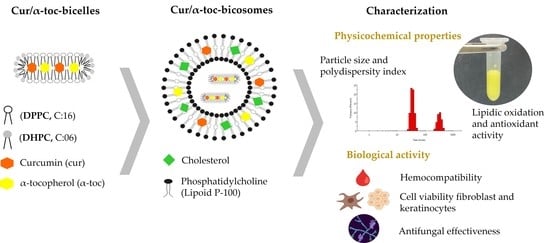
Co-Encapsulation of Curcumin and α-Tocopherol in Bicosome Systems: Physicochemical Properties and Biological Activity
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Preparation of the Systems
2.2.1. Bicelles
2.2.2. Bicosome Systems
2.3. Characterization of the Systems
2.3.1. Determination of Particle Size and Polydispersity Index (PDI)
2.3.2. Morphology
2.3.3. Fourier-Transform Infrared (FTIR) Spectroscopy
2.3.4. Determination of Encapsulation Efficiency (EE) and Loading Capacity (LC)
2.4. Lipid Oxidation
2.5. Antioxidant Activity
2.6. Hemolytic Activity
2.7. Cell Culture
Biocompatibility and Cell Viability
2.8. Antifungal Activity
2.9. Statistical Analysis
3. Results and Discussion
3.1. Characterization of Bicelles and Bicosomes
3.1.1. Particle Size and Polydispersity Index (PDI)
3.1.2. Visual Appearance and Microscopic Structure
3.1.3. Fourier-Transform Infrared Spectroscopy (FTIR)
3.1.4. Encapsulation Efficiency (EE) and Loading Capacity (LC)
3.2. Lipid Oxidation
3.3. Antioxidant Effectiveness Tests
3.4. Hemolysis and Cell Viability Assays
3.5. In Vitro Antifungal Effectiveness
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lim, H.W.; Kohli, I.; Ruvolo, E.; Kolbe, L.; Hamzavi, I.H. Impact of visible light on skin health: The role of antioxidants and free radical quenchers in skin protection. J. Am. Acad. Dermatol. 2022, 86, S27–S37. [Google Scholar] [CrossRef]
- Jing, Y.; Ruan, L.; Jiang, G.; Nie, L.; Shavandi, A.; Sun, Y.; Xu, J.; Shao, X.; Zhu, J. Regenerated silk fibroin and alginate composite hydrogel dressings loaded with curcumin nanoparticles for bacterial-infected wound closure. Biomater. Res. 2023, 149, 213405. [Google Scholar] [CrossRef] [PubMed]
- Fu, Y.S.; Chen, T.H.; Weng, L.; Huang, L.; Lai, D.; Weng, C.F. Pharmacological properties and underlying mechanisms of curcumin and prospects in medicinal potential. Biomed. Pharmacother. 2021, 141, 111888. [Google Scholar] [CrossRef]
- Shah, M.; Murad, W.; Mubin, S.; Ullah, O.; Rehman, N.U.; Rahman, M.H. Multiple health benefits of curcumin and its therapeutic potential. Environ. Sci. Pollut. Res. 2022, 29, 43732–43744. [Google Scholar] [CrossRef]
- Caddeo, C.; Manca, M.L.; Peris, J.E.; Usach, I.; Diez-Sales, O.; Matos, M.; Fernández-Busquets, X.; Fadda, A.M.; Manconi, M. Tocopherol-loaded transfersomes: In vitro antioxidant activity and efficacy in skin regeneration. Int. J. Pharm. 2018, 551, 34–41. [Google Scholar] [CrossRef]
- Akhtar, N.; Akhtar, N.; Menaa, F.; Alharbi, W.; Alaryani, F.S.S.; Alqahtani, A.M.; Ahmad, F. Fabrication of ethosomes containing tocopherol acetate to enhance transdermal permeation: In vitro and ex vivo characterizations. Gels 2022, 8, 335. [Google Scholar] [CrossRef] [PubMed]
- Cheng, H.; Fan, Q.; Liu, T.; Wusigale, L. Co-encapsulation of α-tocopherol and resveratrol in oil-in-water emulsion stabilized by sodium caseinate: Impact of polysaccharide on the stability and bioaccessibility. J. Food Eng. 2020, 264, 109685. [Google Scholar] [CrossRef]
- Na, Y.; Woo, J.; Choi, W.I.; Lee, J.H.; Hong, J.; Sung, D. α-Tocopherol-loaded reactive oxygen species-scavenging ferrocene nanocapsules with high antioxidant efficacy for wound healing. Int. J. Pharm. 2021, 596, 120205. [Google Scholar] [CrossRef]
- Jain, H.; Geetanjali, D.; Dalvi, H.; Bhat, A.; Godugu, C.; Srivastava, S. Liposome mediated topical delivery of Ibrutinib and curcumin as a synergistic approach to combat Imiquimod induced psoriasis. J. Drug Deliv. Sci. Technol. 2022, 68, 103103. [Google Scholar] [CrossRef]
- Farhoudi, L.; Kesharwani, P.; Majeed, M.; Johnston, T.P.; Sahebkar, A. Polymeric nanomicelles of curcumin: Potential applications in cancer. Int. J. Pharm. 2022, 617, 121622. [Google Scholar] [CrossRef]
- Zheng, B.; McClements, D.J. Formulation of more efficacious curcumin delivery systems using colloid science: Enhanced solubility, stability, and bioavailability. Molecules 2020, 25, 2791. [Google Scholar] [CrossRef] [PubMed]
- Tabanelli, R.; Brogi, S.; Calderone, V. Improving Curcumin Bioavailability: Current strategies and future perspectives. Pharmaceutics 2021, 13, 1715. [Google Scholar] [CrossRef] [PubMed]
- Fan, C.; Feng, T.; Wang, X.; Xia, S.; Swing, C.J. Liposomes for encapsulation of liposoluble vitamins (A, D, E and K): Comparation of loading ability, storage stability and bilayer dynamics. Food Res. Int. 2023, 163, 112264. [Google Scholar] [CrossRef]
- Abla, M.J.; Banga, A.K. Formulation of tocopherol nanocarriers and in vitro delivery into human skin. Int. J. Cosmet. Sci. 2014, 36, 239–246. [Google Scholar] [CrossRef]
- Kerdpol, K.; Nutho, B.; Krusong, K.; Poo-arporn, R.P.; Rungrotmongkol, T.; Hannongbua, S. Encapsulation of α-tocopherol in large-ring cyclodextrin containing 26 α-D-glucopyranose units: A molecular dynamics study. J. Mol. Liq. 2021, 339, 116802. [Google Scholar] [CrossRef]
- Zhang, L.; Lin, Z.; Chen, Y.; Gao, D.; Wang, P.; Lin, Y.; Wang, Y.; Wang, F.; Han, Y.; Yuan, H. Co-delivery of docetaxel and resveratrol by liposomes synergistically boosts antitumor efficiency against prostate cancer. Eur. J. Pharm. Sci. 2022, 174, 106199. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.J.; Michniak-Kohn, B. Flavosomes, novel deformable liposomes for the co-delivery of anti-inflammatory compounds to skin. Int. J. Pharm. 2020, 585, 119500. [Google Scholar] [CrossRef] [PubMed]
- Rubio, L.; Alonso, C.; Rodríguez, G.; Cócera, M.; Barbosa-Barros, L.; Coderch, L.; de la Maza, A.; Parra, J.L.; López, O. Bicellar systems as vehicle for the treatment of impaired skin. Eur. J. Pharm. Biopharm. 2014, 86, 212–218. [Google Scholar] [CrossRef]
- Rodríguez, G.; Barbosa-Barros, L.; Rubio, L.; Cócera, M.; Fernández-Campos, F.; Calpena, A.; Fernández, E.; de la Maza, A.; López, O. Bicelles: New lipid nanosystems for dermatological applications. J. Biomed. Nanotechnol. 2015, 11, 282–290. [Google Scholar] [CrossRef]
- Rodríguez, G.; Soria, G.; Coll, E.; Rubio, L.; Barbosa-Barros, L.; López-Iglesias, C.; Planas, A.; de la Maza, A.; López, O. Bicosomes: Bicelles in dilute systems. Biophys. J. 2010, 99, 480–488. [Google Scholar] [CrossRef] [Green Version]
- Moner, V.; Fernández, E.; Calpena, A.C.; Garcia-Herrera, A.; Cócera, M.; López, O. A lamellar body mimetic system for the treatment of oxazolone-induced atopic dermatitis in hairless mice. J. Dermatol. Sci. 2018, 90, 172–179. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fernández, E.; Fajarí, L.; Rodríguez, G.; Cócera, M.; Moner, V.; Barbosa Barros, L.; Kamma-Lorger, C.S.; de la Maza, A.; López, O. Reducing the harmful effects of infrared radiation on the skin using bicosomes incorporating β-Carotene. Skin Pharmacol. Physiol. 2015, 29, 169–177. [Google Scholar] [CrossRef]
- Fernández, E.; Hostachy, S.; Sandt, C.; Rodríguez, G.; Bertrand, H.C.; Clede, S.; Cócera, M.; de la Maza, A.; Lambert, F.; Policar, C.; et al. Monitoring bicosomes containing antioxidants innormal and irradiated skin. RSC Adv. 2016, 6, 72559–72567. [Google Scholar] [CrossRef]
- Fernández, E.; Rodríguez, G.; Hostachy, S.; Clede, S.; Cócera, M.; Sandt, C.; Lambert, F.; de la Maza, A.; Policar, C.; López, O. A rhenium tris-carbonyl derivative as a model molecule for incorporation into phospholipid assemblies for skin applications. Colloids Surf. B 2015, 131, 102–107. [Google Scholar] [CrossRef]
- Fernández, E.; Rodríguez, G.; Cócera, M.; Barbosa-Barros, L.; Alonso, C.; López-Iglesias, C.; Jawhari, T.; de la Maza, A.; López, O. Advanced lipid systems containing β-carotene: Stability under UV-vis radiation and application on porcine skin in vitro. Phys. Chem. Chem. Phys. 2015, 17, 18710–18721. [Google Scholar] [CrossRef] [PubMed]
- Moner, V.; Fernández, E.; Rodríguez, G.; Cócera, M.; Barbosa-Barros, L.; de la Maza, A.; López, O. Lamellar body mimetic system: An up-to-down repairing strategy of the stratum corneum lipid structure. Int. J. Pharm. 2016, 519, 135–143. [Google Scholar] [CrossRef] [PubMed]
- de la Maza, A.; López, O.; Rodríguez, G.; Rubio, L.; Barbosa-Barros, L.; Soria, G.; Planas, A. Liposome-Encapsulated Bicelles and Use Thereof in Diluted Systems. Patent EP2543360A1, 9 January 2013. [Google Scholar]
- Campani, V.; Scotti, L.; Silvestri, T.; Biondi, M.; De Rosa, G. Skin permeation and thermodynamic features of curcumin-loaded liposomes. J. Mater. Sci. Mater. Med. 2020, 31, 18. [Google Scholar] [CrossRef]
- Lind, J.; Nordin, J.; Mäler, L. Lipid dynamics in fast-tumbling bicelles with varying bilayer thickness: Effect of model transmembrane peptides. Biochim. Biophys. Acta. 2008, 1778, 2526–2534. [Google Scholar] [CrossRef] [Green Version]
- Liu, W.; Ye, A.; Liu, W.; Liu, C.; Singh, H. Stability during in vitro digestion of lactoferrin-loaded liposomes prepared from milk fat globule membrane-derived phospholipids. J. Dairy Sci. 2013, 96, 2061–2070. [Google Scholar] [CrossRef] [Green Version]
- Vergara, D.; Shene, C. Encapsulation of lactoferrin into rapeseed phospholipids based liposomes: Optimization and physicochemical characterization. J. Food Eng. 2019, 262, 29–38. [Google Scholar] [CrossRef]
- Sarmento, M.; da Silva, F.; Xavier-Júnior, F.; Oliveira, B.; Barbosa, P.; de Oliveira Borba, E.; Gonçalves, T.; Lansky, V.; Pessoa, M.; Carneiro-da-Cunha, M.G. Characterization of curcumin-loaded lecithin-chitosan bioactive nanoparticles. Carbohydr. Polym. Technol. Appl. 2021, 2, 100119. [Google Scholar]
- Talebi, V.; Ghanbarzadeh, B.; Hamishehkar, H.; Pezeshki, A.; Ostadrahimi, A. Effects of different stabilizers on colloidal properties and encapsulation efficiency of vitamin D3 loaded nano-niosomes. J. Drug Deliv. Sci. Technol. 2021, 61, 101284. [Google Scholar] [CrossRef]
- Mangolim, C.S.; Moriwaki, C.; Nogueira, A.C.; Sato, F.; Baesso, M.L.; Neto, A.M.; Matioli, G. Curcumin-β-cyclodextrin inclusion complex: Stability, solubility, characterization by FT-IR, FT-Raman, X-ray diffraction and photoacoustic spectroscopy, and food application. Food Chem. 2014, 153, 361–370. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Singh, G.; Sachdeva, R.; Rai, B.; Saini, G.G.S. Structure and vibrational spectroscopic study of alpha-tocopherol. J. Mol. Struct. 2017, 1144, 347–354. [Google Scholar] [CrossRef]
- Zhang, L.; Song, X.; Qi, Q.; Liu, W. Interaction of DPPC liposomes with cholesterol and food protein during in vitro digestion using Dynamic Light Scattering and FTIR spectroscopy analysis. Food Chem. 2022, 375, 131893. [Google Scholar] [CrossRef]
- Ng, Z.Y.; Wong, J.Y.; Panneerselvam, J.; Madheswaran, T.; Kumar, P.; Pillay, V.; Hsu, A.; Hansbro, N.; Bebawy, M.; Warke, P.; et al. Assessing the potential of liposomes loaded with curcumin as a therapeutic intervention in asthma. Colloids Surf. B Biointerfaces 2018, 172, 51–59. [Google Scholar] [CrossRef] [PubMed]
- Sepúlveda, C.T.; Alemán, A.; Zapata, J.E.; Montero, M.P.; Gómez-Guillén, M.C. Characterization and storage stability of spray dried soy-rapeseed lecithin/trehalose liposomes loaded with a tilapia viscera hydrolysate. Innov. Food Sci. Emerg. Technol. 2021, 71, 102708. [Google Scholar] [CrossRef]
- Wu, Y.; Mou, B.; Song, S.; Tan, C.P.; Lai, O.M.; Shen, C.; Cheong, L.Z. Curcumin-loaded liposomes prepared from bovine milk and krill phospholipids: Effects of chemical composition on storage stability, in-vitro digestibility and anti-hyperglycemic properties. Food Res. Int. 2020, 136, 109301. [Google Scholar] [CrossRef] [PubMed]
- Chaves, M.A.; Baldino, L.; Pinhoa, S.C.; Reverchon, E. Co-encapsulation of curcumin and vitamin D3 in mixed phospholipid nanoliposomes using a continuous supercritical CO2 assisted process. J. Taiwan Inst. Chem. Eng. 2022, 132, 104120. [Google Scholar] [CrossRef]
- Basiri, L.; Rajabzadeh, G.; Bostan, A. α-Tocopherol-loaded niosome prepared by heating method and its release behavior. Food Chem. 2017, 221, 620–628. [Google Scholar] [CrossRef] [Green Version]
- Musakhanian, J.; Rodier, J.D.; Dave, M. Oxidative stability in lipid formulations: A review of the mechanisms, drivers, and inhibitors of oxidation. AAPS PharmSciTech 2022, 23, 151. [Google Scholar] [CrossRef]
- Taladrid, D.; Marín, D.; Alemán, A.; Álvarez-Acero, I.; Montero, P.; Gómez-Guillén, M.C. Effect of chemical composition and sonication procedure on properties of food-grade soy lecithin liposomes with added glycerol. Food Res. Int. 2017, 100, 541–550. [Google Scholar] [CrossRef] [Green Version]
- Coradini, K.; Lima, F.O.; Oliveira, C.M.; Chaves, P.S.; Athayde, M.L.; Carvalho, L.M.; Beck, R.C.R. Co-encapsulation of resveratrol and curcumin in lipid-core nanocapsules improves their in vitro antioxidant effects. Eur. J. Pharm. Biopharm. 2014, 88, 178–185. [Google Scholar] [CrossRef] [PubMed]
- Barry, J.; Fritz, M.; Brender, J.R.; Smith, P.S.E.; Lee, D.K.; Ramamoorthy, A. Determining the effects of lipophilic drugs on membrane structure by solid-state NMR spectroscopy: The case of the antioxidant curcumin. J. Am. Chem. Soc. 2009, 131, 4490–4498. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, W.; Chen, X.D.; Zeneng, C.; Selomulya, C. On enhancing the solubility of curcumin by microencapsulation in whey protein isolate via spray drying. J. Food Eng. 2016, 169, 189–195. [Google Scholar] [CrossRef]
- Krilov, D.; Kosovic, M.; Serec, K. Spectroscopic studies of alpha tocopherol interaction with a model liposome and its influence on oxidation dynamics, Spectrochim. Acta A Mol. Biomol. Spectrosc. 2014, 129, 588–593. [Google Scholar] [CrossRef] [PubMed]
- Sahreen, D.; Khan, M.R.; Khan, R.A. Evaluation of antioxidant activities of various solvent extracts of Carissa opaca fruits. Food Chem. 2010, 122, 1205–1211. [Google Scholar] [CrossRef]
- Tan, C.; Xia, S.; Xue, J.; Xie, J.; Feng, B.; Zhang, X. Liposomes as vehicles for lutein: Preparation, stability, liposomal membrane dynamics, and structure. J. Agric. Food Chem. 2013, 61, 8175–8184. [Google Scholar] [CrossRef]
- Zhao, Z.; Chen, C.; Xie, C.; Zhao, Y. Design, synthesis and evaluation of liposomes modified with dendritic aspartic acid for bone-specific targeting. Chem. Phys. Lipids 2020, 226, 104832. [Google Scholar] [CrossRef]
- Lundvig, D.M.S.; Pennings, S.W.C.; Brouwer, K.M.; Mtaya-Mlangwa, M.; Mugonzibwa, E.; Kuijpers-Jagtman, A.M.; Wagener, F.A.D.T.G.; Von den Hof, J.W. Cytoprotective responses in HaCaT keratinocytes exposed to high doses of curcumin. Exp. Cell Res. 2015, 336, 298–307. [Google Scholar] [CrossRef]
- Bonferoni, M.C.; Riva, F.; Invernizzi, A.; Dellera, E.; Sandri, G.; Rossi, S.; Marrubini, G.; Bruni, G.; Vigani, B.; Caramella, C.; et al. Alpha tocopherol loaded chitosan oleate nanoemulsions for wound healing. Evaluation on cell lines and ex vivo human biopsies, and stabilization in spray dried Trojan microparticles. Eur. J. Pharm. Biopharm. 2018, 123, 31–41. [Google Scholar] [CrossRef]
- Alalwan, H.; Rajendran, R.; Lappin, D.F.; Combet, E.; Shahzad, M.; Robertson, D.; Nile, C.J.; Williams, C.; Ramage, G. The anti-adhesive effect of curcumin on candida albicans biofilms on denture materials. Front. Microbiol. 2017, 20, 659. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Al-Asmaria, F.; Mereddy, R.; Sultanbawa, Y. A novel photosensitization treatment for the inactivation of fungal spores and cells mediated by curcumin. J. Photochem. Photobiol. B Biol. 2017, 173, 301–306. [Google Scholar] [CrossRef] [PubMed]
- Rajasekar, V.; Darne, P.; Prabhune, A.; Kao, R.Y.T.; Princy Solomon, A.; Ramage, G.; Samaranayake, L.; Neelakantan, P. A curcumin-sophorolipid nanocomplex inhibits Candida albicans filamentation and biofilm development. Colloids Surf. B Biointerfaces 2021, 200, 111617. [Google Scholar] [CrossRef] [PubMed]
- Shahzad, M.; Sherry, L.; Rajendran, R.; Edwards, C.A.; Combet, E.; Ramage, G. Utilising polyphenols for the clinical management of Candida albicans biofilms. Int. J. Antimicrob. Agents 2014, 44, 269–273. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xie, Y.; Gong, X.; Jin, Z.; Xu, W.; Zhao, K. Curcumin encapsulation in self-assembled nanoparticles based on amphiphilic palmitic acid-grafted-quaternized chitosan with enhanced cytotoxic, antimicrobial and antioxidant properties. Int. J. Biol. Macromol. 2022, 222, 2855–2867. [Google Scholar] [CrossRef]






| Bicosome System | DHPC/DPPC Relación Molar (q) | Cur (µM) | α-toc (µM) | Lipoid P-100/chol Ratio | Total Bicelle Lipid Concentration (% w/v) | Total Liposome Lipid Concentration (% w/v) | Total Bicosome System Lipid Concentration (% w/v) |
|---|---|---|---|---|---|---|---|
| BA | 3.5:1 | 180 | 600 | 8:2 | 6 | 6 | 12 |
| BB | 10 | 16 | |||||
| BC | 14 | 20 |
| Bicelles | Particle Size (nm) | Volume (%) | Polydispersity Index (PDI) |
|---|---|---|---|
| Unloaded | 15 ± 0.1 a | 99 ± 1 a | 0.26 ± 0.07 a |
| Loaded | 16 ± 1 a | 99 ± 1 a | 0.28 ± 0.02 a |
| Bicosome Systems | Peak 1 | Peak 2 | ||
|---|---|---|---|---|
| Particle Size (nm) | Volume (%) | Particle Size (nm) | Volume (%) | |
| BA | 31 ± 0.5 b | 63 ± 14 a | 420 ± 7 a | 20 ± 9 a |
| BB | 55 ± 5 a | 57 ± 5 a | 388 ± 6 a | 38 ± 7 a |
| BC | 60 ± 8 a | 69 ± 4 a | 303 ± 1 b | 27 ± 1 a |
| Bicosome Systems | EE cur (%) | EE α-toc (%) | LC cur/α-toc (%) | Hemolysis (%) |
|---|---|---|---|---|
| BA | 56 ± 5 b | 58 ± 8 b | 52 ± 0.4 b | ND |
| BB | 69 ± 4 a | 64 ± 2 a | 65 ± 2 a | ND |
| BC | 77 ± 2 a | 65 ± 3 a | 67 ± 2 a | ND |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vergara, D.; López, O.; Sanhueza, C.; Chávez-Aravena, C.; Villagra, J.; Bustamante, M.; Acevedo, F. Co-Encapsulation of Curcumin and α-Tocopherol in Bicosome Systems: Physicochemical Properties and Biological Activity. Pharmaceutics 2023, 15, 1912. https://doi.org/10.3390/pharmaceutics15071912
Vergara D, López O, Sanhueza C, Chávez-Aravena C, Villagra J, Bustamante M, Acevedo F. Co-Encapsulation of Curcumin and α-Tocopherol in Bicosome Systems: Physicochemical Properties and Biological Activity. Pharmaceutics. 2023; 15(7):1912. https://doi.org/10.3390/pharmaceutics15071912
Chicago/Turabian StyleVergara, Daniela, Olga López, Claudia Sanhueza, Catalina Chávez-Aravena, José Villagra, Mariela Bustamante, and Francisca Acevedo. 2023. "Co-Encapsulation of Curcumin and α-Tocopherol in Bicosome Systems: Physicochemical Properties and Biological Activity" Pharmaceutics 15, no. 7: 1912. https://doi.org/10.3390/pharmaceutics15071912
APA StyleVergara, D., López, O., Sanhueza, C., Chávez-Aravena, C., Villagra, J., Bustamante, M., & Acevedo, F. (2023). Co-Encapsulation of Curcumin and α-Tocopherol in Bicosome Systems: Physicochemical Properties and Biological Activity. Pharmaceutics, 15(7), 1912. https://doi.org/10.3390/pharmaceutics15071912