Synergistic Sustained Drug-Release System Based on Immobilized Rhamnus frangula L. Phytoextract into Layered Double Hydroxide Covered by Biocompatible Hydrogel
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
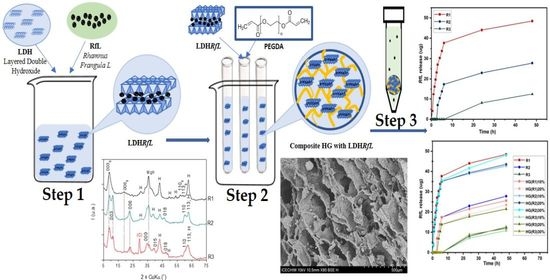
2.2. Synthesis of the LDH Matrix
2.3. The Immobilization of RfL Phytocomplex in the LDH Matrix
2.4. Synthesis of Nanocomposite Hydrogels Based on PEGDA and LDHs with Immobilized RfL Phytoextract
2.5. Equilibrium Swelling Degree (ESD) Determination
2.6. Characterization Methods and Instruments
3. Results and Discussion
3.1. Identification of the Interactions between the PEGDA Matrix and LDH-RfL by FT-IR Spectroscopy
3.2. Identification of the Interactions between the PEGDA Matrix and LDH-RfL by DR-UV-Vis Spectroscopy
3.3. Thermogravimetric (TGA/DTG) Investigation of the PEGDA/LDH-RfL Composite HGs
3.4. Morphological Characterization by XRD of the PEGDA/LDH-RfL Composite HGs
3.5. Morphological Characterization by SEM of the PEGDA/LDH-RfL Composite HGs
3.6. Equilibrium Swelling Degree
3.7. Rheological Behavior
3.8. Controlled-Release Experiments
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Mudshinge, S.R.; Deore, A.B.; Patil, S.; Bhalgat, C.M. Nanoparticles: Emerging Carriers for Drug Delivery. Saudi Pharm. J. 2011, 19, 129–141. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nagaraj, V.; Sun, X.; Mehta, J.; Martin, M.; Ngo, T.; Dey, S. Synthesis, Characterization, and In Vitro Drug Delivery Capabilities of (Zn, Al)-Based Layered Double Hydroxide Nanoparticles. J. Nanotechnol. 2015, 2015, 350370. [Google Scholar] [CrossRef] [Green Version]
- Ameena Shirin, V.K.; Sankar, R.; Johnson, A.P.; Gangadharappa, H.V.; Pramod, K. Advanced Drug Delivery Applications of Layered Double Hydroxide. J. Control. Release 2021, 330, 398–426. [Google Scholar] [CrossRef] [PubMed]
- Cavallaro, G.; Lazzara, G.; Fakhrullin, R. Mesoporous Inorganic Nanoscale Particles for Drug Adsorption and Con-trolled Release. Ther. Deliv. 2018, 9, 287–301. [Google Scholar] [CrossRef] [PubMed]
- Luo, X.; Matranga, C.; Tan, S.; Alba, N.; Cui, X.T. Carbon Nanotube Nanoreservior for Controlled Release of An-ti-Inflammatory Dexamethasone. Biomaterials 2011, 32, 6316–6323. [Google Scholar] [CrossRef] [Green Version]
- Pauli, G.; Tang, W.-L.; Li, S.-D. Development and Characterization of the Solvent-Assisted Active Loading Technology (SALT) for Liposomal Loading of Poorly Water-Soluble Compounds. Pharmaceutics 2019, 11, 465. [Google Scholar] [CrossRef] [Green Version]
- Craparo, E.F.; Drago, S.E.; Costabile, G.; Ferraro, M.; Pace, E.; Scaffaro, R.; Ungaro, F.; Cavallaro, G. Sustained-Release Powders Based on Polymer Particles for Pulmonary Delivery of Beclomethasone Dipropionate in the Treatment of Lung Inflammation. Pharmaceutics 2023, 15, 1248. [Google Scholar] [CrossRef]
- Arrabito, G.; Bonasera, A.; Prestopino, G.; Orsini, A.; Mattoccia, A.; Martinelli, E.; Pignataro, B.; Medaglia, P.G. Layered Double Hydroxides: A Toolbox for Chemistry and Biology. Crystals 2019, 9, 361. [Google Scholar] [CrossRef] [Green Version]
- Pavlovic, M.; Szerlauth, A.; Muráth, S.; Varga, G.; Szilagyi, I. Surface Modification of Two-Dimensional Layered Double Hydroxide Nanoparticles with Biopolymers for Biomedical Applications. Adv. Drug Deliv. Rev. 2022, 191, 114590. [Google Scholar] [CrossRef]
- McNamara, K.; Tofail, S.A.M. Nanoparticles in Biomedical Applications. Adv. Phys. X 2017, 2, 54–88. [Google Scholar] [CrossRef]
- Ahmed, E.M. Hydrogel: Preparation, Characterization, and Applications: A Review. J. Adv. Res. 2015, 6, 105–121. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vigata, M.; Meinert, C.; Hutmacher, D.W.; Bock, N. Hydrogels as Drug Delivery Systems: A Review of Current Char-acterization and Evaluation Techniques. Pharmaceutics 2020, 12, 1188. [Google Scholar] [CrossRef]
- Nairon, K.G.; DePalma, T.; Sivakumar, H.; Skardal, A. Tunable Hydrogel Systems for Delivery and Release of Cell-Secreted and Synthetic Therapeutic Products. In Controlled Drug Delivery Systems, 1st ed.; Opara, E., Ed.; CRC Press: Boca Raton, FL, USA, 2020; pp. 29–53. [Google Scholar]
- Naahidi, S.; Jafari, M.; Logan, M.; Wang, Y.; Yuan, Y.; Bae, H.; Dixon, B.; Chen, P. Biocompatibility of Hydrogel-Based Scaffolds for Tissue Engineering Applications. Biotechnol. Adv. 2017, 35, 530–544. [Google Scholar] [CrossRef] [PubMed]
- Loessner, D.; Meinert, C.; Kaemmerer, E.; Martine, L.C.; Yue, K.; Levett, P.A.; Klein, T.J.; Melchels, F.P.W.; Khademhosseini, A.; Hutmacher, D.W. Functionalization, Preparation and Use of Cell-Laden Gelatin Methacrylo-yl–Based Hydrogels as Modular Tissue Culture Platforms. Nat. Protoc. 2016, 11, 727–746. [Google Scholar] [CrossRef] [Green Version]
- Li, J.; Mooney, D.J. Designing Hydrogels for Controlled Drug Delivery. Nat. Rev. Mater. 2016, 1, 16071. [Google Scholar] [CrossRef] [PubMed]
- Bayat, M.R.; Baghani, M. A review on swelling theories of pH-sensitive hydrogels. J. Intell. Mat. Syst. Struct. 2021, 32. [Google Scholar] [CrossRef]
- García-Fernández, L.; Olmeda-Lozano, M.; Benito-Garzón, L.; Pérez-Caballer, A.; San Román, J.; Vázquez-Lasa, B. In-jectable Hydrogel-Based Drug Delivery System for Cartilage Regeneration. Mater. Sci. Eng. C 2020, 110, 110702. [Google Scholar] [CrossRef]
- Spatarelu, C.P.; (Radu) Chiriac, A.-L.; Cursaru, B.; Iordache, T.-V.; Gavrila, A.-M.; Cojocaru, C.-T.; Botez, R.-E.; Trica, B.; Sarbu, A.; Teodorescu, M.; et al. Composite Nanogels Based on Zeolite-Poly(Ethylene Glycol) Diacrylate for Controlled Drug Delivery. Nanomaterials 2020, 10, 195. [Google Scholar] [CrossRef] [Green Version]
- Zhang, H.; Zou, K.; Guo, S.; Duan, X. Nanostructural Drug-Inorganic Clay Composites: Structure, Thermal Property and in Vitro Release of Captopril-Intercalated Mg Al-Layered Double Hydroxides. J. Solid State Chem. 2006, 179, 1792–1801. [Google Scholar] [CrossRef]
- Mohanambe, L.; Vasudevan, S. Anionic Clays Containing Anti-Inflammatory Drug Molecules: Comparison of Mo-lecular Dynamics Simulation and Measurements. J. Phys. Chem. B 2005, 109, 15651–15658. [Google Scholar] [CrossRef]
- Chiriac, A.-L.; Gavrila, A.-M.; Cursaru, B.; Spatarelu, C.; Sandu, T.; Sarbu, A.; Teodorescu, M.; Perrin, F.; Nicolescu (Iordache), T.-V.; Zaharia (Lungu), A. Poly(Ethylene Glycol) Diacrylate-Nanogels Synthesized by Mini-Emulsion Polymer-ization. Mater. Plast. 2019, 56, 514–519. [Google Scholar] [CrossRef]
- Neblea, E.-I.; Gavrila, A.-M.; Iordache, T.-V.; Zaharia, A.; Stanescu, P.; Radu, I.-C.; Burlacu, S.; Neagu, G.; Chiriac, A.-L.; Sarbu, A. Interpenetrating Networks of Bacterial Cellulose and Poly (Ethylene Glycol) Diacrylate as Potential Cephalexin Carriers in Wound Therapy. J. Polym. Res. 2022, 29, 406. [Google Scholar] [CrossRef]
- Sârbu, A.; Zaharia, A.; Radu, A.L.; Iancu, S.; Stoica, A.; Stroescu, M.C.; Tebrencu, C.; Ionescu, E.; Sandu, T.; Apostol, S. Process for Obtaining Hybrid Hydrogels Containing Bioactive Phytoextracts. Patent RO 132253, 29 November 2021. [Google Scholar]
- Council of Europe. European Pharmacopoeia, 8th ed.; Council of Europe: Strasbourg, France, 2007; Volume 2, pp. 1949–1951. ISBN 978-92-871-7525-0. [Google Scholar]
- Chapter VIII “Frangula bark”. In Romanian Pharmacopoeia, 10th ed.; EdituraMedicala: Bucharest, Romania, 2000; p. 427.
- Chapter 6.8 “Frangulae cortex”. In European Pharmacopoeia, 6th ed.; Council of Europe: Strasbourg, France, 2008; p. 1516.
- Wagner, H.; Bladt, S. Plant Drug Analysis:A Thin Layer Chromatography Atlas, 2nd ed.; Springer: Dordrecht, The Netherlands; New York, NY, USA, 2009. [Google Scholar]
- Reich, E.; Schibli, A. High-Performance Thin-Layer Chromatography for the Analysis of Medicinal Plants, 1st ed.; Thieme: New York, NY, USA, 2007. [Google Scholar]
- Ainsworth, E.A.; Gillespie, K.M. Estimation of total phenolic content and other oxidation substrates in plant tissues using Folin-Ciocalteu reagent. Nat. Protoc. 2007, 2, 875–877. [Google Scholar] [CrossRef]
- Everette, J.D.; Bryant, Q.M.; Green, A.M.; Abbey, Y.A.; Wanglia, G.W.; Walker, R.B. Thorough study of reactivity of various classes toward the Folin-Ciocalteu reagent. J. Agr. Food Chem. 2010, 58, 8139–8181. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wiesneth, S.; Jürgenliemk, G. Total phenolic and tannins determination: A modification of Ph. Eur. 2.8. 14 for higher throughput. Die Pharm. Int. J. Pharm. Sci. 2017, 72, 195–196. [Google Scholar] [CrossRef]
- EMA (European Medicines Agency). Assessment Report on Rhamnus frangula L. cortex.EMA/HMPC/483550/2018. Committee on Herbal Medicinal Products (HMPC). 2019. Available online: www.ema.europa.eu/contact (accessed on 20 January 2023).
- Paneitz, A.; Westendorf, J. Anthranoid contents of rhubarb (Rheum undulatum L.) and other Rheum species and their toxicological relevance. Eur. Food Res. Techn. 1999, 210, 97–101. [Google Scholar] [CrossRef]
- Rauwald, H.D. Chapter 9: Herbal Laxatives: Influence of Anthrones—Anthraquinones on Energy Metabolism and Ion Transport in a Model System. In Phytomedicines of Europe; ACS Symposium Series; ACS Publications: New York, NY, USA, 1998; pp. 97–116. [Google Scholar] [CrossRef]
- Pavel, O.D.; Şerban, A.; Zăvoianu, R.; Bacalum, E.; Bîrjega, R. Curcumin Incorporation into Zn3Al Layered Double Hydroxides—Preparation, Characterization and Curcumin Release. Crystals 2020, 10, 244. [Google Scholar] [CrossRef] [Green Version]
- Teodorescu, M.; Lungu, A.; Stanescu, P.O. Constantin Neamţu Preparation and Properties of Novel Slow-Release NPK Agrochemical Formulations Based on Poly(Acrylic Acid) Hydrogels and Liquid Fertilizers. Ind. Eng. Chem. Res. 2009, 48, 6527–6534. [Google Scholar] [CrossRef]
- Cursaru, B.; Radu, A.-L.; Perrin, F.-X.; Sarbu, A.; Teodorescu, M.; Gavrilă, A.-M.; Damian, C.-M.; Sandu, T.; Iordache, T.-V.; Zaharia, A. Poly(Ethylene Glycol) Composite Hydrogels with Natural Zeolite as Filler for Controlled Delivery Applications. Macromol. Res. 2020, 28, 211–220. [Google Scholar] [CrossRef]
- Flores-Cano, D.A.; Checca-Huaman, N.-R.; Castro-Merino, I.-L.; Pinotti, C.N.; Passamani, E.C.; Litterst, F.J.; Ramos-Guivar, J.A. Progress toward Room-Temperature Synthesis and Functionalization of Iron-Oxide Nanoparticles. Int. J. Mol. Sci. 2022, 23, 8279. [Google Scholar] [CrossRef]
- Pálinkó, I.; Sipos, P.; Berkesi, O.; Varga, G. Distinguishing Anionic Species That Are Intercalated in Layered Double Hydroxides from Those Bound to Their Surface: A Comparative IR Study. J. Phys. Chem. C 2022, 126, 15254–15262. [Google Scholar] [CrossRef]
- Štěpán, V.; Vodehnal, J. Preparation and Infrared Spectra of Anthraquinone and Its Methyl Derivatives and of Quinones Related to Polynuclear Aromatic Hydrocarbons. Collect. Czech. Chem. Commun. 1971, 36, 3964–3979. [Google Scholar] [CrossRef]
- Lesiak, B.; Rangam, N.; Jiricek, P.; Gordeev, I.; Tóth, J.; Kövér, L.; Mohai, M.; Borowicz, P. Surface Study of Fe3O4 Nanoparticles Functionalized with Biocompatible Adsorbed Molecules. Front. Chem. 2019, 7, 642. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Heriyanto, H.; Heraldy, E.; Nugrahaningtyas, K.D. X-RAY Diffraction and Fourier Transform Infrared Study of Ca-Mg-Al Hydrotalcite from Artificial Brine Water with Synthesis Hydrothermal Treatments. IOP Conf. Ser. Mater. Sci. Eng. 2018, 333, 012006. [Google Scholar] [CrossRef]
- Joseph, T.; Varghese, H.T.; Panicker, C.Y.; Thiemann, T.; Viswanathan, K.; Van Alsenoy, C. Infrared and Raman Spectroscopic Analysis and Theoretical Computation of the First Hyperpolarizability of a Monoarylated Anthraqui-none, 1-(4-Methoxyphenyl)-4-Methylanthraquinone. J. Mol. Struct. 2011, 1005, 17–24. [Google Scholar] [CrossRef]
- Strens, R.G.J.; Wood, B.J. Diffuse Reflectance Spectra and Optical Properties of Some Iron and Titanium Oxides and Oxyhydroxides. Mineral. Mag. 1979, 43, 347–354. [Google Scholar] [CrossRef] [Green Version]
- Sherman, D.M.; Waite, T.D. Electronic Spectra of Fe3+ Oxides and Oxide Hydroxides in the near IR to near UV. Am. Min. 1985, 70, 1262–1269. [Google Scholar]
- Sherman, D.M. The Electronic Structures of Fe3+ Coordination Sites in Iron Oxides: Applications to Spectra, Bonding, and Magnetism. Phys. Chem. Miner. 1985, 12, 161–175. [Google Scholar] [CrossRef]
- Scheinost, A.C.; Schulze, D.G.; Schwertmann, U. Diffuse Reflectance Spectra of Al Substituted Goethite: A Ligand Field Approach. Clays Clay Miner. 1999, 47, 156–164. [Google Scholar] [CrossRef]
- Scott, A.I. Chapter 8—Application of spectral data to investigation of gross molecular structure. In Interpretation of the Ultraviolet Spectra of Natural Products; Scott, A.I., Ed.; International Series of Monographs on Organic Chemistry: Pergamon, Greek, 1964; pp. 228–312. ISBN 978-0-08-013615-8. [Google Scholar]
- Hájek, M.; Kocík, J.; Frolich, K.; Vávra, A. Mg-Fe Mixed Oxides and Their Rehydrated Mixed Oxides as Catalysts for Transesterification. J. Clean. Prod. 2017, 161, 1423–1431. [Google Scholar] [CrossRef]
- Browning, M.B.; Cereceres, S.N.; Luong, P.T.; Cosgriff-Hernandez, E.M. Determination of the In Vivo Degradation Mechanism of PEGDA Hydrogels. J. Biomed. Mater. Res. A 2014, 102, 4244–4251. [Google Scholar] [CrossRef] [Green Version]
- Royer, A.; Barriere, T.; Gelin, J.C. The Degradation of Poly(Ethylene Glycol) in an Inconel 718 Feedstock in the Metal Injection Moulding Process. Powder Technol. 2015, 284, 467–474. [Google Scholar] [CrossRef]
- Murakami, T.; Kasai, E. Reduction Mechanism of Iron Oxide–Carbon Composite with Polyethylene at Lower Temperature. ISIJ Int. 2011, 51, 9–13. [Google Scholar] [CrossRef] [Green Version]
- Spreitzer, D.; Schenk, J. Reduction of Iron Oxides with Hydrogen—A Review. Steel Res. Int. 2019, 90, 1900108. [Google Scholar] [CrossRef] [Green Version]
- Nia, S.; Pooresmaeil, M.; Namazi, H. Carboxymethylcellulose/Layered Double Hydroxides Bio-Nanocomposite Hydrogel: A Controlled Amoxicillin Nanocarrier for Colonic Bacterial Infections Treatment. Int. J. Biol. Macromol. 2020, 155, 1401–1409. [Google Scholar] [CrossRef]
- Zhang, Y.-T.; Fan, L.-H.; Zhi, T.-T.; Zhang, L.; Huang, H.; Chen, H.-L. Synthesis and Characterization of Poly(Acrylic Acid-Co-Acrylamide)/Hydrotalcite Nanocomposite Hydrogels for Carbonic Anhydrase Immobilization. J. Polym. Sci. Part A Polym. Chem. 2009, 47, 3232–3240. [Google Scholar] [CrossRef]
- Lee, W.-F.; Chen, Y.-C. Effects of Intercalated Hydrotalcite on Drug Release Behavior for Poly(Acrylic Ac-id-Co-N-Isopropyl Acrylamide)/Intercalated Hydrotalcite Hydrogels. Eur. Polym. J. 2006, 42, 1634–1642. [Google Scholar] [CrossRef]
- Shivakumara, L.R.; Demappa, T. Synthesis and Swelling Behavior of Sodium Alginate/Poly(Vinyl Alcohol) Hydrogels. Turk. J. Pharm. Sci. 2019, 16, 252–260. [Google Scholar] [CrossRef]
- Patel, S.K.; Malone, S.; Cohen, C.; Gillmor, J.R.; Colby, R.H. Elastic Modulus and Equilibrium Swelling of Poly(Dimethylsiloxane) Networks. Macromolecules 1992, 25, 5241–5251. [Google Scholar] [CrossRef]
- Ninciuleanu, C.M.; Ianchiș, R.; Alexandrescu, E.; Mihăescu, C.I.; Burlacu, S.; Trică, B.; Nistor, C.L.; Preda, S.; Scomoroscenco, C.; Gîfu, C.; et al. Adjusting Some Properties of Poly(Methacrylic Acid) (Nano)Composite Hydrogels by Means of Silicon-Containing Inorganic Fillers. Int. J. Mol. Sci. 2022, 23, 10320. [Google Scholar] [CrossRef]
- Jeung, D.-G.; Kim, H.-J.; Oh, J.-M. Incorporation of Glycine Max Merrill Extract into Layered Double Hydroxide through Ion-Exchange and Reconstruction. Nanomaterials 2019, 9, 1262. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, W.-F.; Lee, S.-C. Effect of Hydrotalcite on the Swelling and Mechanical Behaviors for the Hybrid Nano-composite Hydrogels Based on Gelatin and Hydrotalcite. J. Appl. Polym. Sci. 2006, 100, 500–507. [Google Scholar] [CrossRef]
- Rizzo, S.; Zingale, E.; Romeo, A.; Lombardo, R.; Pignatello, R. Colon Delivery of Nutraceutical Ingredients by Food-Grade Polymeric Systems: An Overview of Technological Characterization and Biological Evaluation. Appl. Sci. 2023, 13, 5443. [Google Scholar] [CrossRef]
- Rojas, R.; Mosconi, G.; Zanin, J.P.; Gil, G.A. Layered double hydroxide applications in biomedical implants. App. Clay Sci. 2022, 224, 106514. [Google Scholar] [CrossRef]
- Bhattarai, N.; Gunn, J.; Zhang, M. Chitosan-based hydrogels for controlled, localized drug delivery. Adv. Drug Deliv. Rev. 2010, 62, 83–99. [Google Scholar] [CrossRef]
- Tilman, B. Spectroscopic and kinetic study of the alkylation of phenol with dimethyl carbonate over NaX zeolite. J. Chem. Soc. Faraday Trans. 1998, 94, 985–993. [Google Scholar] [CrossRef]
- Peppas, N.A.; Brannon-Peppas, L. Water Diffusion and Sorption in Amorphous Macromolecular Systems and Foods. J. Food Eng. 1994, 22, 189–210. [Google Scholar] [CrossRef]
- Ghosal, K.; Chandra, A.; Rajabalaya, R.; Chakraborty, S.; Nanda, A. Mathematical modeling of drug release profiles for modified hydrophobic HPMC based gels. Pharmazie 2012, 67, 147–155. [Google Scholar] [CrossRef]










| Sample Code | Cationic Composition of LDHs | PEGDA (g) | TMEDA (g) | APS (g) | LDHs (g) | LDHsRfL (g) | DW (g) |
|---|---|---|---|---|---|---|---|
| HG | 0 | 0.5 | 0.00645 | 0.01 | - | - | 4.99 |
| HG (H1)10% | Mg, Fe | 0.5 | 0.00645 | 0.01 | 0.05 | - | 4.99 |
| HG (H1)20% | Mg, Fe | 0.5 | 0.00645 | 0.01 | 0.1 | - | 4.99 |
| HG (H2)10% | Mg, Fe, Al | 0.5 | 0.00645 | 0.01 | 0.05 | - | 4.99 |
| HG (H2)20% | Mg, Fe, Al | 0.5 | 0.00645 | 0.01 | 0.1 | - | 4.99 |
| HG (H2)30% | Mg, Fe, Al | 0.5 | 0.00645 | 0.01 | 0.15 | - | 4.99 |
| HG (H3)10% | Mg, Ca, Fe, Al | 0.5 | 0.00645 | 0.01 | 0.05 | - | 4.99 |
| HG (H3)20% | Mg, Ca, Fe, Al | 0.5 | 0.00645 | 0.01 | 0.1 | - | 4.99 |
| HG (H3)30% | Mg, Ca, Fe, Al | 0.5 | 0.00645 | 0.01 | 0.15 | - | 4.99 |
| HG (R1)10% | Mg, Fe | 0.5 | 0.00645 | 0.01 | - | 0.05 | 4.99 |
| HG (R1)20% | Mg, Fe | 0.5 | 0.00645 | 0.01 | - | 0.1 | 4.99 |
| HG (R2)10% | Mg, Fe, Al | 0.5 | 0.00645 | 0.01 | - | 0.05 | 4.99 |
| HG (R2)20% | Mg, Fe, Al | 0.5 | 0.00645 | 0.01 | - | 0.1 | 4.99 |
| HG (R2)30% | Mg, Fe, Al | 0.5 | 0.00645 | 0.01 | - | 0.15 | 4.99 |
| HG (R3)10% | Mg, Ca, Fe, Al | 0.5 | 0.00645 | 0.01 | - | 0.05 | 4.99 |
| HG (R3)20% | Mg, Ca, Fe, Al | 0.5 | 0.00645 | 0.01 | - | 0.1 | 4.99 |
| HG (R3)30% | Mg, Ca, Fe, Al | 0.5 | 0.00645 | 0.01 | - | 0.15 | 4.99 |
| Sample | Ti—Tf (°C) Δm (%) | Ti—Tf (°C) Δm (%) | Ti—Tf (°C) Δm (%) | Ti—Tf (°C) Δm (%) |
|---|---|---|---|---|
| H1 | RT–209 −11.41% | 209–500 −22.42% | 500–569 −5.01% | - |
| H2 | RT–223 −13.20% | 223–494 −22.71% | 494–543 −2.79% | - |
| H3 | RT–231 −14.00% | 223–550 −19.74% | 664–773 −2.92% | - |
| C1 | RT–500 −2.4% | 500–784 −1.25% | - | - |
| C2 | RT–500 −3.2% | 500–784 −1.65% | - | - |
| C3 | RT–500 −2.5% | 500–784 −5.75% | - | - |
| R1 | RT–198 −6.50% | 198–560 −16.98% | - | - |
| R2 | RT–213 −11.57% | 213–552 −22.34% | - | - |
| R3 | RT–214 −10.62% | 214–556 −18.6% | 656–773 −2.66% | - |
| RfL Dried Extract | RT–184 −5.83% | 184–325 −31.85% | 325–652 −61.35% | - |
| HG | RT–160 +1.37% | 160–291 −34.79% | 291–480 −53.6% | 480–565 −8.46% |
| HG(H1)20% | RT–170 −1.04% | 170–370 −26.39% | 370–427 −42.22% | 427–525 −15.29% |
| HG(H2)30% | RT–167 −1.2% | 167–360 −29.44% | 360–425 −40.46% | 425–525 −15.02% |
| HG(H3)30% | RT–169 −1.77% | 169–360 −48.45% | 360–424 −22.99% | 424–506 −11.89% |
| HG(R1)20% | RT–200 −3.12% | 200–352 −38.75% | 352–411 −22.47% | 411–526 −17.24% |
| HG(R2)30% | RT–190 −1.24% | 190–354 −41.23% | 354–422 −30.4% | 422–523 −15.33% |
| HG(R3)30% | RT–188 −2.68% | 188–355 −34.1% | 355–424 −32.76% | 424–522 −15.03% |
| Sample | α (Å) | χ (Å) |
|---|---|---|
| H1 | 3.043 | 22.997 |
| H2 | 3.083 | 22.992 |
| H3 | 3.081 | 22.636 |
| R1 | 2.960 | 23.084 |
| R2 | 3.017 | 24.056 |
| R3 | 3.048 | 23.057 |
| HG(R1)20% | 2.955 | 24.218 |
| HG(R2)30% | 3.013 | 24.971 |
| HG(R3)30% | 3.010 | 25.325 |
| Sample | Zero-Order | First-Order | Higuchi | Korsmeyer-Peppas | |||||
|---|---|---|---|---|---|---|---|---|---|
| k0, μg/mL | R2 | k1, min−1 | R2 | kH, µg/mL·min−1/2 | R2 | k | n | R2 | |
| R1 | 0.701 | 0.565 | 0.026 | 0.305 | 6.241 | 0.745 | 3.358 | 0.464 | 0.760 |
| R2 | 0.390 | 0.806 | 0.022 | 0.641 | 3.517 | 0.877 | 3.673 | 0.420 | 0.824 |
| R3 | - | - | - | - | - | - | - | - | - |
| HG(R1)10% | 0.338 | 0.760 | 0.020 | 0.6 | 3.059 | 0.83 | 3.654 | 0.387 | 0.787 |
| HG(R1)20% | 0.320 | 0.858 | 0.021 | 0.719 | 2.914 | 0.906 | 3.743 | 0.405 | 0.844 |
| HG(R2)10% | 0.608 | 0.515 | 0.026 | 0.286 | 5.491 | 0.698 | 3.411 | 0.472 | 0.745 |
| HG(R2)20% | 0.628 | 0.534 | 0.027 | 0.292 | 5.640 | 0.716 | 3.416 | 0.478 | 0.751 |
| HG(R2)30% | 0.714 | 0.602 | 0.028 | 0.325 | 6.281 | 0.775 | 3.401 | 0.484 | 0.779 |
| HG(R3)10% | - | - | - | - | - | - | - | - | - |
| HG(R3)20% | 0.273 | 0.813 | 0.019 | 0.67 | 2.501 | 0.867 | 3.717 | 0.368 | 0.808 |
| HG(R3)30% | - | - | - | - | - | - | - | - | - |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Neagu, A.-L.; Zaharia, A.; Pavel, O.D.; Tîrşoaga, A.; Neblea, I.E.; Dolana, S.V.; Ţebrencu, C.E.; Iordache, T.-V.; Sârbu, A.; Zăvoianu, R. Synergistic Sustained Drug-Release System Based on Immobilized Rhamnus frangula L. Phytoextract into Layered Double Hydroxide Covered by Biocompatible Hydrogel. Pharmaceutics 2023, 15, 1888. https://doi.org/10.3390/pharmaceutics15071888
Neagu A-L, Zaharia A, Pavel OD, Tîrşoaga A, Neblea IE, Dolana SV, Ţebrencu CE, Iordache T-V, Sârbu A, Zăvoianu R. Synergistic Sustained Drug-Release System Based on Immobilized Rhamnus frangula L. Phytoextract into Layered Double Hydroxide Covered by Biocompatible Hydrogel. Pharmaceutics. 2023; 15(7):1888. https://doi.org/10.3390/pharmaceutics15071888
Chicago/Turabian StyleNeagu, Ana-Lorena, Anamaria Zaharia, Octavian Dumitru Pavel, Alina Tîrşoaga, Iulia Elena Neblea, Sorin Viorel Dolana, Carmen Elena Ţebrencu, Tanta-Verona Iordache, Andrei Sârbu, and Rodica Zăvoianu. 2023. "Synergistic Sustained Drug-Release System Based on Immobilized Rhamnus frangula L. Phytoextract into Layered Double Hydroxide Covered by Biocompatible Hydrogel" Pharmaceutics 15, no. 7: 1888. https://doi.org/10.3390/pharmaceutics15071888
APA StyleNeagu, A. -L., Zaharia, A., Pavel, O. D., Tîrşoaga, A., Neblea, I. E., Dolana, S. V., Ţebrencu, C. E., Iordache, T. -V., Sârbu, A., & Zăvoianu, R. (2023). Synergistic Sustained Drug-Release System Based on Immobilized Rhamnus frangula L. Phytoextract into Layered Double Hydroxide Covered by Biocompatible Hydrogel. Pharmaceutics, 15(7), 1888. https://doi.org/10.3390/pharmaceutics15071888