Study and Characterization of Polyvinyl Alcohol-Based Formulations for 3D Printlets Obtained via Fused Deposition Modeling
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
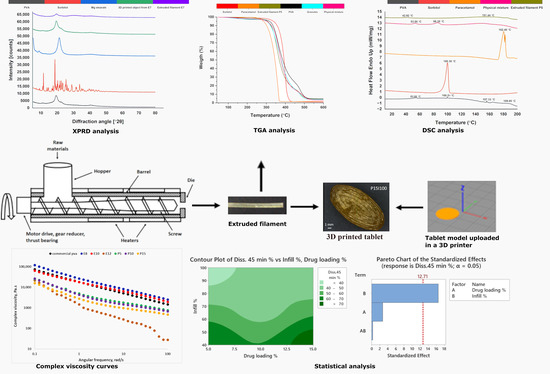
2.2.1. Extrusion and 3D Printing
- Extrusion of the placebo filaments
- Extrusion of the drug-loaded filaments
- 3D printing of the placebo object
- 3D printing of the tablets
2.2.2. Morphology
2.2.3. X-ray Powder Diffraction (XRPD)
2.2.4. Assay of the Extruded Filaments
2.2.5. Thermogravimetric Analysis
2.2.6. Differential Scanning Calorimetry
2.2.7. Rheology
2.2.8. In Vitro Drug Release
2.2.9. Drug Release Kinetics
- Zero-order model—this model refers to drug release processes whose rate does not depend on the drug concentration.
- First-order model—this model describes the release process when the drug release rate is proportional to the drug concentration, i.e., a constant fraction of the drug is released per unit of time.
- Higuchi model—this model assumes that two mechanisms are responsible for controlling the drug release rate: swelling and erosion/degradation. In the Higuchi model, kH is a constant proportional to the burst release rate of the release process.
- Korsmeyer–Peppas model—this model is used to describe the drug release process in occasions when the release follows several kinetics mechanisms.
2.2.10. Design of the Experiments
3. Results and Discussion
3.1. Extrudability and Printability
3.2. Morphology
3.3. X-ray Powder Diffraction (XRPD)
3.4. Assay of the Extruded Filaments
3.5. Thermogravimetric Analysis
3.6. Differential Scanning Calorimetry
3.7. Rheology
3.8. In Vitro Drug Release
3.9. Drug Release Kinetics
3.10. Statistical Evaluation
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Awad, A.; Trenfield, S.J.; Gaisford, S.; Basit, A.W. 3D Printed Medicines: A New Branch of Digital Healthcare. Int. J. Pharm. 2018, 548, 586–596. [Google Scholar] [CrossRef] [PubMed]
- Alhnan, M.A.; Okwuosa, T.C.; Sadia, M.; Wan, K.W.; Ahmed, W.; Arafat, B. Emergence of 3D Printed Dosage Forms: Opportunities and Challenges. Pharm. Res. 2016, 33, 1817–1832. [Google Scholar] [CrossRef] [PubMed]
- Norman, J.; Madurawe, R.D.; Moore, C.M.V.; Khan, M.A.; Khairuzzaman, A. A New Chapter in Pharmaceutical Manufacturing: 3D-Printed Drug Products. Adv. Drug Deliv. Rev. 2017, 108, 39–50. [Google Scholar] [CrossRef] [PubMed]
- Singh, D.; Singh, R.; Boparai, K.S.; Farina, I.; Feo, L.; Verma, A.K. In-Vitro Studies of SS 316 L Biomedical Implants Prepared by FDM, Vapor Smoothing and Investment Casting. Compos. Part B Eng. 2018, 132, 107–114. [Google Scholar] [CrossRef]
- Genina, N.; Holländer, J.; Jukarainen, H.; Mäkilä, E.; Salonen, J.; Sandler, N. Ethylene Vinyl Acetate (EVA) as a New Drug Carrier for 3D Printed Medical Drug Delivery Devices. Eur. J. Pharm. Sci. 2016, 90, 53–63. [Google Scholar] [CrossRef]
- Liang, K.; Carmone, S.; Brambilla, D.; Leroux, J.C. 3D Printing of a Wearable Personalized Oral Delivery Device: A First-in-Human Study. Sci. Adv. 2018, 4, 1–12. [Google Scholar] [CrossRef] [Green Version]
- Goyanes, A.; Robles Martinez, P.; Buanz, A.; Basit, A.W.; Gaisford, S. Effect of Geometry on Drug Release from 3D Printed Tablets. Int. J. Pharm. 2015, 494, 657–663. [Google Scholar] [CrossRef]
- Scoutaris, N.; Ross, S.A.; Douroumis, D. 3D Printed “Starmix” Drug Loaded Dosage Forms for Paediatric Applications. Pharm. Res. 2018, 35, 1–11. [Google Scholar] [CrossRef]
- Trenfield, S.J.; Awad, A.; Goyanes, A.; Gaisford, S.; Basit, A.W. 3D Printing Pharmaceuticals: Drug Development to Frontline Care. Trends Pharmacol. Sci. 2018, 39, 440–451. [Google Scholar] [CrossRef]
- Goyanes, A.; Fina, F.; Martorana, A.; Sedough, D.; Gaisford, S.; Basit, A.W. Development of Modified Release 3D Printed Tablets (Printlets) with Pharmaceutical Excipients Using Additive Manufacturing. Int. J. Pharm. 2017, 527, 21–30. [Google Scholar] [CrossRef]
- Nukala, P.K.; Palekar, S.; Patki, M.; Patel, K. Abuse Deterrent Immediate Release Egg-Shaped Tablet (Egglets) Using 3D Printing Technology: Quality by Design to Optimize Drug Release and Extraction. AAPS PharmSciTech 2019, 20, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Goyanes, A.; Buanz, A.B.M.; Basit, A.W.; Gaisford, S. Fused-Filament 3D Printing (3DP) for Fabrication of Tablets. Int. J. Pharm. 2014, 476, 88–92. [Google Scholar] [CrossRef] [PubMed]
- Pietrzak, K.; Isreb, A.; Alhnan, M.A. A Flexible-Dose Dispenser for Immediate and Extended Release 3D Printed Tablets. Eur. J. Pharm. Biopharm. 2015, 96, 380–387. [Google Scholar] [CrossRef]
- Palekar, S.; Nukala, P.K.; Mishra, S.M.; Kipping, T.; Patel, K. Application of 3D Printing Technology and Quality by Design Approach for Development of Age-Appropriate Pediatric Formulation of Baclofen. Int. J. Pharm. 2019, 556, 106–116. [Google Scholar] [CrossRef]
- Zhang, J.; Feng, X.; Patil, H.; Tiwari, R.V.; Repka, M.A. Coupling 3D Printing with Hot-Melt Extrusion to Produce Controlled-Release Tablets. Int. J. Pharm. 2017, 519, 186–197. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Wang, H.; Li, H.; Ou, Z.; Yang, G. 3D Printed Tablets with Internal Scaffold Structure Using Ethyl Cellulose to Achieve Sustained Ibuprofen Release; Elsevier: Amsterdam, The Netherlands, 2018; Volume 115, ISBN 8605718887107. [Google Scholar]
- Sadia, M.; Sośnicka, A.; Arafat, B.; Isreb, A.; Ahmed, W.; Kelarakis, A.; Alhnan, M.A. Adaptation of Pharmaceutical Excipients to FDM 3D Printing for the Fabrication of Patient-Tailored Immediate Release Tablets. Int. J. Pharm. 2016, 513, 659–668. [Google Scholar] [CrossRef] [PubMed]
- Brough, C.; Miller, D.A.; Keen, J.M.; Kucera, S.A.; Lubda, D.; Williams, R.O. Use of Polyvinyl Alcohol as a Solubility-Enhancing Polymer for Poorly Water Soluble Drug Delivery (Part 1). AAPS PharmSciTech 2016, 17, 167–179. [Google Scholar] [CrossRef] [Green Version]
- De Jaeghere, W.; De Beer, T.; Van Bocxlaer, J.; Remon, J.P.; Vervaet, C. Hot-Melt Extrusion of Polyvinyl Alcohol for Oral Immediate Release Applications. Int. J. Pharm. 2015, 492, 1–9. [Google Scholar] [CrossRef] [Green Version]
- Jang, J.; Lee, D.K. Plasticizer Effect on the Melting and Crystallization Behavior of Polyvinyl Alcohol. Polymer 2003, 44, 8139–8146. [Google Scholar] [CrossRef]
- Gioumouxouzis, C.I.; Katsamenis, O.L.; Bouropoulos, N.; Fatouros, D.G. 3D Printed Oral Solid Dosage Forms Containing Hydrochlorothiazide for Controlled Drug Delivery. J. Drug Deliv. Sci. Technol. 2017, 40, 164–171. [Google Scholar] [CrossRef] [Green Version]
- Obeid, S.; Madžarević, M.; Krkobabić, M.; Ibrić, S. Predicting Drug Release from Diazepam FDM Printed Tablets Using Deep Learning Approach: Influence of Process Parameters and Tablet Surface/Volume Ratio. Int. J. Pharm. 2021, 601, 120507. [Google Scholar] [CrossRef] [PubMed]
- Windolf, H.; Chamberlain, R.; Delmotte, A.; Quodbach, J. Blind-Watermarking—Proof-of-Concept of a Novel Approach to Ensure Batch Traceability for 3D Printed Tablets. Pharmaceutics 2022, 14, 432. [Google Scholar] [CrossRef] [PubMed]
- Chamberlain, R.; Windolf, H.; Geissler, S.; Quodbach, J.; Breitkreutz, J. Precise Dosing of Pramipexole for Low-Dosed Filament Production by Hot Melt Extrusion Applying Various Feeding Methods. Pharmaceutics 2022, 14, 216. [Google Scholar] [CrossRef] [PubMed]
- dos Santos, J.; Deon, M.; da Silva, G.S.; Beck, R.C.R. Multiple Variable Effects in the Customisation of Fused Deposition Modelling 3D-Printed Medicines: A Design of Experiments (DoE) Approach. Int. J. Pharm. 2021, 597, 120331. [Google Scholar] [CrossRef] [PubMed]
- Korte, C.; Quodbach, J. Formulation Development and Process Analysis of Drug-Loaded Filaments Manufactured via Hot-Melt Extrusion for 3D-Printing of Medicines. Pharm. Dev. Technol. 2018, 23, 1117–1127. [Google Scholar] [CrossRef]
- Than, Y.M.; Titapiwatanakun, V. Tailoring Immediate Release FDM 3D Printed Tablets Using a Quality by Design (QbD) Approach. Int. J. Pharm. 2021, 599, 120402. [Google Scholar] [CrossRef]
- Pires, F.Q.; Alves-Silva, I.; Pinho, L.A.G.; Chaker, J.A.; Sa-Barreto, L.L.; Gelfuso, G.M.; Gratieri, T.; Cunha-Filho, M. Predictive Models of FDM 3D Printing Using Experimental Design Based on Pharmaceutical Requirements for Tablet Production. Int. J. Pharm. 2020, 588, 119728. [Google Scholar] [CrossRef]
- CH Harmonised Tripartite Guideline. Pharmaceutical Development. Q8 (2R). As Revised in August 2009. Available online: https://database.ich.org/sites/default/files/Q8_R2_Guideline.pdf (accessed on 2 May 2023).
- Shah, S.R.; Parikh, R.H.; Chavda, J.R.; Sheth, N.R. Application of Plackett-Burman Screening Design for Preparing Glibenclamide Nanoparticles for Dissolution Enhancement. Powder Technol. 2013, 235, 405–411. [Google Scholar] [CrossRef]
- Goo, Y.T.; Park, S.Y.; Chae, B.R.; Yoon, H.Y.; Kim, C.H.; Choi, J.Y.; Song, S.H.; Choi, Y.W. Optimization of Solid Self-Dispersing Micelle for Enhancing Dissolution and Oral Bioavailability of Valsartan Using Box-Behnken Design. Int. J. Pharm. 2020, 585, 119483. [Google Scholar] [CrossRef]
- Goyanes, A.; Wang, J.; Buanz, A.; Martínez-Pacheco, R.; Telford, R.; Gaisford, S.; Basit, A.W. 3D Printing of Medicines: Engineering Novel Oral Devices with Unique Design and Drug Release Characteristics. Mol. Pharm. 2015, 12, 4077–4084. [Google Scholar] [CrossRef] [Green Version]
- Shi, K.; Slavage, J.P.; Maniruzzaman, M.; Nokhodchi, A. Role of Release Modifiers to Modulate Drug Release from Fused Deposition Modelling (FDM) 3D Printed Tablets. Int. J. Pharm. 2021, 597, 120315. [Google Scholar] [CrossRef] [PubMed]
- Arrigo, R.; Frache, A. FDM Printability of PLA Based-Materials: The Key Role of the Rheological Behavior. Polymers 2022, 14, 1754. [Google Scholar] [CrossRef] [PubMed]
- (CVMP), Q.W.P. (QWP) C. for M.P. for H. Use (CHMP) C. for M.P. for V. Use Reflection Paper on the Dissolution Specification for Generic Solid Oral Immediate Release Products with Systemic. Available online: www.ema.europa.eu/contact (accessed on 27 April 2023).
- Skowyra, J.; Pietrzak, K.; Alhnan, M.A. Fabrication of Extended-Release Patient-Tailored Prednisolone Tablets via Fused Deposition Modelling (FDM) 3D Printing. Eur. J. Pharm. Sci. 2015, 68, 11–17. [Google Scholar] [CrossRef] [PubMed]









| Placebo Filament | PVA 1 | PVA 2 | PVA 3 | PEG | Sorb | MgSt | Comment | Printable |
|---|---|---|---|---|---|---|---|---|
| Stage 1 | ||||||||
| E1 | 10 | - | - | 1.11 | - | - | Rough surface Uneven diameter Too brittle to load into printer gear | No |
| E2 | 10 | - | - | - | 1.11 | - | Rough surface Uneven diameter Too brittle to load into printer gear | No |
| E3 | - | 10 | - | 1.11 | - | - | Rough surface Uneven diameter Can be loaded into printer gear | No |
| E4 | - | 10 | - | - | 1.11 | - | Smooth surface Even diameter Can be loaded into printer gear | Yes |
| E5 | - | - | 10 | 1.11 | - | - | Rough surface Uneven diameter Nozzle blockage | No |
| E6 | - | - | 10 | - | 1.11 | - | Smooth surface Even diameter Nozzle blockage | No |
| Stage 2 | ||||||||
| E7 | - | 10 | - | - | 1.25 | 0.15 | Smooth surface Even diameter | Yes |
| E8 | - | 10 | - | - | 1.25 | - | Smooth surface Even diameter | Yes |
| E9 | - | 10 | - | - | 1.5 | 0.15 | Smooth surface Even diameter | Yes |
| E10 | - | 10 | - | - | 1.5 | - | Smooth surface Even diameter | Yes |
| E11 | - | 10 | - | - | 1.75 | 0.15 | Rough surface Uneven diameter | No |
| E12 | - | 10 | - | - | 1.75 | - | Rough surface Uneven diameter | No |
| Experimental Run | Factors | Response | |
|---|---|---|---|
| Drug Loading, % | Infill, % | Diss 45 min, % | |
| P5I40 | 5 | 40 | 69.36 |
| P15I40 | 15 | 40 | 75.81 |
| P5I100 | 5 | 100 | 35.00 |
| P15I100 | 15 | 100 | 40.24 |
| P10I70-1 | 10 | 70 | 44.36 |
| P10I70-2 | 10 | 70 | 47.36 |
| Kinetic Model Printed Tablets | Zero-Order | First-Order | Higuchi | Korsmeyer-Peppas |
|---|---|---|---|---|
| P5I40 | k0 = 0.815 R2 = 0.930 | k1 = 0.03 R2 = 0.994 | kH = 10.708 R2 = 0.985 | n = 0.064 R2 = 0.989 |
| P15I40 | k0 = 0.839 R2 = 0.907 | k1 = 0.082 R2 = 0.978 | kH = 10.411 R2 = 0.985 | n = 0.024 R2 = 0.984 |
| P5I100 | k0 = 0.339 R2 = 0.955 | k1 = 0.014 R2 = 0.981 | kH = 6.464 R2 = 0.992 | n = 0.024 R2 = 0.996 |
| P15I100 | k0 = 0.329 R2 = 0.935 | k1 = 0.026 R2 = 0.929 | kH = 6.416 R2 = 0.991 | n = 0.013 R2 =0.995 |
| P10I70-1 | k0 = 0.337 R2 = 0.909 | k1 = 0.027 R2 = 0.949 | kH = 6.594 R2 = 0.980 | n = 0.022 R2 = 0.986 |
| P10I70-2 | k0 = 0.329 R2 = 0.897 | k1 = 0.028 R2 = 0.964 | kH = 6.567 R2 = 0.986 | n = 0.021 R2 = 0.985 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ilieva, S.; Georgieva, D.; Petkova, V.; Dimitrov, M. Study and Characterization of Polyvinyl Alcohol-Based Formulations for 3D Printlets Obtained via Fused Deposition Modeling. Pharmaceutics 2023, 15, 1867. https://doi.org/10.3390/pharmaceutics15071867
Ilieva S, Georgieva D, Petkova V, Dimitrov M. Study and Characterization of Polyvinyl Alcohol-Based Formulations for 3D Printlets Obtained via Fused Deposition Modeling. Pharmaceutics. 2023; 15(7):1867. https://doi.org/10.3390/pharmaceutics15071867
Chicago/Turabian StyleIlieva, Sofiya, Dilyana Georgieva, Valentina Petkova, and Milen Dimitrov. 2023. "Study and Characterization of Polyvinyl Alcohol-Based Formulations for 3D Printlets Obtained via Fused Deposition Modeling" Pharmaceutics 15, no. 7: 1867. https://doi.org/10.3390/pharmaceutics15071867
APA StyleIlieva, S., Georgieva, D., Petkova, V., & Dimitrov, M. (2023). Study and Characterization of Polyvinyl Alcohol-Based Formulations for 3D Printlets Obtained via Fused Deposition Modeling. Pharmaceutics, 15(7), 1867. https://doi.org/10.3390/pharmaceutics15071867