Anal. 2018, 2018, 165–200. [Google Scholar] [CrossRef]
- Kopec, R.E.; Failla, M.L. Recent Advances in the Bioaccessibility and Bioavailability of Carotenoids and Effects of Other Dietary Lipophiles. J. Food Compos. Anal. 2018, 68, 16–30. [Google Scholar] [CrossRef]
- Xavier, A.A.O.; Mercadante, A.Z. The Bioaccessibility of Carotenoids Impacts the Design of Functional Foods. Curr. Opin. Food Sci. 2019, 26, 1–8. [Google Scholar] [CrossRef]
- McClements, D.J. Recent Developments in Encapsulation and Release of Functional Food Ingredients: Delivery by Design. Curr. Opin. Food Sci. 2018, 23, 80–84. [Google Scholar] [CrossRef]
- Soukoulis, C.; Bohn, T. A Comprehensive Overview on the Micro- and Nano-Technological Encapsulation Advances for Enhancing the Chemical Stability and Bioavailability of Carotenoids. Crit. Rev. Food Sci. Nutr. 2018, 58, 1–36. [Google Scholar] [CrossRef]
- Maghsoudi, S.; Taghavi Shahraki, B.; Rabiee, N.; Fatahi, Y.; Bagherzadeh, M.; Dinarvand, R.; Ahmadi, S.; Rabiee, M.; Tahriri, M.; Hamblin, M.R.; et al. The Colorful World of Carotenoids: A Profound Insight on Therapeutics and Recent Trends in Nano Delivery Systems. Crit. Rev. Food Sci. Nutr. 2021, 62, 3658–3697. [Google Scholar] [CrossRef] [PubMed]
- Boonlao, N.; Ruktanonchai, U.R.; Anal, A.K. Enhancing Bioaccessibility and Bioavailability of Carotenoids Using Emulsion-Based Delivery Systems. Colloids Surf. B Biointerfaces 2022, 209, 112211. [Google Scholar] [CrossRef] [PubMed]
- Park, H.; Otte, A.; Park, K. Evolution of Drug Delivery Systems: From 1950 to 2020 and Beyond. J. Control. Release 2022, 342, 53–65. [Google Scholar] [CrossRef] [PubMed]
- Bodade, R.G.; Bodade, A.G. Microencapsulation of Bioactive Compounds and Enzymes for Therapeutic Applications. In Biopolymer-Based Formulations: Biomedical and Food Applications; Elsevier: Amsterdam, The Netherlands, 2020; pp. 381–404. [Google Scholar] [CrossRef]
- Vargason, A.M.; Anselmo, A.C.; Mitragotri, S. The Evolution of Commercial Drug Delivery Technologies. Nat. Biomed. Eng. 2021, 5, 951–967. [Google Scholar] [CrossRef]
- Gaspar, R.S.; Silva-Lima, B.; Magro, F.; Alcobia, A.; da Costa, F.L.; Feio, J. Non-Biological Complex Drugs (NBCDs): Complex Pharmaceuticals in Need of Individual Robust Clinical Assessment Before Any Therapeutic Equivalence Decision. Front. Med. 2020, 7, 590527. [Google Scholar] [CrossRef]
- Zagalo, D.M.; Simões, S.; Sousa, J. Regulatory Science Approach in Pharmaceutical Development of Follow-on Versions of Non-Biological Complex Drug Products. J. Pharm. Sci. 2022, 111, 2687–2713. [Google Scholar] [CrossRef]
- Al-Zoubi, M.S.; Al-Zoubi, R.M. Nanomedicine Tactics in Cancer Treatment: Challenge and Hope. Crit. Rev. Oncol. Hematol. 2022, 174, 103677. [Google Scholar] [CrossRef]
- Hashida, M. Role of Pharmacokinetic Consideration for the Development of Drug Delivery Systems: A Historical Overview. Adv. Drug Deliv. Rev. 2020, 157, 71–82. [Google Scholar] [CrossRef]
- Venditto, V.J.; Szoka, F.C. Cancer Nanomedicines: So Many Papers and so Few Drugs! Adv. Drug Deliv. Rev. 2013, 65, 80–88. [Google Scholar] [CrossRef] [Green Version]
- Zhou, Q.; Li, J.; Xiang, J.; Shao, S.; Zhou, Z.; Tang, J.; Shen, Y. Transcytosis-Enabled Active Extravasation of Tumor Nanomedicine. Adv. Drug Deliv. Rev. 2022, 189, 114480. [Google Scholar] [CrossRef] [PubMed]
- Patel, S.; Kim, J.; Herrera, M.; Mukherjee, A.; Kabanov, A.V.; Sahay, G. Brief Update on Endocytosis of Nanomedicines. Adv. Drug Deliv. Rev. 2019, 144, 90–111. [Google Scholar] [CrossRef] [PubMed]
- Maakaron, J.E.; Mims, A.S. Daunorubicin-Cytarabine Liposome (CPX-351) in the Management of Newly Diagnosed Secondary AML: A New Twist on an Old Cocktail. Best Pract. Res. Clin. Haematol. 2019, 32, 127–133. [Google Scholar] [CrossRef] [PubMed]
- Romero, E.L.; Morilla, M.J. Drug Delivery Systems against Leishmaniasis? Still an Open Question. Expert Opin. Drug Deliv. 2008, 5, 805–823. [Google Scholar] [CrossRef] [PubMed]
- Bonaccorso, A.; Russo, G.; Pappalardo, F.; Carbone, C.; Puglisi, G.; Pignatello, R.; Musumeci, T. Quality by Design Tools Reducing the Gap from Bench to Bedside for Nanomedicine. Eur. J. Pharm. Biopharm. 2021, 169, 144–155. [Google Scholar] [CrossRef]
- Jensen, G.M.; Hoo, L.S. Regulatory Aspects of Pharmaceutical Nanopreparations. In Handbook of Nanobiomedical Research: Fundamentals, Applications and Recent Developments: Volume 4. Biological Requirement and Safety Assessment of Nanomedicines; World Scientific: Singapore, 2014; pp. 119–143. [Google Scholar] [CrossRef]
- Nanotechnology Fact Sheet—FDA. Available online: https://www.fda.gov/science-research/nanotechnology-programs-fda/nanotechnology-fact-sheet (accessed on 18 May 2023).
- Zhang, X.; Zhao, X.; Tie, S.; Li, J.; Su, W.; Tan, M. A Smart Cauliflower-like Carrier for Astaxanthin Delivery to Relieve Colon Inflammation. J. Control. Release 2022, 342, 372–387. [Google Scholar] [CrossRef]
- Chen, Y.; Su, W.; Tie, S.; Cui, W.; Yu, X.; Zhang, L.; Hua, Z.; Tan, M. Orally Deliverable Sequence-Targeted Astaxanthin Nanoparticles for Colitis Alleviation. Biomaterials 2023, 293, 121976. [Google Scholar] [CrossRef]
- Zhang, X.; Zhao, X.; Hua, Z.; Xing, S.; Li, J.; Fei, S.; Tan, M. ROS-Triggered Self-Disintegrating and PH-Responsive Astaxanthin Nanoparticles for Regulating the Intestinal Barrier and Colitis. Biomaterials 2023, 292, 121937. [Google Scholar] [CrossRef]
- Zhang, C.; Xu, Y.; Wu, S.; Zheng, W.; Song, S.; Ai, C. Fabrication of Astaxanthin-Enriched Colon-Targeted Alginate Microspheres and Its Beneficial Effect on Dextran Sulfate Sodium-Induced Ulcerative Colitis in Mice. Int. J. Biol. Macromol. 2022, 205, 396–409. [Google Scholar] [CrossRef]
- Lee, Y.S.; Jeon, S.H.; Ham, H.J.; Lee, H.P.; Song, M.J.; Hong, J.T. Improved Anti-Inflammatory Effects of Liposomal Astaxanthin on a Phthalic Anhydride-Induced Atopic Dermatitis Model. Front. Immunol. 2020, 11, 565285. [Google Scholar] [CrossRef]
- Hama, S.; Takahashi, K.; Inai, Y.; Shiota, K.; Sakamoto, R.; Yamada, A.; Tsuchiya, H.; Kanamura, K.; Yamashita, E.; Kogure, K. Protective Effects of Topical Application of a Poorly Soluble Antioxidant Astaxanthin Liposomal Formulation on Ultraviolet-Induced Skin Damage. J. Pharm. Sci. 2012, 101, 2909–2916. [Google Scholar] [CrossRef] [PubMed]
- The Preliminary Study on Anti-Photodamaged Effect of Astaxanthin Liposomes in Mice Skin. Available online: https://pubmed.ncbi.nlm.nih.gov/30378331/ (accessed on 17 May 2023).
- Shimokawa, T.; Fukuta, T.; Inagi, T.; Kogure, K. Protective Effect of High-Affinity Liposomes Encapsulating Astaxanthin against Corneal Disorder in the in Vivo Rat Dry Eye Disease Model. J. Clin. Biochem. Nutr. 2020, 66, 224. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xu, L.; Yu, H.; Sun, H.; Yu, X.; Tao, Y. Optimized Nonionic Emulsifier for the Efficient Delivery of Astaxanthin Nanodispersions to Retina: In Vivo and Ex Vivo Evaluations. Drug Deliv. 2019, 26, 1222–1234. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gu, J.; Chen, Y.; Tong, L.; Wang, X.; Yu, D.; Wu, H. Astaxanthin-Loaded Polymer-Lipid Hybrid Nanoparticles (ATX-LPN): Assessment of Potential Otoprotective Effects. J. Nanobiotechnol. 2020, 18, 53. [Google Scholar] [CrossRef] [Green Version]
- Fukuta, T.; Hirai, S.; Yoshida, T.; Maoka, T.; Kogure, K. Protective Effect of Antioxidative Liposomes Co-Encapsulating Astaxanthin and Capsaicin on CCl4-Induced Liver Injury. Biol. Pharm. Bull. 2020, 43, 1272–1274. [Google Scholar] [CrossRef] [PubMed]
- Chiu, C.H.; Chang, C.C.; Lin, S.T.; Chyau, C.C.; Peng, R.Y. Improved Hepatoprotective Effect of Liposome-Encapsulated Astaxanthin in Lipopolysaccharide-Induced Acute Hepatotoxicity. Int. J. Mol. Sci. 2016, 17, 1128. [Google Scholar] [CrossRef] [Green Version]
- Hua, Z.; Zhang, X.; Zhao, X.; Zhu, B.W.; Liu, D.; Tan, M. Hepatic-Targeted Delivery of Astaxanthin for Enhanced Scavenging Free Radical Scavenge and Preventing Mitochondrial Depolarization. Food Chem. 2023, 406, 135036. [Google Scholar] [CrossRef]
- Chen, Z.; Li, W.; Shi, L.; Jiang, L.; Li, M.; Zhang, C.; Peng, H. Kidney-Targeted Astaxanthin Natural Antioxidant Nanosystem for Diabetic Nephropathy Therapy. Eur. J. Pharm. Biopharm. 2020, 156, 143–154. [Google Scholar] [CrossRef]
- Xiong, H.; Wang, S.; Sun, Z.; Li, J.; Zhang, H.; Liu, W.; Ruan, J.; Chen, S.; Gao, C.; Fan, C. The ROS-responsive Scavenger with Intrinsic Antioxidant Capability and Enhanced Immunomodulatory Effects for Cartilage Protection and Osteoarthritis Remission. Appl. Mater Today 2022, 26, 101366. [Google Scholar] [CrossRef]
- Chandra Bhatt, P.; Srivastava, P.; Pandey, P.; Khan, W.; Panda, B.P. Nose to Brain Delivery of Astaxanthin-Loaded Solid Lipid Nanoparticles: Fabrication, Radio Labeling, Optimization and Biological Studies. RSC Adv. 2016, 6, 10001–10010. [Google Scholar] [CrossRef]
- Seyedian, S.S.; Nokhostin, F.; Malamir, M.D. A Review of the Diagnosis, Prevention, and Treatment Methods of Inflammatory Bowel Disease. J. Med. Life 2019, 12, 113. [Google Scholar] [CrossRef] [PubMed]
- Tian, T.; Wang, Z.; Zhang, J. Pathomechanisms of Oxidative Stress in Inflammatory Bowel Disease and Potential Antioxidant Therapies. Oxid. Med. Cell Longev. 2017, 2017, 4535194. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guan, G.; Lan, S. Implications of Antioxidant Systems in Inflammatory Bowel Disease. Biomed. Res. Int. 2018, 2018, 1290179. [Google Scholar] [CrossRef] [Green Version]
- Naserifar, M.; Hosseinzadeh, H.; Abnous, K.; Mohammadi, M.; Taghdisi, S.M.; Ramezani, M.; Alibolandi, M. Oral Delivery of Folate-Targeted Resveratrol-Loaded Nanoparticles for Inflammatory Bowel Disease Therapy in Rats. Life Sci. 2020, 262, 118555. [Google Scholar] [CrossRef]
- Suzuki, Y.; Matsumoto, T.; Okamoto, S.; Hibi, T. A Lecithinized Superoxide Dismutase (PC-SOD) Improves Ulcerative Colitis. Colorectal Dis. 2008, 10, 931. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ng, S.C.; Lam, Y.T.; Tsoi, K.K.F.; Chan, F.K.L.; Sung, J.J.Y.; Wu, J.C.Y. Systematic Review: The Efficacy of Herbal Therapy in Inflammatory Bowel Disease. Aliment. Pharmacol. Ther. 2013, 38, 854–863. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-Fidalgo, S.; Cárdeno, A.; Sánchez-Hidalgo, M.; Aparicio-Soto, M.; Villegas, I.; Rosillo, M.A.; De La Lastra, C.A. Dietary Unsaponifiable Fraction from Extra Virgin Olive Oil Supplementation Attenuates Acute Ulcerative Colitis in Mice. Eur. J. Pharm. Sci. 2013, 48, 572–581. [Google Scholar] [CrossRef]
- Jiang, G.L.; Zhu, M.J. Preparation of Astaxanthin-Encapsulated Complex with Zein and Oligochitosan and Its Application in Food Processing. LWT 2019, 106, 179–185. [Google Scholar] [CrossRef]
- Komai, K.; Shichita, T.; Ito, M.; Kanamori, M.; Chikuma, S.; Yoshimura, A. Role of Scavenger Receptors as Damage-Associated Molecular Pattern Receptors in Toll-like Receptor Activation. Int. Immunol. 2017, 29, 59–70. [Google Scholar] [CrossRef] [Green Version]
- Zong, G.; Zhu, Y.; Zhang, Y.; Wang, Y.; Bai, H.; Yang, Q.; Ben, J.; Zhang, H.; Li, X.; Zhu, X.; et al. SR-A1 Suppresses Colon Inflammation and Tumorigenesis through Negative Regulation of NF-ΚB Signaling. Biochem. Pharmacol. 2018, 154, 335–343. [Google Scholar] [CrossRef]
- Altube, M.J.; Selzer, S.M.; De Farias, M.A.; Portugal, R.V.; Morilla, M.J.; Romero, E.L. Surviving Nebulization-Induced Stress: Dexamethasone in PH-Sensitive Archaeosomes. Nanomedicine 2016, 11, 2103–2117. [Google Scholar] [CrossRef]
- Corcelli, A.; Lobasso, S. 25 Characterization of Lipids of Halophilic Archaea. Methods Microbiol. 2006, 35, 585–613. [Google Scholar] [CrossRef]
- Khan, A.Q.; Agha, M.V.; Sheikhan, K.S.A.M.; Younis, S.M.; Tamimi, M.A.; Alam, M.; Ahmad, A.; Uddin, S.; Buddenkotte, J.; Steinhoff, M. Targeting Deregulated Oxidative Stress in Skin Inflammatory Diseases: An Update on Clinical Importance. Biomed. Pharmacother. 2022, 154, 113601. [Google Scholar] [CrossRef]
- Boos, A.C.; Hagl, B.; Schlesinger, A.; Halm, B.E.; Ballenberger, N.; Pinarci, M.; Heinz, V.; Kreilinger, D.; Spielberger, B.D.; Schimke-Marques, L.F.; et al. Atopic Dermatitis, STAT3- and DOCK8-Hyper-IgE Syndromes Differ in IgE-Based Sensitization Pattern. Allergy 2014, 69, 943–953. [Google Scholar] [CrossRef]
- Sutton, S.; Clutterbuck, A.; Harris, P.; Gent, T.; Freeman, S.; Foster, N.; Barrett-Jolley, R.; Mobasheri, A. The Contribution of the Synovium, Synovial Derived Inflammatory Cytokines and Neuropeptides to the Pathogenesis of Osteoarthritis. Veter. J. 2009, 179, 10–24. [Google Scholar] [CrossRef]
- Thomson, A.; Hilkens, C.M.U. Synovial Macrophages in Osteoarthritis: The Key to Understanding Pathogenesis? Front. Immunol. 2021, 12, 1831. [Google Scholar] [CrossRef]
- Roman-Blas, J.A.; Jimenez, S.A. NF-KappaB as a Potential Therapeutic Target in Osteoarthritis and Rheumatoid Arthritis. Osteoarthritis Cartil. 2006, 14, 839–848. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fukuta, T.; Hirai, S.; Yoshida, T.; Maoka, T.; Kogure, K. Enhancement of Antioxidative Activity of Astaxanthin by Combination with an Antioxidant Capable of Forming Intermolecular Interactions. Free Radic. Res. 2020, 54, 818–828. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Zhao, X.; Yang, S.; Chen, B.; Shi, J. Salidroside Alleviates High Glucose-Induced Oxidative Stress and Extracellular Matrix Accumulation in Rat Glomerular Mesangial Cells by the TXNIP-NLRP3 Inflammasome Pathway. Chem. Biol. Interact. 2017, 278, 48–53. [Google Scholar] [CrossRef]
- Ding, T.; Wang, S.; Zhang, X.; Zai, W.; Fan, J.; Chen, W.; Bian, Q.; Luan, J.; Shen, Y.; Zhang, Y.; et al. Kidney Protection Effects of Dihydroquercetin on Diabetic Nephropathy through Suppressing ROS and NLRP3 Inflammasome. Phytomedicine 2018, 41, 45–53. [Google Scholar] [CrossRef] [PubMed]
- Paller, M.S.; Hoidal, J.R.; Ferris, T.F. Oxygen Free Radicals in Ischemic Acute Renal Failure in the Rat. J. Clin. Invest. 1984, 74, 1156–1164. [Google Scholar] [CrossRef] [Green Version]
- Sun, T.; Jiang, D.; Rosenkrans, Z.T.; Ehlerding, E.B.; Ni, D.; Qi, C.; Kutyreff, C.J.; Barnhart, T.E.; Engle, J.W.; Huang, P.; et al. A Melanin-Based Natural Antioxidant Defense Nanosystem for Theranostic Application in Acute Kidney Injury. Adv. Funct. Mater. 2019, 29, 201904833. [Google Scholar] [CrossRef]
- Nasim, N.; Sandeep, I.S.; Mohanty, S. Plant-Derived Natural Products for Drug Discovery: Current Approaches and Prospects. Nucleus 2022, 65, 399–411. [Google Scholar] [CrossRef] [PubMed]
- Atanasov, A.G.; Zotchev, S.B.; Dirsch, V.M.; Orhan, I.E.; Banach, M.; Rollinger, J.M.; Barreca, D.; Weckwerth, W.; Bauer, R.; Bayer, E.A.; et al. Natural Products in Drug Discovery: Advances and Opportunities. Nat. Rev. Drug Discov. 2021, 20, 200–216. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Ouyang, Z.; Du, H.; Wang, M.; Wang, J.; Sun, H.; Kong, L.; Xu, Q.; Ma, H.; Sun, Y. New Opportunities and Challenges of Natural Products Research: When Target Identification Meets Single-Cell Multiomics. Acta Pharm. Sin. B 2022, 12, 4011–4039. [Google Scholar] [CrossRef] [PubMed]
- Dinkova-Kostova, A.T.; Liby, K.T.; Stephenson, K.K.; Holtzclaw, W.D.; Gao, X.; Suh, N.; Williams, C.; Risingsong, R.; Honda, T.; Gribble, G.W.; et al. Extremely Potent Triterpenoid Inducers of the Phase 2 Response: Correlations of Protection against Oxidant and Inflammatory Stress. Proc. Natl. Acad. Sci. USA 2005, 102, 4584–4589. [Google Scholar] [CrossRef] [Green Version]
- Niu, T.; Xuan, R.; Jiang, L.; Wu, W.; Zhen, Z.; Song, Y.; Hong, L.; Zheng, K.; Zhang, J.; Xu, Q.; et al. Astaxanthin Induces the Nrf2/HO-1 Antioxidant Pathway in Human Umbilical Vein Endothelial Cells by Generating Trace Amounts of ROS. J. Agric. Food Chem. 2018, 66, 1551–1559. [Google Scholar] [CrossRef]
- Banerjee, S.; Ramaswamy, S. Process Model and Techno-Economic Analysis of Natural Astaxanthin Production from Microalgae Incorporating Geospatial Variabilities. Bioresour. Technol. Rep. 2022, 20, 101260. [Google Scholar] [CrossRef]
- Rather, L.J.; Mir, S.S.; Ganie, S.A.; Islam, S.U.; Li, Q. Research Progress, Challenges, and Perspectives in Microbial Pigment Production for Industrial Applications—A Review. Dyes Pigments 2023, 210, 110989. [Google Scholar] [CrossRef]
- Younis, M.A.; Tawfeek, H.M.; Abdellatif, A.A.H.; Abdel-Aleem, J.A.; Harashima, H. Clinical Translation of Nanomedicines: Challenges, Opportunities, and Keys. Adv. Drug Deliv. Rev. 2022, 181, 114083. [Google Scholar] [CrossRef]
- Donoso, A.; González-Durán, J.; Muñoz, A.A.; González, P.A.; Agurto-Muñoz, C. Therapeutic Uses of Natural Astaxanthin: An Evidence-Based Review Focused on Human Clinical Trials. Pharmacol. Res. 2021, 166, 105479. [Google Scholar] [CrossRef] [PubMed]
- Sinniah, A.; Yazid, S.; Flower, R.J. From NSAIDs to Glucocorticoids and Beyond. Cells 2021, 10, 3524. [Google Scholar] [CrossRef] [PubMed]
- Yasir, M.; Goyal, A.; Sonthalia, S. Corticosteroid Adverse Effects; StatPearls: Tampa, FL, USA, 2018. [Google Scholar]
- Aljebab, F.; Choonara, I.; Conroy, S. Systematic Review of the Toxicity of Long-Course Oral Corticosteroids in Children. PLoS ONE 2017, 12, e170259. [Google Scholar] [CrossRef] [Green Version]
- Saad, J.; Pellegrini, M.V. Nonsteroidal Anti-Inflammatory Drugs Toxicity; StatPearls: Tampa, FL, USA, 2022. [Google Scholar]
- Świacka, K.; Michnowska, A.; Maculewicz, J.; Caban, M.; Smolarz, K. Toxic Effects of NSAIDs in Non-Target Species: A Review from the Perspective of the Aquatic Environment. Environ. Pollut. 2021, 273, 115891. [Google Scholar] [CrossRef] [PubMed]

 ) and
) and  ) (highly reactive (
) (highly reactive ( ); highly oxidant (
); highly oxidant ( ); less reactive (
); less reactive ( )) are generated both by endogenous (mitochondrial respiratory chain, prostaglandin synthesis, and phagocytosis) and exogenous (exposure to environmental pollutions, heavy metals, certain drugs, organic solvents, alcohol, and radiations) stimuli. By direct reactions, •OH, ONOO-, and HOCl oxidate proteins and generate nucleic acid fragmentation, mutagenic lesions, and peroxidase lipids, which lead to malfunction and cellular death (
)) are generated both by endogenous (mitochondrial respiratory chain, prostaglandin synthesis, and phagocytosis) and exogenous (exposure to environmental pollutions, heavy metals, certain drugs, organic solvents, alcohol, and radiations) stimuli. By direct reactions, •OH, ONOO-, and HOCl oxidate proteins and generate nucleic acid fragmentation, mutagenic lesions, and peroxidase lipids, which lead to malfunction and cellular death ( ). To defend against oxidative damage, organisms have endogenous antioxidant systems, which include (i) antioxidant enzymes (superoxide dismutase (SOD), catalase (CAT), glutathione peroxidases (GPx), and peroxiredoxins (Prxs) [remove H2O2]), (ii) substrates of antioxidant enzymes (glutathione (GSH), thioredoxin (Trx), and NADPH), and (iii) low-molecular-mass antioxidants (bilirubin, albumin, uric acid, α-lipoic acid, melatonin, and coenzyme Q10). In addition, organisms employed exogenous antioxidants only present in microbial or plant cell such as vitamin C, vitamin E, carotenoids, polyphenols, flavonoids, and metals Se, Cu, Zn, and Mn (co-factors of enzymes). The effectivity of the antioxidant system depends on the capacity to maintain redox homeostasis controlling the generation and elimination of O2•−, H2O2, and •NO at levels that limit the production of •OH and ONOO- [37]. The only effective strategy to reduce the damage induced by •OH is to avoid its formation by preventing O2•− formation and eliminating O2•− and H2O2. Remotion of O2•− prevents the formation of ONOO−, while remotion of H2O2 prevents the formation of •OH and HOX [X: halogen] [35].
). To defend against oxidative damage, organisms have endogenous antioxidant systems, which include (i) antioxidant enzymes (superoxide dismutase (SOD), catalase (CAT), glutathione peroxidases (GPx), and peroxiredoxins (Prxs) [remove H2O2]), (ii) substrates of antioxidant enzymes (glutathione (GSH), thioredoxin (Trx), and NADPH), and (iii) low-molecular-mass antioxidants (bilirubin, albumin, uric acid, α-lipoic acid, melatonin, and coenzyme Q10). In addition, organisms employed exogenous antioxidants only present in microbial or plant cell such as vitamin C, vitamin E, carotenoids, polyphenols, flavonoids, and metals Se, Cu, Zn, and Mn (co-factors of enzymes). The effectivity of the antioxidant system depends on the capacity to maintain redox homeostasis controlling the generation and elimination of O2•−, H2O2, and •NO at levels that limit the production of •OH and ONOO- [37]. The only effective strategy to reduce the damage induced by •OH is to avoid its formation by preventing O2•− formation and eliminating O2•− and H2O2. Remotion of O2•− prevents the formation of ONOO−, while remotion of H2O2 prevents the formation of •OH and HOX [X: halogen] [35].
 ) and
) and  ) (highly reactive (
) (highly reactive ( ); highly oxidant (
); highly oxidant ( ); less reactive (
); less reactive ( )) are generated both by endogenous (mitochondrial respiratory chain, prostaglandin synthesis, and phagocytosis) and exogenous (exposure to environmental pollutions, heavy metals, certain drugs, organic solvents, alcohol, and radiations) stimuli. By direct reactions, •OH, ONOO-, and HOCl oxidate proteins and generate nucleic acid fragmentation, mutagenic lesions, and peroxidase lipids, which lead to malfunction and cellular death (
)) are generated both by endogenous (mitochondrial respiratory chain, prostaglandin synthesis, and phagocytosis) and exogenous (exposure to environmental pollutions, heavy metals, certain drugs, organic solvents, alcohol, and radiations) stimuli. By direct reactions, •OH, ONOO-, and HOCl oxidate proteins and generate nucleic acid fragmentation, mutagenic lesions, and peroxidase lipids, which lead to malfunction and cellular death ( ). To defend against oxidative damage, organisms have endogenous antioxidant systems, which include (i) antioxidant enzymes (superoxide dismutase (SOD), catalase (CAT), glutathione peroxidases (GPx), and peroxiredoxins (Prxs) [remove H2O2]), (ii) substrates of antioxidant enzymes (glutathione (GSH), thioredoxin (Trx), and NADPH), and (iii) low-molecular-mass antioxidants (bilirubin, albumin, uric acid, α-lipoic acid, melatonin, and coenzyme Q10). In addition, organisms employed exogenous antioxidants only present in microbial or plant cell such as vitamin C, vitamin E, carotenoids, polyphenols, flavonoids, and metals Se, Cu, Zn, and Mn (co-factors of enzymes). The effectivity of the antioxidant system depends on the capacity to maintain redox homeostasis controlling the generation and elimination of O2•−, H2O2, and •NO at levels that limit the production of •OH and ONOO- [37]. The only effective strategy to reduce the damage induced by •OH is to avoid its formation by preventing O2•− formation and eliminating O2•− and H2O2. Remotion of O2•− prevents the formation of ONOO−, while remotion of H2O2 prevents the formation of •OH and HOX [X: halogen] [35].
). To defend against oxidative damage, organisms have endogenous antioxidant systems, which include (i) antioxidant enzymes (superoxide dismutase (SOD), catalase (CAT), glutathione peroxidases (GPx), and peroxiredoxins (Prxs) [remove H2O2]), (ii) substrates of antioxidant enzymes (glutathione (GSH), thioredoxin (Trx), and NADPH), and (iii) low-molecular-mass antioxidants (bilirubin, albumin, uric acid, α-lipoic acid, melatonin, and coenzyme Q10). In addition, organisms employed exogenous antioxidants only present in microbial or plant cell such as vitamin C, vitamin E, carotenoids, polyphenols, flavonoids, and metals Se, Cu, Zn, and Mn (co-factors of enzymes). The effectivity of the antioxidant system depends on the capacity to maintain redox homeostasis controlling the generation and elimination of O2•−, H2O2, and •NO at levels that limit the production of •OH and ONOO- [37]. The only effective strategy to reduce the damage induced by •OH is to avoid its formation by preventing O2•− formation and eliminating O2•− and H2O2. Remotion of O2•− prevents the formation of ONOO−, while remotion of H2O2 prevents the formation of •OH and HOX [X: halogen] [35].






| Carotenoid/ Property | AST | BR | |
|---|---|---|---|
| Production | Natural source | H. pluvialis: high cost and low production | Halophilic archaea |
| Chemical synthesis | Low-cost labor Inexpensive chemicals | Not reported | |
| Production volume/market | 190 Tn 2021: USD 647 million | Not reported | |
| Isomers | Optical | H. pluvialis: 3S, 3′S Synthetic: 3S,3′S: 3R,3′S: 3R,3′R 1:2:1 ratio | None |
| Geometric | All-trans (all-E) and cis isomers (9-Z, 13-Z, 15-Z) H. pluvialis: 73% all-E-AST and 27% cis-AST | All-trans, cis isomers (5-Z, 9-Z, 13-Z), and double isomers (5-Z-26-Z, and 9-Z-26-Z) | |
| Esterification | Yes | No | |
| Biological role | Oxygenic photosynthesis (light harvesting) and photoprotection | Defense against osmotic stress and radiation [47] Structural support to rhodopsin complexes (a retinal protein-carotenoid complex) [48] | |
| Physicochemical role on membrane | None | ↑ membrane rigidity ↓ water permeability | |
| Antioxidant role | 1O2 quenching | 800 times > coenzyme Q 6000 times > vitamin C 550 times > green tea catechins, 11 times > β-carotene [49], natural 50 times > synthetic | Not reported |
| Free radical scavenger | 65 times > vitamin C 50 times > vitamin E [50] Natural 20 times > synthetic [51] | DPPH (IC50) Hfx. mediterranei extract: 40–74 μg/mL, Haloterrigena sp. [52], H. tebenquichense [53], Halorubrum sp. BS2 [54] extracts: 3–6 µg/mL Haloarchaeal strains from Atacama Desert [55]: 4.2–34.7 µg/mL | |
| Preventing lipid peroxidation | 100–500 times > vitamin E | H. tebenquichense extract protected red cells against peroxyl radical-induced hemolysis (IC50: 1.9 μg/mL) [55] | |
| Toxicity | NOAEL (no observed adverse effect levels) natural AST is 465 and 557 mg/kg/day in male and female rats, respectively. The repeated-dose oral toxicity in pregnant mice showed LD50 > 20 g/kg [56]. | Up to 500 mg/kg/day for 14 days on Wistar rats, no observed adverse effects were registered. | |
| In vivo/in vitro activity | In vivo animal models: anti-neurodegenerative diseases [57], hepatoprotective [58], anti-cardiovascular diseases [59], inhibited the development of COPD and acute lung injury [60], improve dyslipidemia and metabolic syndrome [61], anti-diabetic nephropathy [62], burn wounds healing [63], and immunostimulation [64] In vitro: anti-fibrotic, and bone disease healing | In vivo: not reported In vitro: antioxidant, anti-inflammatory [55]; antiviral and anti-cancer activity [65] Cholinesterase [66], cyclooxygenase-2 [67], α-glucosidase, α-amylase, and pancreatic lipase [68] inhibition Beneficial effects on sperm cell viability [69] Antimicrobial activity against pathogenic bacteria and fungi [54,70,71] | |
| Metabolic role | In animals ↑ fecundity, growth rate, egg yolk volume and quantity, intensity of flesh color, and strengthening of immune responses [72] ↑ lipids and glucose metabolism [73]. | Not reported | |
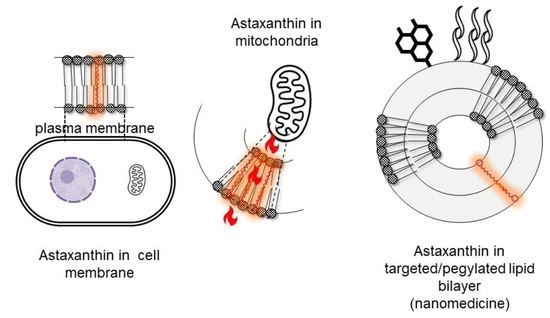
| Intracellular target | Mitochondria | Not reported | |
| Signaling pathways | Nrf2 | ↑ NADPH, GSH, and OS-responsive enzymes [74,75] in brain, heart, kidney, eyes, lungs, skin, and liver [76]. | Not reported |
| PI3K/Akt | ↑ downstream signaling mediators, including mTOR, and Nrf2 [77]. | Not reported | |
| NF-κB | ↓ TNF-α, IL-6, IL-1β prostaglandin E, inducible nitric oxide synthase (iNOS), and COX-2, in macrophages and neutrophils, ↓ inflammation in vivo [78]. | Not reported | |
| Others | ↓ JAK/STAT-3, PPARγ, and p38 MAPKs [79] | Not reported | |
| Apoptosis | Proapoptotic and antiapoptotic Anti-ROS generated-apoptosis: blocks caspase 3 and 9, cytochrome c, p-ERK/ERK, and decrease the Bax/Bcl2 ratio [75,77]. | Proapoptotic: induced caspase-mediated apoptosis and inhibit MMP-9 in cancer cells [80] | |
| Animal uses | Aquaculture feed (synthetic) | Aquaculture feed (artemia) | |
| Human uses | Food supplements (natural), nutraceutical (natural), cosmetic ingredient (synthetic) | Cosmetic ingredient | |
| Clinical trials | Several trials with dietary AST Preventive effects against atherosclerosis [78]; neuroprotective against cognitive impairment [81]; improved visual acuity and retinal blood flow [82]; reduced the signs of skin aging [83]. | Not reported | |
| Disease/Route of Administration | Carotenoid/ Source | Np Type, Composition, and Structural Features | Type Studies/Dose | Reference |
|---|---|---|---|---|
| IBD oral | AST crude (5% purity) from Shandong Wefirst Biotechnology Co., Ltd. (Shandong, China) Natural AST from H. pluvialis | Polymeric microparticles: caseinate, chitosan-TPP and sodium alginate 1.7 μm (10 mg AST) EE: 55% LC: 50 μg/mg Neutral ξ potential | Raw264.7 macrophages Murine DSS model 12.5 mg/kg/day 7 days of treatment, then 6 days DSS + treatment | [198] |
| IBD oral | AST crude (5% purity) from Shandong Wefirst Biotechnology Co., Ltd. (Weihai, Shandong, China) Natural AST from H. pluvialis | Polymeric Np: poly (propylene sulfide) and Rhodamine 123 covalently modified sodium alginate 260 nm EE: 69%; LC: 3.6 μg/mg | Raw264.7 macrophages Murine DSS model 1.25 mg/kg/day 11 days of treatment, then 6 days DSS + treatments | [199] |
| IBD oral | AST crude (10% purity) from Xi’an Realin Biotechnology Co., Ltd. (Xi’an, China) Natural AST from H. pluvialis | Polymeric Np: TPP-modified whey protein isolate-dextran conjugate, covered by lipoic acid-modified HA 380 nm; −31 mVξ potential EE: 81%; LC: 2.7% | Raw264.7 macrophages Murine DSS model 10 mg/kg/day 14 days of treatment, then 7 days DSS + treatment | [200] |
| IBD oral | AST (purity >95%) from Solarbio Life Sciences (Beijing, China). The origin is not specified. Synthetic. | Olive oil-lecithin o/w emulsion encapsulated in sodium alginate microparticles 0.5–3.2 μm EE: 87% | Murine DSS model 30 ppm DSS and Np treatments at the same time once a day for 9 weeks | [201] |
| IBD oral | BR extracted from H. tebenquichense | NAC Compritol, BR, polar archaeolipids, and Tween 80 (2: 2: 1.2: 3% w/w) 66 nm; −32 mV ξ potential | THP-1 derived macrophages, Caco-2 cells gut inflammation model 3.5.10−4 μg/mL | [53] |
| Atopic dermatitis topical | AST from GDE Co., Ltd. (Siheung, Republic of Korea). The origin is not specified. Synthetic. | Liposomes SPC 65 nm | PA-induced (three times a week for 4 weeks) AD on mice 0.2 mg Liposomal treatment 3 h after PA induction | [202] |
| Psoriasis topical | BR extracted from H. tebenquichense | NAC 70 nm; −39 mV ξ potential | CaCl2 differentiated HaCaT cells imiquimod stimulated psoriasis model 3.5.10−4 μg/mL | [71] |
| UV-induced skin damage topical | AST from Sigma-Aldrich (USA) * | Liposomes EPC and DOTAP/EPC/Chol (2:2:1) 326 nm; −2 mV ξ potential and 170 nm; 43 mV ξ potential, respectively | UV treatment on Hos:HR-1 hairless mice dorsal skin once a day for five consecutive days 18 μg | [203] |
| UV-induced skin damage topical | No information available | Liposomes | C57BL/6J mice UVB irradiated one time per day for the first five days, and one time every other day for the next nine days | [204] |
| Dry eye disease topical | AST from FUJIFILM Wako Pure Chemical Corporation (Japan). The origin is not specified (3S,3’S). | Liposomes EPC or EPC/DOTAP (18:2 molar ratio) 130 nm; −0.4 mV ξ potential and 85 nm; 9 mV ξ potential, respectively | Rat DED model 0.6 μg Six times a day for 13 days | [205] |
| Retinal degeneration oral | AST (>90%) from Xian Zelang Biotech, China. The origin is not specified. Synthetic. | Micelles Polysorbate 20 (Tween 20) 76 nm; −16.5 mV ξ potential | N-methyl-N-nitrosourea (MNU) retinal degeneration mouse model 100 mg/kg Eight times 6 h before and at 0, 6, 12, 24, 36, 48, and 72 h after MNU IP administration | [206] |
| Otoprotection local administration | AST from Sigma-Aldrich (USA) * | Lipid–polymer hybrid Np (mPEG-PLA-DMPC) 91 nm; −10 mV ξ potential | Zebrafish and guinea pig exposure to cisplatin 5 μg | [207] |
| Liver injury endovenous | AST (3R, 3’R) from Sigma-Aldrich (USA) Synthetic | Liposomes EPC 114 nm | CCl4-induced liver injury rat model 298 μg/kg | [208] |
| Liver injury oral | AST (>99%) from the Fuji Chemical Industry Co., Ltd. (Toyama Prefecture, Japan) Natural AST from H. pluvialis | Liposomes SPC/Chol (4:1 w/w) 240 nm | LPS-induced liver injury rat model 2, 5, or 10 mg/kg/day Administered once a day 8 days before LPS IP challenge | [209] |
| Liver injury oral | AST (10% purity) was bought from Xi’an Realin Biotechnology Co., Ltd. Natural AST from H. pluvialis. | Hydroxypropyl-β-cyclodextrin 98 nm; EE: 74% | HepaRG cells Mice biodistribution | [210] |
| Diabetic nephropathy (DN) endovenous | AST from Innochem (Beijing, China). The origin is not specified. Synthetic. | Liposomes EPC/Chol/Glucose-PEG600-DSPE 95:20:5 molar ratio 120 nm; −31 mV ξ potential EE: 80%; LC: 6.8% | HRMCs cells 10 mg/kg DN rat model rats fed with high-sugar and high-fat fodder for 6 weeks followed by streptozocin injection | [211] |
| Osteoarthritis intra-articular injection | AST from Shanghai Macklin Biochemical Co., Ltd. (Shanghai, China). The origin is not specified. Synthetic. | Polymeric micelles poly (ethylene glycol)-polythioketal-poly (ethylene glycol) EE: 94%; LC: 9.4% | Bone marrow-derived macrophages 37 μg Rat OA model (intra-articular injection of monosodium iodoacetate into each left knee) Single injection 3 days after OA induction | [212] |
| Nose to brain | AST from Algaltech, Israel Natural AST from H. pluvialis | SLN stearic acid (50 mg), % AST (6.11%) and poloxamer 188: lecithin (1: 6) 206 nm; EE: 77%; LC: 47% | PC-12 cells 4 mg/kg Rat biodistribution | [213] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Morilla, M.J.; Ghosal, K.; Romero, E.L. More Than Pigments: The Potential of Astaxanthin and Bacterioruberin-Based Nanomedicines. Pharmaceutics 2023, 15, 1828. https://doi.org/10.3390/pharmaceutics15071828
Morilla MJ, Ghosal K, Romero EL. More Than Pigments: The Potential of Astaxanthin and Bacterioruberin-Based Nanomedicines. Pharmaceutics. 2023; 15(7):1828. https://doi.org/10.3390/pharmaceutics15071828
Chicago/Turabian StyleMorilla, Maria Jose, Kajal Ghosal, and Eder Lilia Romero. 2023. "More Than Pigments: The Potential of Astaxanthin and Bacterioruberin-Based Nanomedicines" Pharmaceutics 15, no. 7: 1828. https://doi.org/10.3390/pharmaceutics15071828
APA StyleMorilla, M. J., Ghosal, K., & Romero, E. L. (2023). More Than Pigments: The Potential of Astaxanthin and Bacterioruberin-Based Nanomedicines. Pharmaceutics, 15(7), 1828. https://doi.org/10.3390/pharmaceutics15071828