Comparative Studies of the Uptake and Internalization Pathways of Different Lipid Nano-Systems Intended for Brain Delivery
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
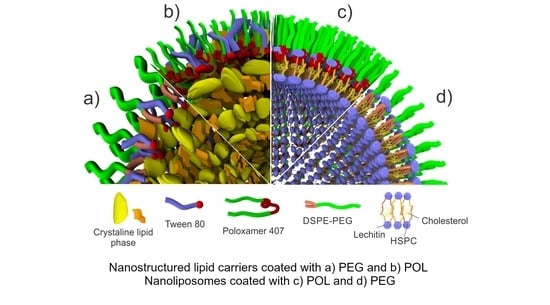
2.2. Preparation of Nanoliposomes (NL)
2.3. Preparation of Nanostructured Lipid Carriers (NLC)
2.4. Morphological Properties of Prepared Nano-Carriers
2.5. Particle Size, Particle Size Distribution, and Surface Potential of the Prepared Nano-Carriers
2.6. ATR-FTIR Spectroscopy Analysis
2.7. Protein Adsorption Studies
2.8. In Vitro Cell Culture Studies
2.8.1. hCMEC/D3 Cell Culture Lines
2.8.2. SH-SY5Y Cell Culture Lines
2.8.3. Cell Viability Assay (MTS Assay)
2.8.4. Cell Cytotoxicity Assay (LDH Assay)
2.8.5. Life-Cell Imaging (Fluorescent Microscopy)
2.8.6. Confocal Microscopy
2.8.7. Quantitative Cell Uptake Studies
2.8.8. Statistical Analysis
3. Results and Discussion
3.1. Morphological Properties
3.2. Particle Size, Particle Size Distribution, and Surface Potential
3.3. ATR-FTIR Spectroscopy Analysis
3.4. Protein Adsorption Studies
3.5. Cell Viability Assay (MTS Assay)
3.6. Cell Cytotoxicity Assay (LDH Assay)
3.7. Internalization Studies
3.8. Cellular Uptake Studies
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Alajangi, H.K.; Kaur, M.; Sharma, A.; Rana, S.; Thakur, S.; Chatterjee, M.; Singla, N.; Jaiswal, P.K.; Singh, G.; Barnwal, R.P. Blood–Brain Barrier: Emerging Trends on Transport Models and New-Age Strategies for Therapeutics Intervention against Neurological Disorders. Mol. Brain 2022, 15, 49. [Google Scholar] [CrossRef] [PubMed]
- Achar, A.; Myers, R.; Ghosh, C. Drug Delivery Challenges in Brain Disorders across the Blood–Brain Barrier: Novel Methods and Future Considerations for Improved Therapy. Biomedicines 2021, 9, 1834. [Google Scholar] [CrossRef] [PubMed]
- Zeeshan, M.; Mukhtar, M.; Ul Ain, Q.; Khan, S.; Ali, H. Nanopharmaceuticals: A Boon to the Brain-Targeted Drug Delivery. In Pharmaceutical Formulation Design—Recent Practices; IntechOpen: London, UK, 2020; p. 164. ISBN 978-1-78985-839-6. [Google Scholar]
- Puglisi-Allegra, S.; Andolina, D. Serotonin and Stress Coping. Behav. Brain Res. 2015, 277, 58–67. [Google Scholar] [CrossRef]
- Rhea, E.M.; Banks, W.A. Role of the Blood-Brain Barrier in Central Nervous System Insulin Resistance. Front. Neurosci. 2019, 13, 521. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Haque, S.; Md, S.; Alam, M.I.; Sahni, J.K.; Ali, J.; Baboota, S. Nanostructure-Based Drug Delivery Systems for Brain Targeting. Drug Dev. Ind. Pharm. 2012, 38, 387–411. [Google Scholar] [CrossRef]
- Bellettato, C.M.; Scarpa, M. Possible Strategies to Cross the Blood-Brain Barrier. Ital. J. Pediatr. 2018, 44, 131. [Google Scholar] [CrossRef] [Green Version]
- Begley, D.J. Delivery of Therapeutic Agents to the Central Nervous System: The Problems and the Possibilities. Pharmacol. Ther. 2004, 104, 29–45. [Google Scholar] [CrossRef]
- Vieira, D.B.; Gamarra, L.F. Getting into the Brain: Liposome-Based Strategies for Effective Drug Delivery across the Blood-Brain Barrier. Int. J. Nanomedicine 2016, 11, 5381–5414. [Google Scholar] [CrossRef] [Green Version]
- Skotland, T.; Iversen, T.G.; Llorente, A.; Sandvig, K. Biodistribution, Pharmacokinetics and Excretion Studies of Intravenously Injected Nanoparticles and Extracellular Vesicles: Possibilities and Challenges. Adv. Drug Deliv. Rev. 2022, 186, 114326. [Google Scholar] [CrossRef]
- Mullis, A.S.; Schlichtmann, B.W.; Narasimhan, B.; Cademartiri, R.; Mallapragada, S.K. Ligand-Cascading Nano-Delivery Devices to Enable Multiscale Targeting of Anti-Neurodegenerative Therapeutics. Biomed. Mater. 2018, 13, 034102. [Google Scholar] [CrossRef]
- Stupp, S.I. Self-Assembly and Biomaterials. Nano Lett. 2010, 10, 4783–4786. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huang, J.; Lu, Y.; Wang, H.; Liu, J.; Liao, M.; Hong, L.; Tao, R.; Ahmed, M.M.; Liu, P.; Liu, S.; et al. The Effect of Lipid Nanoparticle PEGylation on Neuroinflammatory Response in Mouse Brain. Biomaterials 2013, 34, 7960–7970. [Google Scholar] [CrossRef] [PubMed]
- Dhiman, N.; Awasthi, R.; Sharma, B.; Kharkwal, H.; Kulkarni, G.T. Lipid Nanoparticles as Carriers for Bioactive Delivery. Front. Chem. 2021, 9, 580118. [Google Scholar] [CrossRef]
- Lu, H.; Zhang, S.; Wang, J.; Chen, Q. A Review on Polymer and Lipid-Based Nanocarriers and Its Application to Nano-Pharmaceutical and Food-Based Systems. Front. Nutr. 2021, 8, 783831. [Google Scholar] [CrossRef] [PubMed]
- Jenkins, S.I.; Weinberg, D.; Al-Shakli, A.F.; Fernandes, A.R.; Yiu, H.H.P.; Telling, N.D.; Roach, P.; Chari, D.M. “Stealth” Nanoparticles Evade Neural Immune Cells but Also Evade Major Brain Cell Populations: Implications for PEG-Based Neurotherapeutics. J. Control. Release 2016, 224, 136–145. [Google Scholar] [CrossRef] [Green Version]
- Foroozandeh, P.; Aziz, A.A. Insight into Cellular Uptake and Intracellular Trafficking of Nanoparticles. Nanoscale Res. Lett. 2018, 13, 339. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hallan, S.S.; Sguizzato, M.; Esposito, E.; Cortesi, R. Challenges in the Physical Characterization of Lipid Nanoparticles. Pharmaceutics 2021, 13, 549. [Google Scholar] [CrossRef]
- Mihailova, L.; Tchekalarova, J.; Shalabalija, D.; Geskovski, N.; Stoilkovska Gjorgievska, V.; Stefkov, G.; Krasteva, P.; Simonoska Crcarevska, M.; Glavas Dodov, M. Lipid Nano-Carriers Loaded with Cannabis Sativa Extract for Epilepsy Treatment—In Vitro Characterization and In Vivo Efficacy Studies. J. Pharm. Sci. 2022, 111, 3384–3396. [Google Scholar] [CrossRef]
- Dimchevska, S.; Geskovski, N.; Koliqi, R.; Matevska-Geskovska, N.; Gomez Vallejo, V.; Szczupak, B.; Sebastian, E.S.; Llop, J.; Hristov, D.R.; Monopoli, M.P.; et al. Efficacy Assessment of Self-Assembled PLGA-PEG-PLGA Nanoparticles: Correlation of Nano-Bio Interface Interactions, Biodistribution, Internalization and Gene Expression Studies. Int. J. Pharm. 2017, 533, 389–401. [Google Scholar] [CrossRef]
- Shalabalija, D.; Mihailova, L.; Crcarevska, M.S.; Karanfilova, I.C.; Ivanovski, V.; Nestorovska, A.K.; Novotni, G.; Dodov, M.G. Formulation and Optimization of Bioinspired Rosemary Extract Loaded PEGylated Nanoliposomes for Potential Treatment of Alzheimer’s Disease Using Design of Experiments. J. Drug Deliv. Sci. Technol. 2021, 63, 102434. [Google Scholar] [CrossRef]
- Bozzuto, G.; Molinari, A. Liposomes as Nanomedical Devices. Int. J. Nanomed. 2015, 10, 975–999. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, Y.; Tan, J.; Thomas, A.; Ou-Yang, D.; Muzykantov, V.R. The Shape of Things to Come: Importance of Design in Nanotechnology for Drug Delivery. Ther. Deliv. 2012, 3, 181–194. [Google Scholar] [CrossRef] [Green Version]
- Wang, S.; Allen, N.; Vickers, T.A.; Revenko, A.S.; Sun, H.; Liang, X.-H.; Crooke, S.T. Cellular Uptake Mediated by Epidermal Growth Factor Receptor Facilitates the Intracellular Activity of Phosphorothioate-Modified Antisense Oligonucleotides. Nucleic Acids Res. 2018, 46, 3579–3594. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Singh, S.; Gupta, B. Size-Controlled Preparation of Nanosoy for Potential Biomedical Applications. Polym. Int. 2016, 65, 1373–1381. [Google Scholar] [CrossRef]
- Patil, S.; Sandberg, A.; Heckert, E.; Self, W.; Seal, S. Protein Adsorption and Cellular Uptake of Cerium Oxide Nanoparticles as a Function of Zeta Potential. Biomaterials 2007, 28, 4600–4607. [Google Scholar] [CrossRef] [Green Version]
- Abstiens, K.; Maslanka Figueroa, S.; Gregoritza, M.; Goepferich, A.M. Interaction of Functionalized Nanoparticles with Serum Proteins and Its Impact on Colloidal Stability and Cargo Leaching. Soft Matter 2019, 15, 709–720. [Google Scholar] [CrossRef]
- Wang, W.; Huang, Z.; Li, Y.; Wang, W.; Shi, J.; Fu, F.; Huang, Y.; Pan, X.; Wu, C. Impact of Particle Size and PH on Protein Corona Formation of Solid Lipid Nanoparticles: A Proof-of-Concept Study. Acta Pharm. Sin. B 2021, 11, 1030–1046. [Google Scholar] [CrossRef]
- Alayoubi, A.; Alqahtani, S.; Kaddoumi, A.; Nazzal, S. Effect of PEG Surface Conformation on Anticancer Activity and Blood Circulation of Nanoemulsions Loaded with Tocotrienol-Rich Fraction of Palm Oil. AAPS J. 2013, 15, 1168–1179. [Google Scholar] [CrossRef] [Green Version]
- Yu, Y.; Luan, Y.; Dai, W. Dynamic Process, Mechanisms, Influencing Factors and Study Methods of Protein Corona Formation. Int. J. Biol. Macromol. 2022, 205, 731–739. [Google Scholar] [CrossRef]
- Guo, C.; Liu, H.; Wang, J.; Chen, J. Conformational Structure of Triblock Copolymers by FT-Raman and FTIR Spectroscopy. J. Colloid Interface Sci. 1999, 209, 368–373. [Google Scholar] [CrossRef]
- Markova, E.; Taneska, L.; Kostovska, M.; Shalabalija, D.; Mihailova, L.; Glavas Dodov, M.; Makreski, P.; Geskovski, N.; Petrushevska, M.; Taravari, A.N.; et al. Design and Evaluation of Nanostructured Lipid Carriers Loaded with Salvia Officinalis Extract for Alzheimer’s Disease Treatment. J. Biomed. Mater. Res. Part B Appl. Biomater. 2022, 110, 1368–1390. [Google Scholar] [CrossRef] [PubMed]
- Bros, M.; Nuhn, L.; Simon, J.; Moll, L.; Mailänder, V.; Landfester, K.; Grabbe, S. The Protein Corona as a Confounding Variable of Nanoparticle-Mediated Targeted Vaccine Delivery. Front. Immunol. 2018, 9, 1760. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Guan, J.; Jiang, Z.; Yang, Y.; Liu, J.; Hua, W.; Mao, Y.; Li, C.; Lu, W.; Qian, J.; et al. Brain-Targeted Drug Delivery by Manipulating Protein Corona Functions. Nat. Commun. 2019, 10, 3561. [Google Scholar] [CrossRef] [Green Version]
- Tekie, F.S.M.; Hajiramezanali, M.; Geramifar, P.; Raoufi, M.; Dinarvand, R.; Soleimani, M.; Atyabi, F. Controlling Evolution of Protein Corona: A Prosperous Approach to Improve Chitosan-Based Nanoparticle Biodistribution and Half-Life. Sci. Rep. 2020, 10, 9664. [Google Scholar] [CrossRef] [PubMed]
- Pelaz, B.; del Pino, P.; Maffre, P.; Hartmann, R.; Gallego, M.; Rivera-Fernández, S.; de la Fuente, J.M.; Nienhaus, G.U.; Parak, W.J. Surface Functionalization of Nanoparticles with Polyethylene Glycol: Effects on Protein Adsorption and Cellular Uptake. ACS Nano 2015, 9, 6996–7008. [Google Scholar] [CrossRef] [PubMed]
- Göppert, T.M.; Müller, R.H. Protein Adsorption Patterns on Poloxamer- and Poloxamine-Stabilized Solid Lipid Nanoparticles (SLN). Eur. J. Pharm. Biopharm. 2005, 60, 361–372. [Google Scholar] [CrossRef]
- Simonoska Crcarevska, M.; Geskovski, N.; Calis, S.; Dimchevska, S.; Kuzmanovska, S.; Petruševski, G.; Kajdžanoska, M.; Ugarkovic, S.; Goracinova, K. Definition of Formulation Design Space, in Vitro Bioactivity and in Vivo Biodistribution for Hydrophilic Drug Loaded PLGA/PEO-PPO-PEO Nanoparticles Using OFAT Experiments. Eur. J. Pharm. Sci. 2013, 49, 65–80. [Google Scholar] [CrossRef]
- Stolnik, S.; Daudali, B.; Arien, A.; Whetstone, J.; Heald, C.R.; Garnett, M.C.; Davis, S.S.; Illum, L. The Effect of Surface Coverage and Conformation of Poly(Ethylene Oxide) (PEO) Chains of Poloxamer 407 on the Biological Fate of Model Colloidal Drug Carriers. Biochim. Biophys. Acta (BBA) Biomembr. 2001, 1514, 261–279. [Google Scholar] [CrossRef] [Green Version]
- Strachan, J.B.; Dyett, B.P.; Nasa, Z.; Valery, C.; Conn, C.E. Toxicity and Cellular Uptake of Lipid Nanoparticles of Different Structure and Composition. J. Colloid Interface Sci. 2020, 576, 241–251. [Google Scholar] [CrossRef]
- Gaspar, D.P.; Faria, V.; Gonçalves, L.M.D.; Taboada, P.; Remuñán-López, C.; Almeida, A.J. Rifabutin-Loaded Solid Lipid Nanoparticles for Inhaled Antitubercular Therapy: Physicochemical and in Vitro Studies. Int. J. Pharm. 2016, 497, 199–209. [Google Scholar] [CrossRef]
- Silva, S.; Marto, J.; Gonçalves, L.; Almeida, A.J.; Vale, N. Formulation, Characterization and Evaluation against SH-SY5Y Cells of New Tacrine and Tacrine-MAP Loaded with Lipid Nanoparticles. Nanomaterials 2020, 10, 2089. [Google Scholar] [CrossRef] [PubMed]
- Benson, B.A.; Vercellotti, G.M.; Dalmasso, A.P. IL-4 and IL-13 Induce Protection from Complement and Melittin in Endothelial Cells despite Initial Loss of Cytoplasmic Proteins: Membrane Resealing Impairs Quantifying Cytotoxicity with the Lactate Dehydrogenase Permeability Assay. Xenotransplantation 2015, 22, 295–301. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chan, F.K.-M.; Moriwaki, K.; De Rosa, M.J. Detection of Necrosis by Release of Lactate Dehydrogenase (LDH) Activity. Methods Mol. Biol. 2013, 979, 65–70. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rai, Y.; Pathak, R.; Kumari, N.; Sah, D.K.; Pandey, S.; Kalra, N.; Soni, R.; Dwarakanath, B.S.; Bhatt, A.N. Mitochondrial Biogenesis and Metabolic Hyperactivation Limits the Application of MTT Assay in the Estimation of Radiation Induced Growth Inhibition. Sci. Rep. 2018, 8, 1531. [Google Scholar] [CrossRef] [Green Version]
- Smith, B.H.; Gazda, L.S.; Conn, B.L.; Jain, K.; Asina, S.; Levine, D.M.; Parker, T.S.; Laramore, M.A.; Martis, P.C.; Vinerean, H.V.; et al. Three-Dimensional Culture of Mouse Renal Carcinoma Cells in Agarose Macrobeads Selects for a Subpopulation of Cells with Cancer Stem Cell or Cancer Progenitor Properties. Cancer Res. 2011, 71, 716–724. [Google Scholar] [CrossRef] [Green Version]
- Chenthamara, D.; Subramaniam, S.; Ramakrishnan, S.G.; Krishnaswamy, S.; Essa, M.M.; Lin, F.-H.; Qoronfleh, M.W. Therapeutic Efficacy of Nanoparticles and Routes of Administration. Biomater. Res. 2019, 23, 20. [Google Scholar] [CrossRef]
- Nguyen, V.H.; Thuy, V.N.; Van, T.V.; Dao, A.H.; Lee, B.-J. Nanostructured Lipid Carriers and Their Potential Applications for Versatile Drug Delivery via Oral Administration. OpenNano 2022, 8, 100064. [Google Scholar] [CrossRef]
- Torchilin, V.P.; Levchenko, T.S.; Rammohan, R.; Volodina, N.; Papahadjopoulos-Sternberg, B.; D’Souza, G.G.M. Cell Transfection in Vitro and in Vivo with Nontoxic TAT Peptide-Liposome-DNA Complexes. Proc. Natl. Acad. Sci. USA 2003, 100, 1972–1977. [Google Scholar] [CrossRef]
- Wang, J.; Wang, W. Significance of Physicochemical and Uptake Kinetics in Controlling the Toxicity of Metallic Nanomaterials to Aquatic Organisms. J. Zhejiang Univ. Sci. A 2014, 15, 573–592. [Google Scholar] [CrossRef] [Green Version]
- Neves, A.R.; Queiroz, J.F.; Lima, S.A.C.; Reis, S. Apo E-Functionalization of Solid Lipid Nanoparticles Enhances Brain Drug Delivery: Uptake Mechanism and Transport Pathways. Bioconjug Chem. 2017, 28, 995–1004. [Google Scholar] [CrossRef]
- Lombardo, S.M.; Schneider, M.; Türeli, A.E.; Türeli, N.G. Key for Crossing the BBB with Nanoparticles: The Rational Design. Beilstein J. Nanotechnol. 2020, 11, 866–883. [Google Scholar] [CrossRef] [PubMed]
- Shang, W.; Zhang, X.; Zhang, M.; Fan, Z.; Sun, Y.; Han, M.; Fan, L. The Uptake Mechanism and Biocompatibility of Graphene Quantum Dots with Human Neural Stem Cells. Nanoscale 2014, 6, 5799–5806. [Google Scholar] [CrossRef]
- Wu, M.; Guo, H.; Liu, L.; Liu, Y.; Xie, L. Size-Dependent Cellular Uptake and Localization Profiles of Silver Nanoparticles. Int. J. Nanomed. 2019, 14, 4247–4259. [Google Scholar] [CrossRef] [Green Version]
- Li, Y.; Wang, J.; Gao, Y.; Zhu, J.; Wientjes, M.G.; Au, J.L.-S. Relationships between Liposome Properties, Cell Membrane Binding, Intracellular Processing, and Intracellular Bioavailability. AAPS J. 2011, 13, 585–597. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shubhra, Q.T.H.; Tóth, J.; Gyenis, J.; Feczkó, T. Surface Modification of HSA Containing Magnetic PLGA Nanoparticles by Poloxamer to Decrease Plasma Protein Adsorption. Colloids Surf. B Biointerfaces 2014, 122, 529–536. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nie, S.; Hsiao, W.L.W.; Pan, W.; Yang, Z. Thermoreversible Pluronic F127-Based Hydrogel Containing Liposomes for the Controlled Delivery of Paclitaxel: In Vitro Drug Release, Cell Cytotoxicity, and Uptake Studies. Int. J. Nanomed. 2011, 6, 151–166. [Google Scholar] [CrossRef] [Green Version]
- Markoutsa, E.; Pampalakis, G.; Niarakis, A.; Romero, I.A.; Weksler, B.; Couraud, P.-O.; Antimisiaris, S.G. Uptake and Permeability Studies of BBB-Targeting Immunoliposomes Using the HCMEC/D3 Cell Line. Eur. J. Pharm. Biopharm. 2011, 77, 265–274. [Google Scholar] [CrossRef]
- Strother, L.; Miles, G.B.; Holiday, A.R.; Cheng, Y.; Doherty, G.H. Long-Term Culture of SH-SY5Y Neuroblastoma Cells in the Absence of Neurotrophins: A Novel Model of Neuronal Ageing. J. Neurosci. Methods 2021, 362, 109301. [Google Scholar] [CrossRef]
- Ottenbrite, R.M.; Javan, R. Biological Structures. In Encyclopedia of Condensed Matter Physics; Bassani, F., Liedl, G.L., Wyder, P., Eds.; Elsevier: Oxford, UK, 2005; pp. 99–108. ISBN 978-0-12-369401-0. [Google Scholar]
- Ivanov, A.I. Exocytosis and Endocytosis; Humana: Totowa, NJ, USA, 2008; Volume 440, ISBN 978-1-59745-178-9. [Google Scholar]
- Akinc, A.; Battaglia, G. Exploiting Endocytosis for Nanomedicines. Cold Spring Harb. Perspect. Biol. 2013, 5, a016980. [Google Scholar] [CrossRef] [Green Version]
- Vu, K.; Weksler, B.; Romero, I.; Couraud, P.-O.; Gelli, A. Immortalized Human Brain Endothelial Cell Line HCMEC/D3 as a Model of the Blood-Brain Barrier Facilitates In Vitro Studies of Central Nervous System Infection by Cryptococcus Neoformans. Eukaryot. Cell 2009, 8, 1803–1807. [Google Scholar] [CrossRef] [Green Version]
- Kovalevich, J.; Langford, D. Considerations for the Use of SH-SY5Y Neuroblastoma Cells in Neurobiology. Methods Mol. Biol. 2013, 1078, 9. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Harris, J.R.; Milton, N.G.N. Cholesterol in Alzheimer’s Disease and Other Amyloidogenic Disorders. Subcell. Biochem. 2010, 51, 47–75. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.; Ashizawa, A.T.; Kim, K.S.; Falk, D.J.; Notterpek, L. Liposomes to Target Peripheral Neurons and Schwann Cells. PLoS ONE 2013, 8, e78724. [Google Scholar] [CrossRef] [PubMed]













| Sample | Z-Average Diameter ± SD (nm) | PDI ± SD | ZP ± SD (mV) |
|---|---|---|---|
| NLPEG | 112.900 ± 4.726 | 0.164 ± 0.035 | −26.500 ± 2.928 |
| NLPOL | 123.000 ± 1.365 | 0.270 ± 0.006 | −31.201 ± 3.326 |
| NLCPEG | 104.000 ± 3.200 | 0.191 ± 0.065 | −24.600 ± 0.833 |
| NLCPOL | 106.700 ± 2.612 | 0.181 ± 0.024 | −24.301 ± 0.850 |
| NL | NLC | ||||
|---|---|---|---|---|---|
| 0 | PEG | POL | 0 | PEG | POL |
| 29.254 ± 0.534% | 8.042 ± 4.032% | 14.802 ± 6.609% | 22.727 ± 5.221% | 13.054 ± 2.577% | 13.636 ± 2.293% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mihailova, L.; Shalabalija, D.; Zimmer, A.; Geskovski, N.; Makreski, P.; Petrushevska, M.; Simonoska Crcarevska, M.; Glavas Dodov, M. Comparative Studies of the Uptake and Internalization Pathways of Different Lipid Nano-Systems Intended for Brain Delivery. Pharmaceutics 2023, 15, 2082. https://doi.org/10.3390/pharmaceutics15082082
Mihailova L, Shalabalija D, Zimmer A, Geskovski N, Makreski P, Petrushevska M, Simonoska Crcarevska M, Glavas Dodov M. Comparative Studies of the Uptake and Internalization Pathways of Different Lipid Nano-Systems Intended for Brain Delivery. Pharmaceutics. 2023; 15(8):2082. https://doi.org/10.3390/pharmaceutics15082082
Chicago/Turabian StyleMihailova, Ljubica, Dushko Shalabalija, Andreas Zimmer, Nikola Geskovski, Petre Makreski, Marija Petrushevska, Maja Simonoska Crcarevska, and Marija Glavas Dodov. 2023. "Comparative Studies of the Uptake and Internalization Pathways of Different Lipid Nano-Systems Intended for Brain Delivery" Pharmaceutics 15, no. 8: 2082. https://doi.org/10.3390/pharmaceutics15082082
APA StyleMihailova, L., Shalabalija, D., Zimmer, A., Geskovski, N., Makreski, P., Petrushevska, M., Simonoska Crcarevska, M., & Glavas Dodov, M. (2023). Comparative Studies of the Uptake and Internalization Pathways of Different Lipid Nano-Systems Intended for Brain Delivery. Pharmaceutics, 15(8), 2082. https://doi.org/10.3390/pharmaceutics15082082