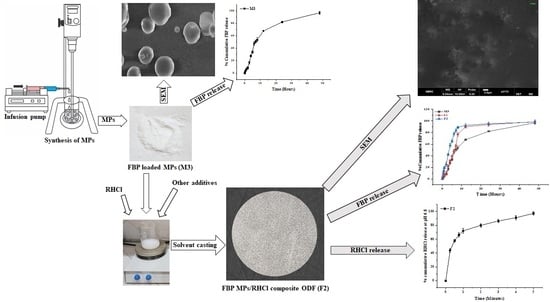
In Vitro and In Vivo Evaluation of Composite Oral Fast Disintegrating Film: An Innovative Strategy for the Codelivery of Ranitidine HCl and Flurbiprofen
Abstract
:1. Introduction
2. Material and Methods
2.1. Material
2.2. Methods
2.2.1. Fabrication of FBP Microparticles (MPs)
2.2.2. Screening for Optimized Blank ODF
2.2.3. Fabrication of Composite ODF
Incorporation of Dual Drug in Composite ODF
2.2.4. Characterization of MPs
Rheological Studies of MPs
Solid State Characterization of MPs
Drug Content in MPs
In Vitro FBP Release from MPs
2.2.5. Characterization of Composite ODF
Physical Properties
- Thickness
- Disintegration Time (DT)
Mechanical Properties
- Folding endurance
- Tensile strength
Solid State Characterization
Drug Contents
In Vitro Drug Release
2.2.6. In Vivo Studies
Anti-Inflammatory Activity
- Ct = left hind paw thickness (mm) at time t
- C0 = left hind paw thickness (mm) before carrageenan injection
- (Ct − C0) control = increase in paw size after carrageenan injection to control rats at time t
- (Ct − C0) treated = increase in paw size after carrageenan injection to treated rats at time t.
Pro-Inflammatory Cytokines
In Vivo Evaluation of Gastroprotective Effect
- Gastric lesion index (GLI)
- Histological evaluation
3. Results and Discussion
3.1. Fabrication and Evaluation FBP Loaded MPs
3.1.1. Solid State Characterization of FBP Loaded MPs
3.1.2. Drug Release from FBP Loaded MPs
3.2. Fabrication and Evaluation of Blank ODF
3.3. Fabrication of Composite ODF
3.4. Physical Properties of Composite ODF
3.4.1. Thickness
3.4.2. In Vitro DT
3.5. Mechanical Properties of Composite ODF
3.5.1. Folding Endurance
3.5.2. Tensile Strength
3.6. Solid State Characterization of Composite ODF
3.6.1. SEM
3.6.2. FTIR
3.6.3. XRD
3.6.4. DSC
3.6.5. TGA
3.7. In Vitro Drug Release from Composite ODF
3.8. In Vivo Studies
3.8.1. Anti-Inflammatory Activity
3.8.2. Detection of Pro-Inflammatory Cytokines
3.8.3. In Vivo Evaluation of Gastroprotective Effect
Gastric Lesion Index (GLI)
Histological Evaluation
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Khan, M.S.; Roberts, M.S. Challenges and innovations of drug delivery in older age. Adv. Drug Deliv. Rev. 2018, 135, 3–38. [Google Scholar] [CrossRef] [PubMed]
- Eedara, B.B.; Nyavanandi, D.; Narala, S.; Veerareddy, P.R.; Bandari, S. Improved Dissolution Rate and Intestinal Absorption of Fexofenadine Hydrochloride by the Preparation of Solid Dispersions: In Vitro and In Situ Evaluation. Pharmaceutics 2021, 13, 310. [Google Scholar] [CrossRef] [PubMed]
- Celebioglu, A.; Uyar, T. Fast-dissolving antioxidant curcumin/cyclodextrin inclusion complex electrospun nanofibrous webs. Food Chem. 2020, 317, 126397. [Google Scholar] [CrossRef] [PubMed]
- Qushawy, M.; Prabahar, K.; Abd-Alhaseeb, M.; Swidan, S.; Nasr, A. Preparation and evaluation of carbamazepine solid lipid nanoparticle for alleviating seizure activity in pentylenetetrazole-kindled mice. Molecules 2019, 24, 3971. [Google Scholar] [CrossRef] [Green Version]
- Elbadawi, M.; Nikjoo, D.; Gustafsson, T.; Gaisford, S.; Basit, A. Pressure-assisted microsyringe 3D printing of oral films based on pullulan and hydroxypropyl methylcellulose. Int. J. Pharm. 2021, 595, 120197. [Google Scholar] [CrossRef]
- Ouda, G.I.; Dahmash, E.Z.; Alyami, H.; Iyire, A. A novel technique to improve drug loading capacity of fast/extended release orally dissolving films with potential for paediatric and geriatric drug delivery. AAPS PharmSciTech 2020, 21, 126. [Google Scholar] [CrossRef]
- Musazzi, U.M.; Khalid, G.M.; Selmin, F.; Minghetti, P.; Cilurzo, F. Trends in the production methods of orodispersible films. Int. J. Pharm. 2020, 576, 118963. [Google Scholar] [CrossRef]
- Shankar Raman, S.; Narayanan, V.H.B.; Durai, R. Lamotrigine Nanoparticle Laden Polymer Composite Oral Dissolving Films for Improving Therapeutic Potential of the Hydrophobic Antiepileptic Molecule. ASSAY Drug Dev. Technol. 2021, 19, 2–16. [Google Scholar] [CrossRef]
- Musazzi, U.M.; Dolci, L.S.; Albertini, B.; Passerini, N.; Cilurzo, F. A new melatonin oral delivery platform based on orodispersible films containing solid lipid microparticles. Int. J. Pharm. 2019, 559, 280–288. [Google Scholar] [CrossRef] [Green Version]
- Łyszczarz, E.; Hofmanová, J.; Szafraniec-Szczęsny, J.; Jachowicz, R. Orodispersible films containing ball milled aripiprazole-poloxamer® 407 solid dispersions. Int. J. Pharm. 2020, 575, 118955. [Google Scholar] [CrossRef]
- Akyüz, L.; Duman, F. Encapsulation of flurbiprofen by chitosan using a spray-drying method with in vitro drug releasing and molecular docking. Turk. J. Pharm. Sci. 2017, 14, 34. [Google Scholar] [CrossRef] [PubMed]
- Chieng, N.; Aaltonen, J.; Saville, D.; Rades, T. Physical characterization and stability of amorphous indomethacin and ranitidine hydrochloride binary systems prepared by mechanical activation. Eur. J. Pharm. Biopharm. 2009, 71, 47–54. [Google Scholar] [CrossRef] [PubMed]
- Rashid, A.; Khan, I.U.; Khalid, S.H.; Asghar, S.; Munir, M.U. Development and evaluation of oral fast disintegrating film of ranitidine HC1 by solvent casting method. Pak. J. Pharm. Sci. 2021, 34, 1527–1535. [Google Scholar] [PubMed]
- Hanif, M.; Nazir, N.; Zia, M.U.; Chudhary, B.A.; Abbas, G.; Rana, S.J.; Zaman, M. Development of high performance liquid chomatography method for determination of flurbiprofen and ranitidine in bilayer tablets. Lat. Am. J. Pharm. 2015, 34, 1737–1742. [Google Scholar]
- Hanif, M.; Usman, Z.; Rasul, A.; Shahid, S.; Nazer, N.; Chaurasiya, V.; Sattar, S. Formulation Development and Optimization of Flurbiprufen and Ranitidine Bilayer Tablet Designed by Central Composite Rotatable Design (CCRD) and Their In Vitro Kinetic Studies. Lat. Am. J. Pharm. 2014, 33, 920–927. [Google Scholar]
- El-Setouhy, D.A.; Abd El-Malak, N.S. Formulation of a novel tianeptine sodium orodispersible film. AAPS PharmSciTech 2010, 11, 1018–1025. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lou, H.; Liu, M.; Qu, W.; Hu, Z.; Brunson, E.; Johnson, J.; Almoazen, H. Evaluation of Chlorpheniramine Maleate microparticles in orally disintegrating film and orally disintegrating tablet for pediatrics. Drug Dev. Ind. Pharm. 2014, 40, 910–918. [Google Scholar] [CrossRef]
- Prissaux, X.; Josh, A.; Dussautois, C.; Le Bihan, G.; Lefevre, P. Functional advantages of a novel modified starch over HPMC in aqueous film coating of tablets. In Proceedings of the AAPS Annual Meeting and Exposition Poster, San Antonio, TX, USA, 29 October–2 November 2006. [Google Scholar]
- Pimparade, M.B.; Vo, A.; Maurya, A.S.; Bae, J.; Morott, J.T.; Feng, X.; Kim, D.W.; Kulkarni, V.I.; Tiwari, R.; Vanaja, K. Development and evaluation of an oral fast disintegrating anti-allergic film using hot-melt extrusion technology. Eur. J. Pharm. Biopharm. 2017, 119, 81–90. [Google Scholar] [CrossRef]
- Arya, A.; Chandra, A.; Sharma, V.; Pathak, K. Fast dissolving oral films: An innovative drug delivery system and dosage form. Int. J. ChemTech Res. 2010, 2, 576–583. [Google Scholar]
- Ahmadi, P.; Jahanban-Esfahlan, A.; Ahmadi, A.; Tabibiazar, M.; Mohammadifar, M. Development of Ethyl Cellulose-based Formulations: A Perspective on the Novel Technical Methods. Food Rev. Int. 2020, 38, 685–732. [Google Scholar] [CrossRef]
- Pi, C.; Yuan, J.; Liu, H.; Zuo, Y.; Feng, T.; Zhan, C.; Wu, J.; Ye, Y.; Zhao, L.; Wei, Y. In vitro and in vivo evaluation of curcumin loaded hollow microspheres prepared with ethyl cellulose and citric acid. Int. J. Biol. Macromol. 2018, 115, 1046–1054. [Google Scholar] [CrossRef] [PubMed]
- Thakare, M.; Israel, B.e.; Garner, S.; Ahmed, H.; Elder, D.; Capomacchia, A. Nonionic surfactant structure on the drug release, formulation and physical properties of ethylcellulose microspheres. Pharm. Dev. Technol. 2017, 22, 418–425. [Google Scholar] [CrossRef] [PubMed]
- Zafar, A.; Afzal, M.; Quazi, A.M.; Yasir, M.; Kazmi, I.; Al-Abaasi, F.A.; Alruwaili, N.K.; Alharbi, K.S.; Alzarea, S.I.; Sharma, S. Chitosan-ethyl cellulose microspheres of domperidone for nasal delivery: Preparation, in-vitro characterization, in-vivo study for pharmacokinetic evaluation and bioavailability enhancement. J. Drug Deliv. Sci. Technol. 2021, 63, 102471. [Google Scholar] [CrossRef]
- Raza, H.; Javeria, S.; Rashid, Z. Sustained released Metformin microparticles for better management of type II diabetes mellitus: In-vitro studies. Mater. Res. Express 2020, 7, 015343. [Google Scholar] [CrossRef]
- Alayoubi, A.; Haynes, L.; Patil, H.; Daihom, B.; Helms, R.; Almoazen, H. Development of a fast dissolving film of epinephrine hydrochloride as a potential anaphylactic treatment for pediatrics. Pharm. Dev. Technol. 2017, 22, 1012–1016. [Google Scholar] [CrossRef] [PubMed]
- Singh, H.; Singla, Y.P.; Narang, R.S.; Pandita, D.; Singh, S.; Narang, J.K. Frovatriptan loaded hydroxy propyl methyl cellulose/treated chitosan based composite fast dissolving sublingual films for management of migraine. J. Drug Deliv. Sci. Technol. 2018, 47, 230–239. [Google Scholar] [CrossRef]
- Lai, F.; Franceschini, I.; Corrias, F.; Sala, M.C.; Cilurzo, F.; Sinico, C.; Pini, E. Maltodextrin fast dissolving films for quercetin nanocrystal delivery. A feasibility study. Carbohydr. Polym. 2015, 121, 217–223. [Google Scholar] [CrossRef] [PubMed]
- Mehmood, Y.; Khan, I.U.; Shahzad, Y.; Khan, R.U.; Iqbal, M.S.; Khan, H.A.; Khalid, I.; Yousaf, A.M.; Khalid, S.H.; Asghar, S. In-vitro and in-vivo evaluation of velpatasvir-loaded mesoporous silica scaffolds. A prospective carrier for drug bioavailability enhancement. Pharmaceutics 2020, 12, 307. [Google Scholar] [CrossRef] [Green Version]
- Chandra, A.; Chondkar, A.D.; Shirodkar, R.; Lewis, S.A. Rapidly dissolving lacidipine nanoparticle strips for transbuccal administration. J. Drug Deliv. Sci. Technol. 2018, 47, 259–267. [Google Scholar] [CrossRef]
- Gajra, B.; Dalwadi, C.; Patel, R. Formulation and optimization of itraconazole polymeric lipid hybrid nanoparticles (Lipomer) using box behnken design. DARU J. Pharm. Sci. 2015, 23, 1–15. [Google Scholar] [CrossRef] [Green Version]
- Islam, N.; Irfan, M.; Khan, S.-U.-D.; Syed, H.K.; Iqbal, M.S.; Khan, I.U.; Mahdy, A.; Raafat, M.; Hossain, M.A.; Inam, S. Poloxamer-188 and d-α-Tocopheryl Polyethylene Glycol Succinate (TPGS-1000) Mixed Micelles Integrated Orodispersible Sublingual Films to Improve Oral Bioavailability of Ebastine; In Vitro and In Vivo Characterization. Pharmaceutics 2021, 13, 54. [Google Scholar] [CrossRef]
- Brniak, W.; Maślak, E.; Jachowicz, R. Orodispersible films and tablets with prednisolone microparticles. Eur. J. Pharm. Sci. 2015, 75, 81–90. [Google Scholar] [CrossRef] [PubMed]
- Ye, X.; Ye, X.; Ye, N. Analgesic effect of flurbiprofen ester and its effect on serum inflammatory factors and Β-endorphin expression in rats with incision pain. Trop. J. Pharm. Res. 2020, 19, 513–517. [Google Scholar] [CrossRef]
- Moon, S.-M.; Lee, S.A.; Hong, J.H.; Kim, J.-S.; Kim, D.K.; Kim, C.S. Oleamide suppresses inflammatory responses in LPS-induced RAW264. 7 murine macrophages and alleviates paw edema in a carrageenan-induced inflammatory rat model. Int. Immunopharmacol. 2018, 56, 179–185. [Google Scholar] [CrossRef] [PubMed]
- Li, W.-x.; Qian, P.; Guo, Y.-t.; Gu, L.; Jurat, J.; Bai, Y.; Zhang, D.-f. Myrtenal and β-caryophyllene oxide screened from Liquidambaris Fructus suppress NLRP3 inflammasome components in rheumatoid arthritis. BMC Complement. Med. Ther. 2021, 21, 242. [Google Scholar] [CrossRef] [PubMed]
- Al-Thamarani, S.; Gardouh, A. Enhanced oral bioavailability and gastroprotective effect of ibuprofen through mixed polymer–lipid nanoparticles. Ther. Deliv. 2021, 12, 363–374. [Google Scholar] [CrossRef]
- Tee, K.S.; Saripan, M.S.; Yap, H.Y.; Soon, C.F. Development of a mechatronic syringe pump to control fluid flow in a microfluidic device based on polyimide film. IOP Conf. Ser. Mater. Sci. Eng. 2017, 226, 012031. [Google Scholar] [CrossRef]
- USP General Chapter. 467> Organic Volatile impurities. In Chemical Tests, United States Pharmacopeia; United States Pharmacopeial Convention: North Bethesda, MD, USA, 2012. [Google Scholar]
- Barbosa, H.D.C.; Santos, B.F.F.d.; Tavares, A.A.; Barbosa, R.C.; Fook, M.V.L.; Canedo, E.L.; Silva, S.M.d.L. Inexpensive apparatus for fabricating microspheres for 5-fluorouracil controlled release systems. Int. J. Chem. Eng. 2018, 2018, 2340249. [Google Scholar] [CrossRef]
- Ranjha, N.M.; Khan, I.U.; Naseem, S. Encapsulation and characterization of flurbiprofen loaded poly (є-caprolactone)–poly (vinylpyrrolidone) blend micropheres by solvent evaporation method. J. Sol-Gel Sci. Technol. 2009, 50, 281–289. [Google Scholar] [CrossRef]
- Kim, R.M.; Jang, D.-J.; Kim, Y.C.; Yoon, J.-H.; Min, K.A.; Maeng, H.-J.; Cho, K.H. Flurbiprofen-loaded solid SNEDDS preconcentrate for the enhanced solubility, in-vitro dissolution and bioavailability in rats. Pharmaceutics 2018, 10, 247. [Google Scholar] [CrossRef] [Green Version]
- Maiti, S.; Dey, P.; Kaity, S.; Ray, S.; Maji, S.; Sa, B. Investigation on processing variables for the preparation of fluconazole-loaded ethyl cellulose microspheres by modified multiple emulsion technique. AAPS PharmSciTech 2009, 10, 703–715. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Abdellatif, A.A.; El Hamd, M.; Ali, R.; Saleh, K.I. Formulation and evaluation of oral sustained release ethyl cellulose microparticles incorporated with flurbiprofen. Bull. Pharm. Sci. Assiut 2016, 39, 27–41. [Google Scholar] [CrossRef] [Green Version]
- Bharti, K.; Mittal, P.; Mishra, B. Formulation and characterization of fast dissolving oral films containing buspirone hydrochloride nanoparticles using design of experiment. J. Drug Deliv. Sci. Technol. 2019, 49, 420–432. [Google Scholar] [CrossRef]
- Karki, S.; Kim, H.; Na, S.-J.; Shin, D.; Jo, K.; Lee, J. Thin films as an emerging platform for drug delivery. Asian J. Pharm. Sci. 2016, 11, 559–574. [Google Scholar] [CrossRef] [Green Version]
- Lai, K.L.; Fang, Y.; Han, H.; Li, Q.; Zhang, S.; Li, H.Y.; Chow, S.F.; Lam, T.N.; Lee, W.Y.T. Orally-dissolving film for sublingual and buccal delivery of ropinirole. Colloids Surf. B Biointerfaces 2018, 163, 9–18. [Google Scholar] [CrossRef]
- Pezik, E.; Gulsun, T.; Sahin, S.; Vural, İ. Development and characterization of pullulan-based orally disintegrating films containing amlodipine besylate. Eur. J. Pharm. Sci. 2021, 156, 105597. [Google Scholar] [CrossRef]
- Dixit, R.; Puthli, S. Oral strip technology: Overview and future potential. J. Control. Release 2009, 139, 94–107. [Google Scholar] [CrossRef]
- EDQM. Oromucosal preparations. In European Pharmacopoeia; European Directorate for the Quality of Medicines & Healthcare (EDQM): Strasbourg, France, 2018. [Google Scholar]
- Song, Q.; Shen, C.; Shen, B.; Lian, W.; Liu, X.; Dai, B.; Yuan, H. Development of a fast dissolving sublingual film containing meloxicam nanocrystals for enhanced dissolution and earlier absorption. J. Drug Deliv. Sci. Technol. 2018, 43, 243–252. [Google Scholar] [CrossRef]
- Prajapati, V.D.; Chaudhari, A.M.; Gandhi, A.K.; Maheriya, P. Pullulan based oral thin film formulation of zolmitriptan: Development and optimization using factorial design. Int. J. Biol. Macromol. 2018, 107, 2075–2085. [Google Scholar] [CrossRef]
- Chonkar, A.D.; Rao, J.V.; Managuli, R.S.; Mutalik, S.; Dengale, S.; Jain, P.; Udupa, N. Development of fast dissolving oral films containing lercanidipine HCl nanoparticles in semicrystalline polymeric matrix for enhanced dissolution and ex vivo permeation. Eur. J. Pharm. Biopharm. 2016, 103, 179–191. [Google Scholar] [CrossRef]
- Rédai, E.-M.; Antonoaea, P.; Todoran, N.; Vlad, R.A.; Bîrsan, M.; Tătaru, A.; Ciurba, A. Development and Evaluation of Fluoxetine Fast Dissolving Films: An Alternative for Noncompliance in Pediatric Patients. Processes 2021, 9, 778. [Google Scholar] [CrossRef]
- Mirzaei-Mohkam, A.; Garavand, F.; Dehnad, D.; Keramat, J.; Nasirpour, A. Physical, mechanical, thermal and structural characteristics of nanoencapsulated vitamin E loaded carboxymethyl cellulose films. Prog. Org. Coat. 2020, 138, 105383. [Google Scholar] [CrossRef]
- Nonsee, K.; Supitchaya, C.; Thawien, W. Antimicrobial activity and the properties of edible hydroxypropyl methylcellulose based films incorporated with encapsulated clove (Eugenia caryophyllata Thunb.) oil. Int. Food Res. J. 2011, 18, 1531. [Google Scholar]
- Shapi’i, R.; Othman, S.H. Effect of concentration of chitosan on the mechanical, morphological and optical properties of tapioca starch film. Int. Food Res. J. 2016, 23, S187. [Google Scholar]
- Darbasizadeh, B.; Motasadizadeh, H.; Foroughi-Nia, B.; Farhadnejad, H. Tripolyphosphate-crosslinked chitosan/poly (ethylene oxide) electrospun nanofibrous mats as a floating gastro-retentive delivery system for ranitidine hydrochloride. J. Pharm. Biomed. Anal. 2018, 153, 63–75. [Google Scholar] [CrossRef]
- Gulsun, T.; Borna, S.E.; Vural, I.; Sahin, S. Preparation and characterization of furosemide nanosuspensions. J. Drug Deliv. Sci. Technol. 2018, 45, 93–100. [Google Scholar] [CrossRef]
- Herrada-Manchón, H.; Rodríguez-González, D.; Fernández, M.A.; Suñé-Pou, M.; Pérez-Lozano, P.; García-Montoya, E.; Aguilar, E. 3D printed gummies: Personalized drug dosage in a safe and appealing way. Int. J. Pharm. 2020, 587, 119687. [Google Scholar] [CrossRef]
- Desai, J.; Alexander, K.; Riga, A. Characterization of polymeric dispersions of dimenhydrinate in ethyl cellulose for controlled release. Int. J. Pharm. 2006, 308, 115–123. [Google Scholar] [CrossRef]
- Trygg, J.; Yildir, E.; Kolakovic, R.; Sandler, N.; Fardim, P. Solid-State Properties and Controlled Release of Ranitidine Hydrochloride from Tailored Oxidised Cellulose Beads. Macromol. Mater. Eng. 2015, 300, 210–217. [Google Scholar] [CrossRef]
- Mirmehrabi, M.; Rohani, S.; Murthy, K.; Radatus, B. Characterization of tautomeric forms of ranitidine hydrochloride: Thermal analysis, solid-state NMR, X-ray. J. Cryst. Growth 2004, 260, 517–526. [Google Scholar] [CrossRef]
- Yasin, H.; Al-Taani, B.; Salem, M.S. Preparation and characterization of ethylcellulose microspheres for sustained-release of pregabalin. Res. Pharm. Sci. 2021, 16, 1. [Google Scholar] [CrossRef] [PubMed]
- Mahajan, H.S.; Kelkar, Y.V. Pregelatinized hydroxypropyl pea starch as matrix forming material for lyophilized orodispersible tablets of tadalafil. J. Drug Deliv. Sci. Technol. 2017, 41, 310–316. [Google Scholar] [CrossRef]
- Murtaza, G.; Ahamd, M.; Akhtar, N.; Rasool, F. A comparative study of various microencapsulation techniques: Effect of polymer viscosity on microcapsule characteristics. Pak. J. Pharm. Sci. 2009, 22. [Google Scholar]
- Ouarga, A.; Noukrati, H.; Iraola-Arregui, I.; Elaissari, A.; Barroug, A. Development of anti-corrosion coating based on phosphorylated ethyl cellulose microcapsules. Prog. Org. Coat. 2020, 148, 105885. [Google Scholar] [CrossRef]
- Zaman, M.; Hassan, R.; Razzaq, S.; Mahmood, A.; Amjad, M.W.; Raja, M.A.G.; Qaisar, A.A.; Majeed, A.; Hanif, M.; Tahir, R.A. Fabrication of polyvinyl alcohol based fast dissolving oral strips of sumatriptan succinate and metoclopramide HCL. Sci. Prog. 2020, 103, 0036850420964302. [Google Scholar] [CrossRef]
- Nalluri, B.N.; Sravani, B.; Anusha, V.S.; Sribramhini, R.; Maheswari, K. Development and evaluation of mouth dissolving films of sumatriptan succinate for better therapeutic efficacy. J. Appl. Pharm. Sci. 2013, 3, 161–166. [Google Scholar]
- Gaitano, R.O.; Calvo, N.L.; Narda, G.E.; Kaufman, T.S.; Maggio, R.M.; Brusau, E.V. Preparation and physical characterization of a diclofenac-ranitidine co-precipitate for improving the dissolution of diclofenac. J. Pharm. Sci. 2016, 105, 1258–1268. [Google Scholar] [CrossRef] [Green Version]
- Mohamed, H.I.; El-Kamel, A.H.; Hammad, G.O.; Heikal, L.A. Design of Targeted Flurbiprofen Biomimetic Nanoparticles for Management of Arthritis: In Vitro and In Vivo Appraisal. Pharmaceutics 2022, 14, 140. [Google Scholar] [CrossRef]
- Li, W.; Huang, H.; Niu, X.; Fan, T.; Mu, Q.; Li, H. Protective effect of tetrahydrocoptisine against ethanol-induced gastric ulcer in mice. Toxicol. Appl. Pharmacol. 2013, 272, 21–29. [Google Scholar] [CrossRef]
- Yang, C.; Li, T. Transdermal delivery of flurbiprofen from polyoxypropylene-polyoxyethylene block copolymer stabilized reduced graphene oxide to manage pain in spondylitis: In vitro and in vivo studies. Eur. J. Pharm. Sci. 2021, 165, 105929. [Google Scholar] [CrossRef]
- Jafar, M.; Mohsin, A.A.; Khalid, M.S.; Alshahrani, A.M.; Alkhateeb, F.S.; Alqarni, A.S. Ranitidine hydrochloride stomach specific bouyant microsponge: Preparation, in-vitro characterization, and in-vivo anti-ulcer activity. J. Drug Deliv. Sci. Technol. 2020, 55, 101453. [Google Scholar] [CrossRef]












| Code | Organic Phase | Aqueous Phase | ||
|---|---|---|---|---|
| Polymer to Drug Ratio | DCM | Surfactant (PVA 1500) | Distilled Water | |
| M1 | 1:1 | 20 mL | 0.5% w/v | 100 mL |
| M2 | 2:1 | |||
| M3 | 3:1 | |||
| Code | Lycoat® RS780 | Pearlitol Flash® | Plasticizer (% w/w of Polymer) | Water (mL) | ||
|---|---|---|---|---|---|---|
| (% w/v) | % w/w of Polymer | PG | PEG 400 | GLY | ||
| L1 | 10 | 10 | 1 | s | 10 | |
| L2 | 5 | |||||
| L3 | 10 | |||||
| L4 | 15 | |||||
| L5 | 10 | 10 | 1 | 10 | ||
| L6 | 5 | |||||
| L7 | 10 | |||||
| L8 | 15 | |||||
| L9 | 10 | 10 | 1 | 10 | ||
| L10 | 5 | |||||
| L11 | 10 | |||||
| L12 | 15 | |||||
| Code | Lycoat® RS780 (%w/v) | Glycerin (%w/w of Polymer) | Pearlitol Flash® (%w/w of Polymer) | Water (mL) |
|---|---|---|---|---|
| L13 | 1 | 10 | 10 | 10 |
| L14 | 5 | |||
| L15 | 10 | |||
| L16 | 15 | |||
| L17 | 20 |
| Code | Lycoat® RS780 (g) | GLY (g) | Pearlitol Flash® (g) | M3 (g) | RHCl (g) | Water (10 mL) |
|---|---|---|---|---|---|---|
| L15 | 1 | 0.1 | 0.1 | - | - | 10 |
| F1 | 1 | 0.1 | 0.1 | 0.15 | - | 10 |
| F2 | 1 | 0.1 | 0.1 | 0.15 | 0.15 | 10 |
| Code | Appearance | Folding Endurance | DT (Seconds) |
|---|---|---|---|
| L1 | No film form | – | – |
| L2 | Fragile and Brittle, Translucent | 137 | 111 ± 0.032 |
| L3 | Flexible, Durable, Translucent | 198 | 164 ± 0.135 |
| L4 | Ductile and Sticky, Translucent | 52 | 210 ± 0.318 |
| L5 | No Film Form | – | – |
| L6 | Fragile and Brittle, Rough Surface | 76 | 30 ± 0.177 |
| L7 | Flexible, Durable, Rough Surface | 302 | 99.30 ± 0.337 |
| L8 | Ductile and very Sticky | Not performed | 190 ± 0.178 |
| L9 | No Film Form | – | – |
| L10 | Fragile and Brittle, Smooth, Transparent | >300 | 20 ± 0.163 |
| L11 | Flexible, Durable, Smooth, Transparent | 300 | 58.59 ± 0.396 |
| L12 | Ductile and Sticky | 100 | 70 ± 0.19 |
| Code | Appearance | Folding Endurance | DT (Seconds) |
|---|---|---|---|
| L13 | No film formation | - | - |
| L14 | Very thin film formed, break during peeling | - | - |
| L15 | Smooth, flexible, durable, transparent and easily peelable film formed | >300 | 20 ± 1.52 |
| L16 | Thick, smooth, transparent and brittle in nature | 180 | 60 ± 1.83 |
| L17 | Thick, smooth, transparent and brittle in nature | 110 | 70.03 ± 1.29 |
| Code | Thickness (µm) | Folding Endurance | Tensile Strength (N/mm2) | DT (Seconds) |
|---|---|---|---|---|
| F1 | 71 ± 0.021 | 350 | 2.75 ± 0.25 | 15.63 ± 1.01 s |
| F2 | 75 ± 0.018 | 300 | 2.49 ± 0.19 | 15.01 ± 0.13 s |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rashid, A.; Khalid, S.H.; Irfan, M.; Asghar, S.; Rizg, W.Y.; Sabei, F.Y.; Alfayez, E.; Alkharobi, H.; Safhi, A.Y.; Hosny, K.M.; et al. In Vitro and In Vivo Evaluation of Composite Oral Fast Disintegrating Film: An Innovative Strategy for the Codelivery of Ranitidine HCl and Flurbiprofen. Pharmaceutics 2023, 15, 1987. https://doi.org/10.3390/pharmaceutics15071987
Rashid A, Khalid SH, Irfan M, Asghar S, Rizg WY, Sabei FY, Alfayez E, Alkharobi H, Safhi AY, Hosny KM, et al. In Vitro and In Vivo Evaluation of Composite Oral Fast Disintegrating Film: An Innovative Strategy for the Codelivery of Ranitidine HCl and Flurbiprofen. Pharmaceutics. 2023; 15(7):1987. https://doi.org/10.3390/pharmaceutics15071987
Chicago/Turabian StyleRashid, Aisha, Syed Haroon Khalid, Muhammad Irfan, Sajid Asghar, Waleed Y. Rizg, Fahad Y. Sabei, Eman Alfayez, Hanaa Alkharobi, Awaji Y. Safhi, Khaled M. Hosny, and et al. 2023. "In Vitro and In Vivo Evaluation of Composite Oral Fast Disintegrating Film: An Innovative Strategy for the Codelivery of Ranitidine HCl and Flurbiprofen" Pharmaceutics 15, no. 7: 1987. https://doi.org/10.3390/pharmaceutics15071987
APA StyleRashid, A., Khalid, S. H., Irfan, M., Asghar, S., Rizg, W. Y., Sabei, F. Y., Alfayez, E., Alkharobi, H., Safhi, A. Y., Hosny, K. M., Arshad, M. S., & Khan, I. U. (2023). In Vitro and In Vivo Evaluation of Composite Oral Fast Disintegrating Film: An Innovative Strategy for the Codelivery of Ranitidine HCl and Flurbiprofen. Pharmaceutics, 15(7), 1987. https://doi.org/10.3390/pharmaceutics15071987