Fabrication and Characterization of an Enzyme-Triggered, Therapeutic-Releasing Hydrogel Bandage Contact Lens Material
Abstract
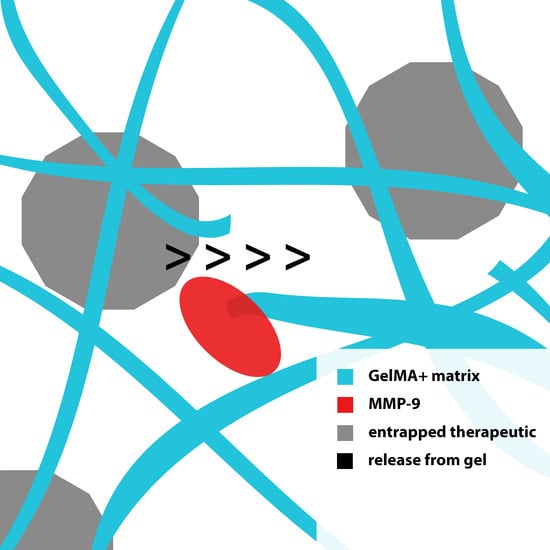
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Gelatin Methacrylate Synthesis
2.3. Preparation of GelMA+ Hydrogels and BLF-Loaded GelMA+ Hydrogels
2.4. Physical Characterization
2.4.1. Scanning Electron Microscopy
2.4.2. Enzymatic Degradation of the GelMA+ Hydrogels
2.4.3. Swelling Percentage and Water Content of the GelMA+ Hydrogels
2.4.4. Mechanical Properties of the GelMA+ Hydrogels
2.4.5. Optical Transmittance of the GelMA+ Hydrogels
2.4.6. In Vitro Release of Bovine Lactoferrin (BLF)
2.5. Biological Characterizations
2.5.1. Cell Culture
2.5.2. Cell Culture in the Presence of GelMA+ Hydrogels
2.5.3. Cell Mortality Assay
2.5.4. Live/Dead Assay
2.5.5. Statistical Analysis
3. Results
3.1. Physical Characterization
3.1.1. Scanning Electron Microscopy Images
3.1.2. Enzymatic Degradation of GelMA+ Hydrogel
3.1.3. Swelling Profile and Water Content of GelMA+ Hydrogel
3.1.4. Tensile Test
3.1.5. Optical Transmittance
3.1.6. In Vitro Release of Bovine Lactoferrin
3.2. Biological Characterization
3.2.1. Cell Growth on GelMA+ Gels
3.2.2. Cell Mortality Assay
3.2.3. Live/Dead Assay
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Whitcher, J.P.; Srinivasan, M.; Upadhyay, M.P. Corneal Blindness: A Global Perspective. Bull. World Health Organ. 2001, 79, 214–221. [Google Scholar] [PubMed]
- Ross, M.; Deschenes, J. Practice Patterns in the Interdisciplinary Management of Corneal Abrasions. Can. J. Ophthalmol. 2017, 52, 548–551. [Google Scholar] [CrossRef] [PubMed]
- Schrader, S.; Wedel, T.; Moll, R.; Geerling, G. Combination of Serum Eye Drops with Hydrogel Bandage Contact Lenses in the Treatment of Persistent Epithelial Defects. Graefe Arch. Clin. Exp. Ophthalmol. 2006, 244, 1345–1349. [Google Scholar] [CrossRef] [PubMed]
- Arora, R.; Jain, S.; Monga, S.; Narayanan, R.; Raina, U.K.; Mehta, D.K. Efficacy of Continuous Wear Purevision Contact Lenses for Therapeutic Use. Contact Lens Anterior Eye 2004, 27, 39–43. [Google Scholar] [CrossRef] [PubMed]
- Blackmore, S.J. The Use of Contact Lenses in the Treatment of Persistent Epithelial Defects. Contact Lens Anterior Eye 2010, 33, 239–244. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.-C.; Inamoto, Y.; Wang, R.-K.; Lee, S.-J.; Hung, K.-F.; Shen, T.-T. The Disposable Bandage Soft Contact Lenses Therapy and Anterior Segment Optical Coherence Tomography for Management of Ocular Graft-Versus-Host Disease. BMC Ophthalmol. 2021, 21, 271. [Google Scholar] [CrossRef]
- Mangan, M.S.; Tekcan, H.; Yurttaser Ocak, S. Efficacy of Bandage Contact Lenses Versus Eye Patching in Early Postoperative Period of Müller’s Muscle-Conjunctival Resection. Ophthalmic Res. 2021, 64, 139–144. [Google Scholar] [CrossRef]
- Busin, M.; Spitznas, M. Sustained Gentamicin Release by Presoaked Medicated Bandage Contact Lenses. Ophthalmology 1988, 95, 796–798. [Google Scholar] [CrossRef]
- Donnenfeld, E.D.; Selkin, B.A.; Perry, H.D.; Moadel, K.; Selkin, G.T.; Cohen, A.J.; Sperber, L.T. Controlled Evaluation of a Bandage Contact Lens and a Topical Nonsteroidal Anti-Inflammatory Drug in Treating Traumatic Corneal Abrasions. Ophthalmology 1995, 102, 979–984. [Google Scholar] [CrossRef]
- Kanpolat, A.; Ucakhan, O.O. Therapeutic Use of Focus Night & Day Contact Lenses. Cornea 2003, 22, 726–734. [Google Scholar]
- Ahad, M.A.; Anandan, M.; Tah, V.; Dhingra, S.; Leyland, M. Randomized Controlled Study of Ocular Lubrication Versus Bandage Contact Lens in the Primary Treatment of Recurrent Corneal Erosion Syndrome. Cornea 2013, 32, 1311–1314. [Google Scholar] [CrossRef] [PubMed]
- Ahmadi, F.; Oveisi, Z.; Samani, S.M.; Amoozgar, Z. Chitosan Based Hydrogels: Characteristics and Pharmaceutical Applications. Res. Pharm. Sci. 2015, 10, 1–16. [Google Scholar] [PubMed]
- Kashyap, N.; Kumar, N.; Kumar, M.N. Hydrogels for Pharmaceutical and Biomedical Applications. Crit. Rev. Ther. Drug Carr. Syst. 2005, 22, 107–149. [Google Scholar] [CrossRef] [PubMed]
- Hoare, T.R.; Kohane, D.S. Hydrogels in Drug Delivery: Progress and Challenges. Polymer 2008, 49, 1993–2007. [Google Scholar] [CrossRef]
- Bajgrowicz, M.; Phan, C.M.; Subbaraman, L.N.; Jones, L. Release of Ciprofloxacin and Moxifloxacin from Daily Disposable Contact Lenses from an in Vitro Eye Model. Investig. Ophthalmol. Vis. Sci. 2015, 56, 2234–2242. [Google Scholar] [CrossRef]
- Hui, A.; Bajgrowicz-Cieslak, M.; Phan, C.M.; Jones, L. In Vitro Release of Two Anti-Muscarinic Drugs from Soft Contact Lenses. Clin. Ophthalmol. 2017, 11, 1657–1665. [Google Scholar] [CrossRef]
- Maulvi, F.A.; Soni, T.G.; Shah, D.O. A Review on Therapeutic Contact Lenses for Ocular Drug Delivery. Drug Deliv. 2016, 23, 3017–3026. [Google Scholar] [CrossRef]
- Phan, C.M.; Subbaraman, L.; Liu, S.; Gu, F.; Jones, L. In Vitro Uptake and Release of Natamycin Dex-B-Pla Nanoparticles from Model Contact Lens Materials. J. Biomater. Sci. Polym. Ed. 2014, 25, 18–31. [Google Scholar] [CrossRef]
- Phan, C.M.; Subbaraman, L.N.; Jones, L. In Vitro Uptake and Release of Natamycin from Conventional and Silicone Hydrogel Contact Lens Materials. Eye Contact Lens 2013, 39, 162–168. [Google Scholar] [CrossRef]
- Yue, K.; Trujillo-de Santiago, G.; Alvarez, M.M.; Tamayol, A.; Annabi, N.; Khademhosseini, A. Synthesis, Properties, and Biomedical Applications of Gelatin Methacryloyl (Gelma) Hydrogels. Biomaterials 2015, 73, 254–271. [Google Scholar] [CrossRef]
- Koshy, S.T.; Desai, R.M.; Joly, P.; Li, J.; Bagrodia, R.K.; Lewin, S.A.; Joshi, N.S.; Mooney, D.J. Click-Crosslinked Injectable Gelatin Hydrogels. Adv. Healthc. Mater. 2016, 5, 541–547. [Google Scholar] [CrossRef] [PubMed]
- Ward, A.J.; Courts, A. The Science and Technology of Gelatin; Academic Press: Cambridge, MA, USA, 1977. [Google Scholar]
- Zimmermann, D.R.; Trüeb, B.; Winterhalter, K.H.; Witmer, R.; Fischer, R.W. Type Vi Collagen is a Major Component of the Human Cornea. FEBS Lett. 1986, 197, 55–58. [Google Scholar] [CrossRef] [PubMed]
- Barbetta, A.; Dentini, M.; Zannoni, E.M.; De Stefano, M.E. Tailoring the Porosity and Morphology of Gelatin-Methacrylate Polyhipe Scaffolds for Tissue Engineering Applications. Langmuir 2005, 21, 12333–12341. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.C.; Lin, R.Z.; Qi, H.; Yang, Y.; Bae, H.; Melero-Martin, J.M.; Khademhosseini, A. Functional Human Vascular Network Generated in Photocrosslinkable Gelatin Methacrylate Hydrogels. Adv. Funct. Mater. 2012, 22, 2027–2039. [Google Scholar] [CrossRef] [PubMed]
- Singh, D.; Tripathi, A.; Nayak, V.; Kumar, A. Proliferation of Chondrocytes on a 3-D Modelled Macroporous Poly(Hydroxyethyl Methacrylate)-Gelatin Cryogel. J. Biomater. Sci. Polym. Ed. 2011, 22, 1733–1751. [Google Scholar] [CrossRef] [PubMed]
- Gómez-Guillén, M.C.; Turnay, J.; Fernández-Dıaz, M.; Ulmo, N.; Lizarbe, M.A.; Montero, P. Structural and Physical Properties of Gelatin Extracted from Different Marine Species: A Comparative Study. Food Hydrocoll. 2002, 16, 25–34. [Google Scholar] [CrossRef]
- Nichol, J.W.; Koshy, S.T.; Bae, H.; Hwang, C.M.; Yamanlar, S.; Khademhosseini, A. Cell-Laden Microengineered Gelatin Methacrylate Hydrogels. Biomaterials 2010, 31, 5536–5544. [Google Scholar] [CrossRef]
- Gnavi, S.; di Blasio, L.; Tonda-Turo, C.; Mancardi, A.; Primo, L.; Ciardelli, G.; Gambarotta, G.; Geuna, S.; Perroteau, I. Gelatin-Based Hydrogel for Vascular Endothelial Growth Factor Release in Peripheral Nerve Tissue Engineering. J. Tissue Eng. Regen. Med. 2017, 11, 459–470. [Google Scholar] [CrossRef]
- Sharifi, S.; Islam, M.M.; Sharifi, H.; Islam, R.; Koza, D.; Reyes-Ortega, F.; Alba-Molina, D.; Nilsson, P.H.; Dohlman, C.H.; Mollnes, T.E.; et al. Tuning Gelatin-Based Hydrogel Towards Bioadhesive Ocular Tissue Engineering Applications. Bioact. Mater. 2021, 6, 3947–3961. [Google Scholar] [CrossRef]
- Tronci, G.; Neffe, A.T.; Pierce, B.F.; Lendlein, A. An Entropy–Elastic Gelatin-Based Hydrogel System. J. Mater. Chem. 2010, 20, 8875–8884. [Google Scholar] [CrossRef]
- Maitra, J.; Shukla, V.K. Cross-Linking in Hydrogels—A Review. Am. J. Polym. Sci. 2014, 4, 25–31. [Google Scholar]
- Jia, X.; Kiick, K.L. Hybrid Multicomponent Hydrogels for Tissue Engineering. Macromol. Biosci. 2009, 9, 140–156. [Google Scholar] [CrossRef] [PubMed]
- Rizwan, M.; Peh, G.S.; Ang, H.-P.; Lwin, N.C.; Adnan, K.; Mehta, J.S.; Tan, W.S.; Yim, E.K. Sequentially-Crosslinked Bioactive Hydrogels as Nano-Patterned Substrates with Customizable Stiffness and Degradation for Corneal Tissue Engineering Applications. Biomaterials 2017, 120, 139–154. [Google Scholar] [CrossRef] [PubMed]
- Van den Steen, P.E.; Dubois, B.; Nelissen, I.; Rudd, P.M.; Dwek, R.A.; Opdenakker, G. Biochemistry and Molecular Biology of Gelatinase B or Matrix Metalloproteinase-9 (Mmp-9). Crit. Rev. Biochem. Mol. Biol. 2002, 37, 375–536. [Google Scholar] [CrossRef]
- Xu, F.; Inci, F.; Mullick, O.; Gurkan, U.A.; Sung, Y.; Kavaz, D.; Li, B.; Denkbas, E.B.; Demirci, U. Release of Magnetic Nanoparticles from Cell-Encapsulating Biodegradable Nanobiomaterials. ACS Nano 2012, 6, 6640–6649. [Google Scholar] [CrossRef]
- Xiao, W.; He, J.; Nichol, J.W.; Wang, L.; Hutson, C.B.; Wang, B.; Du, Y.; Fan, H.; Khademhosseini, A. Synthesis and Characterization of Photocrosslinkable Gelatin and Silk Fibroin Interpenetrating Polymer Network Hydrogels. Acta Biomater. 2011, 7, 2384–2393. [Google Scholar] [CrossRef]
- Sivak, J.M.; Fini, M.E. Mmps in the Eye: Emerging Roles for Matrix Metalloproteinases in Ocular Physiology. Prog. Retin. Eye Res. 2002, 21, 1–14. [Google Scholar] [CrossRef]
- Matsubara, M.; Girard, M.T.; Kublin, C.L.; Cintron, C.; Fini, M.E. Differential Roles for Two Gelatinolytic Enzymes of the Matrix Metalloproteinase Family in the Remodelling Cornea. Dev. Biol. 1991, 147, 425–439. [Google Scholar] [CrossRef]
- Mulholland, B.; Tuft, S.J.; Khaw, P.T. Matrix Metalloproteinase Distribution During Early Corneal Wound Healing. Eye 2005, 19, 584–588. [Google Scholar] [CrossRef]
- Fini, M.E.; Girard, M.T.; Matsubara, M. Collagenolytic/Gelatinolytic Enzymes in Corneal Wound Healing. Acta Ophthalmol. 1992, 70 (Suppl. 1985), 26–33. [Google Scholar] [CrossRef]
- Derakhshanfar, S.; Mbeleck, R.; Xu, K.; Zhang, X.; Zhong, W.; Xing, M. 3d Bioprinting for Biomedical Devices and Tissue Engineering: A Review of Recent Trends and Advances. Bioact. Mater. 2018, 3, 144–156. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.-Y.; Choi, A.-J.; Park, J.-E.; Jang, Y.-S.; Lee, M.-H. Antibacterial Activity and Biocompatibility with the Concentration of Ginger Fraction in Biodegradable Gelatin Methacryloyl (Gelma) Hydrogel Coating for Medical Implants. Polymers 2022, 14, 5317. [Google Scholar] [CrossRef] [PubMed]
- Feng, M.; Hu, S.; Qin, W.; Tang, Y.; Guo, R.; Han, L. Bioprinting of a Blue Light-Cross-Linked Biodegradable Hydrogel Encapsulating Amniotic Mesenchymal Stem Cells for Intrauterine Adhesion Prevention. ACS Omega 2021, 6, 23067–23075. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.-H.; Tsai, C.-W.; Tsai, N.-Y.; Chiang, C.-Y.; Lin, R.-S.; Pereira, R.-F.; Li, Y.-C.-E. An Injectable, Dual Crosslinkable Hybrid Pectin Methacrylate (Pecma)/Gelatin Methacryloyl (Gelma) Hydrogel for Skin Hemostasis Applications. Int. J. Biol. Macromol. 2021, 185, 441–450. [Google Scholar] [CrossRef] [PubMed]
- Ukmar, T.; Maver, U.; Planinsek, O.; Kaučič, V.; Gaberšček, M.; Godec, A. Understanding Controlled Drug Release from Mesoporous Silicates: Theory and Experiment. J. Control. Release 2011, 155, 409–417. [Google Scholar] [CrossRef]
- Siboro, S.A.; Anugrah, D.S.; Ramesh, K.; Park, S.H.; Kim, H.R.; Lim, K.T. Tunable Porosity of Covalently Crosslinked Alginate-Based Hydrogels and Its Significance in Drug Release Behavior. Carbohydr. Polym. 2021, 260, 117779. [Google Scholar] [CrossRef]
- Noshadi, I.; Hong, S.; Sullivan, K.E.; Sani, E.S.; Portillo-Lara, R.; Tamayol, A.; Shin, S.R.; Gao, A.E.; Stoppel, W.L.; Black, L.D., III; et al. In Vitro and in Vivo Analysis of Visible Light Crosslinkable Gelatin Methacryloyl (Gelma) Hydrogels. Biomater. Sci. 2017, 5, 2093–2105. [Google Scholar] [CrossRef]
- ur Rehman, S.R.; Augustine, R.; Zahid, A.A.; Ahmed, R.; Tariq, M.; Hasan, A. Reduced Graphene Oxide Incorporated Gelma Hydrogel Promotes Angiogenesis for Wound Healing Applications. Int. J. Nanomed. 2019, 14, 9603. [Google Scholar] [CrossRef]
- Yang, J.; Wang, F.; Tan, T. Controlling Degradation and Physical Properties of Chemical Sand Fixing Agent-Poly (Aspartic Acid) by Crosslinking Density and Composites. J. Appl. Polym. Sci. 2009, 111, 1557–1563. [Google Scholar] [CrossRef]
- Peng, Z.; Chen, F. Hydroxyethyl Cellulose-Based Hydrogels with Various Pore Sizes Prepared by Freeze-Drying. J. Macromol. Sci. Part B Phys. 2010, 50, 340–349. [Google Scholar] [CrossRef]
- Tranoudis, I.; Efron, N. Tensile Properties of Soft Contact Lens Materials. Contact Lens Anterior Eye 2004, 27, 177–191. [Google Scholar] [CrossRef] [PubMed]
- Bhamra, T.S.; Tighe, B.J. Mechanical Properties of Contact Lenses: The Contribution of Measurement Techniques and Clinical Feedback to 50 Years of Materials Development. Contact Lens Anterior Eye 2017, 40, 70–81. [Google Scholar] [CrossRef] [PubMed]
- Peppas, N.A.; Hoffman, A.S. Hydrogels. In Biomaterials Science; Wagner, W.R., Sakiyama-Elbert, S.E., Zhang, G., Yaszemski, M.J., Eds.; Academic Press: Cambridge, MA, USA, 2020; pp. 153–166. [Google Scholar]
- Wang, Y.; Nian, G.; Kim, J.; Suo, Z. Polyacrylamide Hydrogels. Vi. Synthesis-Property Relation. J. Mech. Phys. Solids 2023, 170, 105099. [Google Scholar] [CrossRef]
- Yacob, N.; Hashim, K. Morphological Effect on Swelling Behaviour of Hydrogel. AIP Conf. Proc. 2014, 1584, 153–159. [Google Scholar]
- Wang, Z.; Li, X.; Zhang, X.; Sheng, R.; Lin, Q.; Song, W.; Hao, L. Novel Contact Lenses Embedded with Drug-Loaded Zwitterionic Nanogels for Extended Ophthalmic Drug Delivery. Nanomaterials 2021, 11, 2328. [Google Scholar] [CrossRef] [PubMed]
- Gupta, S.; Goswami, S.; Sinha, A. A Combined Effect of Freeze--Thaw Cycles and Polymer Concentration on the Structure and Mechanical Properties of Transparent Pva Gels. Biomed. Mater. 2012, 7, 015006. [Google Scholar] [CrossRef] [PubMed]
- Lira, M.; Pereira, C.; Real Oliveira, M.E.; Castanheira, E.M. Importance of Contact Lens Power and Thickness in Oxygen Transmissibility. Contact Lens Anterior Eye 2015, 38, 120–126. [Google Scholar] [CrossRef]
- Kapfelsberger, A.; Eckstein, J.; von Ahrentschildt, A.; Bischoff, J.; Marx, S.; Sickenberger, W. Ultraviolet and Visible Transmittance of Soft Contact Lenses with and without Ultraviolet Blockers. Optom. Vis. Sci. 2021, 98, 1270–1278. [Google Scholar] [CrossRef]
- Wang, H.; Zhou, L.; Liao, J.; Tan, Y.; Ouyang, K.; Ning, C.; Ni, G.; Tan, G. Cell-Laden Photocrosslinked Gelma-Dexma Copolymer Hydrogels with Tunable Mechanical Properties for Tissue Engineering. J. Mater. Sci. Mater. Med. 2014, 25, 2173–2183. [Google Scholar] [CrossRef]
- Augustine, R.; Hasan, A.; Dalvi, Y.B.; Rehman, S.R.U.; Varghese, R.; Unni, R.N.; Yalcin, H.C.; Alfkey, R.; Thomas, S.; Al Moustafa, A.E. Growth Factor Loaded In Situ Photocrosslinkable Poly(3-Hydroxybutyrate-Co-3-Hydroxyvalerate)/Gelatin Methacryloyl Hybrid Patch for Diabetic Wound Healing. Mater. Sci. Eng. C-Mater. Biol. Appl. 2021, 118, 111519. [Google Scholar] [CrossRef]
- Fathi, A.; Lee, S.; Breen, A.; Shirazi, A.N.; Valtchev, P.; Dehghani, F. Enhancing the Mechanical Properties and Physical Stability of Biomimetic Polymer Hydrogels for Micro-Patterning and Tissue Engineering Applications. Eur. Polym. J. 2014, 59, 161–170. [Google Scholar] [CrossRef]
- Liu, B.; Wang, Y.; Miao, Y.; Zhang, X.; Fan, Z.; Singh, G.; Zhang, X.; Xu, K.; Li, B.; Hu, Z.; et al. Hydrogen Bonds Autonomously Powered Gelatin Methacrylate Hydrogels with Super-Elasticity, Self-Heal and Underwater Self-Adhesion for Sutureless Skin and Stomach Surgery and E-Skin. Biomaterials 2018, 171, 83–96. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Deng, R.; Ren, X.; Zhang, K.; Li, J. 2d Gelatin Methacrylate Hydrogels with Tunable Stiffness for Investigating Cell Behaviors. ACS Appl. Bio Mater. 2018, 2, 570–576. [Google Scholar] [CrossRef] [PubMed]
- Pattamatta, U.; Willcox, M.; Stapleton, F.; Cole, N.; Garrett, Q. Bovine Lactoferrin Stimulates Human Corneal Epithelial Alkali Wound Healing in Vitro. Investig. Ophthalmol. Vis. Sci. 2009, 50, 1636–1643. [Google Scholar] [CrossRef] [PubMed]
- Groves, M.L. The Isolation of a Red Protein from Milk2. J. Am. Chem. Soc. 1960, 82, 3345–3350. [Google Scholar] [CrossRef]
- Flanagan, J.L.; Willcox, M.D. Role of Lactoferrin in the Tear Film. Biochimie 2009, 91, 35–43. [Google Scholar] [CrossRef] [PubMed]
- Gruden, S.; Poklar Ulrih, N. Diverse Mechanisms of Antimicrobial Activities of Lactoferrins, Lactoferricins, and Other Lactoferrin-Derived Peptides. Int. J. Mol. Sci. 2021, 22, 11264. [Google Scholar] [CrossRef]
- Masson, P.L.; Heremans, J.F. Lactoferrin in Milk from Different Species. Comp. Biochem. Physiol. Part B 1971, 39, 119–129. [Google Scholar] [CrossRef]
- Masson, P.; Heremans, J.; Dive, C. An Iron-Binding Protein Common to Many External Secretions. Clin. Chim. Acta 1966, 14, 735–739. [Google Scholar] [CrossRef]
- Tang, L.; Wu, J.J.; Ma, Q.; Cui, T.; Andreopoulos, F.M.; Gil, J.; Valdes, J.; Davis, S.C.; Li, J. Human Lactoferrin Stimulates Skin Keratinocyte Function and Wound Re-Epithelialization. Br. J. Dermatol. 2010, 163, 38–47. [Google Scholar] [CrossRef]
- Engelmayer, J.; Blezinger, P.; Varadhachary, A. Talactoferrin Stimulates Wound Healing with Modulation of Inflammation. J. Surg. Res. 2008, 149, 278–286. [Google Scholar] [CrossRef] [PubMed]
- Mouritzen, M.V.; Petkovic, M.; Qvist, K.; Poulsen, S.S.; Alarico, S.; Leal, E.C.; Dalgaard, L.T.; Empadinhas, N.; Carvalho, E.; Jenssen, H. Improved Diabetic Wound Healing by Lfcinb Is Associated with Relevant Changes in the Skin Immune Response and Microbiota. Mol. Ther. Methods Clin. Dev. 2021, 20, 726–739. [Google Scholar] [CrossRef] [PubMed]
- Ashby, B.; Garrett, Q.; Willcox, M. Bovine Lactoferrin Structures Promoting Corneal Epithelial Wound Healing in Vitro. Investig. Ophthalmol. Vis. Sci. 2011, 52, 2719–2726. [Google Scholar] [CrossRef] [PubMed]
- Li, D.Q.; Pflugfelder, S.C. Matrix Metalloproteinases in Corneal Inflammation. Ocul. Surf. 2005, 3, S198–S202. [Google Scholar] [CrossRef] [PubMed]
- Behzadian, M.A.; Wang, X.L.; Windsor, L.J.; Ghaly, N.; Caldwell, R.B. Tgf-Beta Increases Retinal Endothelial Cell Permeability by Increasing Mmp-9: Possible Role of Glial Cells in Endothelial Barrier Function. Investig. Ophthalmol. Vis. Sci. 2001, 42, 853–859. [Google Scholar]
- Sternlicht, M.D.; Werb, Z. How Matrix Metalloproteinases Regulate Cell Behavior. Annu. Rev. Cell Dev. Biol. 2001, 17, 463–516. [Google Scholar] [CrossRef] [PubMed]
- Pflugfelder, S.C. Antiinflammatory Therapy for Dry Eye. Am. J. Ophthalmol. 2004, 137, 337–342. [Google Scholar] [CrossRef]
- Afonso, A.A.; Sobrin, L.; Monroy, D.C.; Selzer, M.; Lokeshwar, B.; Pflugfelder, S.C. Tear Fluid Gelatinase B Activity Correlates with Il-1alpha Concentration and Fluorescein Clearance in Ocular Rosacea. Investig. Ophthalmol. Vis. Sci. 1999, 40, 2506–2512. [Google Scholar]
- Acera, A.; Vecino, E.; Duran, J.A. Tear Mmp-9 Levels as a Marker of Ocular Surface Inflammation in Conjunctivochalasis. Investig. Ophthalmol. Vis. Sci. 2013, 54, 8285–8291. [Google Scholar] [CrossRef]
- Chotikavanich, S.; de Paiva, C.S.; Chen, J.J.; Bian, F.; Farley, W.J.; Pflugfelder, S.C. Production and Activity of Matrix Metalloproteinase-9 on the Ocular Surface Increase in Dysfunctional Tear Syndrome. Investig. Ophthalmol. Vis. Sci. 2009, 50, 3203–3209. [Google Scholar] [CrossRef]
- Tse, J. Gelatin Methacrylate as a Controlled Release Vehicle for Treatment of Recurrent Corneal Erosion. Master’s Thesis, University of Waterloo, Waterloo, ON, Canada, 2018. [Google Scholar]
- Langer, R. Drug Delivery. Drugs on Target. Science 2001, 293, 58–59. [Google Scholar] [CrossRef] [PubMed]
- Ulijn, R.V. Enzyme-Responsive Materials: A New Class of Smart Biomaterials. J. Mater. Chem. 2006, 16, 2217–2225. [Google Scholar] [CrossRef]
- Da Silva, A.A.; Leal-Junior, E.C.; Alves, A.C.; Rambo, C.S.; Dos Santos, S.A.; Vieira, R.P.; De Carvalho, P.D. Wound-Healing Effects of Low-Level Laser Therapy in Diabetic Rats Involve the Modulation of Mmp-2 and Mmp-9 and the Redistribution of Collagen Types I and Iii. J. Cosmet. Laser Ther. 2013, 15, 210–216. [Google Scholar] [CrossRef] [PubMed]
- Eichler, W.; Bechtel, J.M.; Schumacher, J.; Wermelt, J.A.; Klotz, K.F.; Bartels, C. A Rise of Mmp-2 and Mmp-9 in Bronchoalveolar Lavage Fluid Is Associated with Acute Lung Injury after Cardiopulmonary Bypass in a Swine Model. Perfusion 2003, 18, 107–113. [Google Scholar] [CrossRef]








| Formulation | 20% GelMA+ | 30% GelMA+ |
|---|---|---|
| % Swelling | 303.69 ± 6.95 | 234.68 ± 8.98 |
| Water content (%) | 74.85 ± 0.67 | 70.85 ± 1.81 |
| Tensile strain (kPa) | 133.06 ± 8.98 | 181.85 ± 25.25 |
| Young modulus (MPa) | 2.04 ± 0.16 | 2.80 ± 0.74 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bose, S.; Phan, C.-M.; Rizwan, M.; Tse, J.W.; Yim, E.; Jones, L. Fabrication and Characterization of an Enzyme-Triggered, Therapeutic-Releasing Hydrogel Bandage Contact Lens Material. Pharmaceutics 2024, 16, 26. https://doi.org/10.3390/pharmaceutics16010026
Bose S, Phan C-M, Rizwan M, Tse JW, Yim E, Jones L. Fabrication and Characterization of an Enzyme-Triggered, Therapeutic-Releasing Hydrogel Bandage Contact Lens Material. Pharmaceutics. 2024; 16(1):26. https://doi.org/10.3390/pharmaceutics16010026
Chicago/Turabian StyleBose, Susmita, Chau-Minh Phan, Muhammad Rizwan, John Waylon Tse, Evelyn Yim, and Lyndon Jones. 2024. "Fabrication and Characterization of an Enzyme-Triggered, Therapeutic-Releasing Hydrogel Bandage Contact Lens Material" Pharmaceutics 16, no. 1: 26. https://doi.org/10.3390/pharmaceutics16010026
APA StyleBose, S., Phan, C. -M., Rizwan, M., Tse, J. W., Yim, E., & Jones, L. (2024). Fabrication and Characterization of an Enzyme-Triggered, Therapeutic-Releasing Hydrogel Bandage Contact Lens Material. Pharmaceutics, 16(1), 26. https://doi.org/10.3390/pharmaceutics16010026