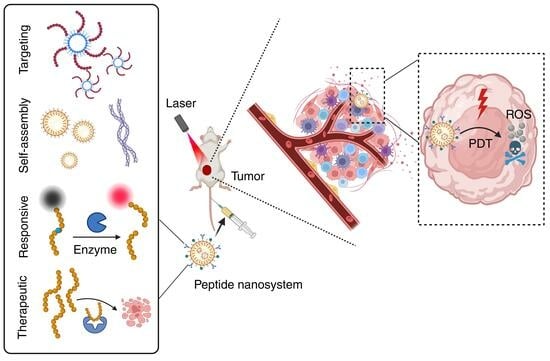
Versatile Peptide-Based Nanosystems for Photodynamic Therapy
Abstract
:1. Introduction

2. Properties and Architecture of Peptides
2.1. Chemical Structures of Peptide
2.2. Function and Bioactivity of Peptide
2.3. Architecture of Peptide Nanosystems
3. Multifunctional Peptide-Based Nanosystems for PDT
3.1. Targeted Peptide-Based Nanosystems for PDT
3.2. Stimuli-Responsive Peptide-Based Nanosystems for PDT
3.3. Self-Assembled Peptide-Based Nanosystems for PDT
3.4. Therapeutic Peptide-Based Nanosystems for PDT
4. Outlook and Perspectives
Funding
Acknowledgments
Conflicts of Interest
References
- Kawczyk-Krupka, A.; Bugaj, A.M.; Latos, W.; Zaremba, K.; Wawrzyniec, K.; Sieron, A. Photodynamic therapy in colorectal cancer treatment: The state of the art in clinical trials. Photodiagnosis Photodyn. Ther. 2015, 12, 545–553. [Google Scholar] [CrossRef]
- Vermandel, M.; Dupont, C.; Lecomte, F.; Leroy, H.A.; Tuleasca, C.; Mordon, S.; Hadjipanayis, C.G.; Reyns, N. Standardized intraoperative 5-ALA photodynamic therapy for newly diagnosed glioblastoma patients: A preliminary analysis of the INDYGO clinical trial. J. Neurooncol. 2021, 152, 501–514. [Google Scholar] [CrossRef]
- Usuda, J.; Inoue, T.; Tsuchida, T.; Ohtani, K.; Maehara, S.; Ikeda, N.; Ohsaki, Y.; Sasaki, T.; Oka, K. Clinical trial of photodynamic therapy for peripheral-type lung cancers using a new laser device in a pilot study. Photodiagnosis Photodyn. Ther. 2020, 30, 101698. [Google Scholar] [CrossRef]
- Marmur, E.S.; Schmults, C.D.; Goldberg, D.J. A review of laser and photodynamic therapy for the treatment of nonmelanoma skin cancer. Dermatol. Surg. Off. Publ. Am. Soc. Dermatol. Surg. 2004, 30, 264–271. [Google Scholar]
- Frochot, C.; Mordon, S. Update of the situation of clinical photodynamic therapy in Europe in the 2003–2018 period. J. Porphyr. Phthalocyanines 2019, 23, 347–357. [Google Scholar] [CrossRef]
- Rigual, N.; Shafirstein, G.; Cooper, M.T.; Baumann, H.; Bellnier, D.A.; Sunar, U.; Tracy, E.C.; Rohrbach, D.J.; Wilding, G.; Tan, W.; et al. Photodynamic Therapy with 3-(1′-Hexyloxyethyl) Pyropheophorbide a for Cancer of the Oral Cavity. Clin. Cancer Res. 2013, 19, 6605–6613. [Google Scholar] [CrossRef]
- Huggett, M.T.; Jermyn, M.; Gillams, A.; Illing, R.; Mosse, S.; Novelli, M.; Kent, E.; Bown, S.G.; Hasan, T.; Pogue, B.W.; et al. Phase I/II study of verteporfin photodynamic therapy in locally advanced pancreatic cancer. Br. J. Cancer 2014, 110, 1698–1704. [Google Scholar] [CrossRef]
- Wang, I.; Bendsoe, N.; Klinteberg, C.A.; Enejder, A.M.K.; Andersson-Engels, S.; Svanberg, S.; Svanberg, K. Photodynamic therapy vs. cryosurgery of basal cell carcinomas: Results of a phase III clinical trial. Br. J. Dermatol. 2001, 144, 832–840. [Google Scholar] [CrossRef]
- Stepp, H.; Stummer, W. 5-ALA in the management of malignant glioma. Lasers Surg. Med. 2018, 50, 399–419. [Google Scholar] [CrossRef]
- Das, K.; Roychoudhury, A. Reactive oxygen species (ROS) and response of antioxidants as ROS-scavengers during environmental stress in plants. Front. Environ. Sci. 2014, 2, 53. [Google Scholar] [CrossRef]
- Felsher, D.W. Photodynamic therapy for cancer. Nat. Rev. Cancer 2003, 3, 375–380. [Google Scholar] [CrossRef]
- Tong, R.; Kohane, D.S. Shedding light on nanomedicine. WIREs Nanomed. Nanobiotechnol. 2012, 4, 638–662. [Google Scholar] [CrossRef]
- Woodhams, J.H.; Macrobert, A.J.; Bown, S.G. The role of oxygen monitoring during photodynamic therapy and its potential for treatment dosimetry. Photochem. Photobiol. Sci. 2007, 6, 1246–1256. [Google Scholar] [CrossRef]
- Chen, D.; Wang, Y.; Zhao, H.; Qiu, H.; Wang, Y.; Yang, J.; Gu, Y. Monitoring perfusion and oxygen saturation in port-wine stains during vascular targeted photodynamic therapy. Ann. Transl. Med. 2021, 9, 214. [Google Scholar] [CrossRef]
- Wan, Y.; Fu, L.H.; Li, C.; Lin, J.; Huang, P. Conquering the Hypoxia Limitation for Photodynamic Therapy. Adv. Mater. 2021, 33, e2103978. [Google Scholar] [CrossRef]
- Kim, M.M.; Darafsheh, A. Light Sources and Dosimetry Techniques for Photodynamic Therapy. Photochem. Photobiol. 2020, 96, 280–294. [Google Scholar] [CrossRef]
- Zhang, R.; Duan, Y.; Liu, B. Recent advances of AIE dots in NIR imaging and phototherapy. Nanoscale 2019, 11, 19241–19250. [Google Scholar] [CrossRef]
- Schrama, D.; Reisfeld, R.A.; Becker, J.C. Antibody targeted drugs as cancer therapeutics. Nat. Rev. Drug Discov. 2006, 5, 147–159. [Google Scholar] [CrossRef]
- Majumdar, S.; Siahaan, T.J. Peptide-mediated targeted drug delivery. Med. Res. Rev. 2012, 32, 637–658. [Google Scholar] [CrossRef]
- Zhao, Y.; Ren, W.; Zhong, T.; Zhang, S.; Huang, D.; Guo, Y.; Yao, X.; Wang, C.; Zhang, W.Q.; Zhang, X.; et al. Tumor-specific pH-responsive peptide-modified pH-sensitive liposomes containing doxorubicin for enhancing glioma targeting and anti-tumor activity. J. Control. Release 2016, 222, 56–66. [Google Scholar] [CrossRef]
- He, H.; Sun, L.; Ye, J.; Liu, E.; Chen, S.; Liang, Q.; Shin, M.C.; Yang, V.C. Enzyme-triggered, cell penetrating peptide-mediated delivery of anti-tumor agents. J. Control. Release 2016, 240, 67–76. [Google Scholar] [CrossRef] [PubMed]
- Celis, E.; Tsai, V.; Crimi, C.; DeMars, R.; Wentworth, P.A.; Chesnut, R.W.; Grey, H.M.; Sette, A.; Serra, H.M. Induction of anti-tumor cytotoxic T lymphocytes in normal humans using primary cultures and synthetic peptide epitopes. Proc. Natl. Acad. Sci. USA 1994, 91, 2105–2109. [Google Scholar] [CrossRef]
- Wu, C.H.; Liu, I.J.; Lu, R.M.; Wu, H.C. Advancement and applications of peptide phage display technology in biomedical science. J. Biomed. Sci. 2016, 23, 8. [Google Scholar] [CrossRef]
- Ruoslahti, E. Tumor penetrating peptides for improved drug delivery. Adv. Drug Deliv. Rev. 2017, 110, 3–12. [Google Scholar] [CrossRef]
- Sheehan, F.; Sementa, D.; Jain, A.; Kumar, M.; Tayarani-Najjaran, M.; Kroiss, D.; Ulijn, R.V. Peptide-Based Supramolecular Systems Chemistry. Chem. Rev. 2021, 121, 13869–13914. [Google Scholar] [CrossRef]
- Tugyi, R.; Uray, K.; Ivan, D.; Fellinger, E.; Perkins, A.; Hudecz, F. Partial D-amino acid substitution: Improved enzymatic stability and preserved Ab recognition of a MUC2 epitope peptide. Proc. Natl. Acad. Sci. USA 2005, 102, 413–418. [Google Scholar] [CrossRef] [PubMed]
- Palomo, J.M. Solid-phase peptide synthesis: An overview focused on the preparation of biologically relevant peptides. RSC Adv. 2014, 4, 32658–32672. [Google Scholar] [CrossRef]
- Vandekerckhove, J.; Deboben, A.; Nassal, M.; Wieland, T. The phalloidin binding site of F-actin. EMBO J. 1985, 4, 2815–2818. [Google Scholar] [CrossRef]
- Melak, M.; Plessner, M.; Grosse, R. Actin visualization at a glance. J. Cell Sci. 2017, 130, 525–530. [Google Scholar] [CrossRef]
- Sheikh, A.; Alhakamy, N.A.; Md, S.; Kesharwani, P. Recent Progress of RGD Modified Liposomes as Multistage Rocket Against Cancer. Front. Pharmacol. 2021, 12, 803304. [Google Scholar] [CrossRef]
- Bogdanowich-Knipp, S.J.; Chakrabarti, S.; Williams, T.D.; Dillman, R.K.; Siahaan, T.J. Solution stability of linear vs. cyclic RGD peptides. J. Pept. Res. Off. J. Am. Pept. Soc. 1999, 53, 530–541. [Google Scholar] [CrossRef]
- Jia, S.; Ji, S.; Zhao, J.; Lv, Y.; Wang, J.; Sun, D.; Ding, D. A Fluorinated Supramolecular Self-Assembled Peptide as Nanovaccine Adjuvant for Enhanced Cancer Vaccine Therapy. Small Methods 2023, 7, e2201409. [Google Scholar] [CrossRef] [PubMed]
- Hamley, I.W. Small Bioactive Peptides for Biomaterials Design and Therapeutics. Chem. Rev. 2017, 117, 14015–14041. [Google Scholar] [CrossRef]
- Felicio, M.R.; Silva, O.N.; Goncalves, S.; Santos, N.C.; Franco, O.L. Peptides with Dual Antimicrobial and Anticancer Activities. Front. Chem. 2017, 5, 5. [Google Scholar] [CrossRef]
- Tornesello, A.L.; Borrelli, A.; Buonaguro, L.; Buonaguro, F.M.; Tornesello, M.L. Antimicrobial Peptides as Anticancer Agents: Functional Properties and Biological Activities. Molecules 2020, 25, 2850. [Google Scholar] [CrossRef]
- Singh, S.B. Discovery and Development of Dolastatin 10-Derived Antibody Drug Conjugate Anticancer Drugs. J. Nat. Prod. 2022, 85, 666–687. [Google Scholar] [CrossRef]
- Shrestha, N.; Araújo, F.; Shahbazi, M.A.; Mäkilä, E.; Gomes, M.J.; Herranz-Blanco, B.; Lindgren, R.; Granroth, S.; Kukk, E.; Salonen, J.; et al. Thiolation and Cell-Penetrating Peptide Surface Functionalization of Porous Silicon Nanoparticles for Oral Delivery of Insulin. Adv. Funct. Mater. 2016, 26, 3405–3416. [Google Scholar] [CrossRef]
- Zhao, H.; Wu, M.; Zhu, L.; Tian, Y.; Wu, M.; Li, Y.; Deng, L.; Jiang, W.; Shen, W.; Wang, Z.; et al. Cell-penetrating Peptide-modified Targeted Drug-loaded Phase-transformation Lipid Nanoparticles Combined with Low-intensity Focused Ultrasound for Precision Theranostics against Hepatocellular Carcinoma. Theranostics 2018, 8, 1892–1910. [Google Scholar] [CrossRef]
- Chen, Y.; Xianyu, Y.; Jiang, X. Surface Modification of Gold Nanoparticles with Small Molecules for Biochemical Analysis. Acc. Chem. Res. 2017, 50, 310–319. [Google Scholar] [CrossRef]
- Chong, K.C.; Hu, F.; Liu, B. AIEgen bioconjugates for specific detection of disease-related protein biomarkers. Mater. Chem. Front. 2019, 3, 12–24. [Google Scholar] [CrossRef]
- Yuan, C.; Ji, W.; Xing, R.; Li, J.; Gazit, E.; Yan, X. Hierarchically oriented organization in supramolecular peptide crystals. Nat. Rev. Chem. 2019, 3, 567–588. [Google Scholar] [CrossRef]
- Liu, K.; Xing, R.; Zou, Q.; Ma, G.; Möhwald, H.; Yan, X. Simple peptide-tuned self-assembly of photosensitizers towards anticancer photodynamic therapy. Angew. Chem. 2016, 128, 3088–3091. [Google Scholar] [CrossRef]
- Lynn, G.M.; Sedlik, C.; Baharom, F.; Zhu, Y.; Ramirez-Valdez, R.A.; Coble, V.L.; Tobin, K.; Nichols, S.R.; Itzkowitz, Y.; Zaidi, N.; et al. Peptide-TLR-7/8a conjugate vaccines chemically programmed for nanoparticle self-assembly enhance CD8 T-cell immunity to tumor antigens. Nat. Biotechnol. 2020, 38, 320–332. [Google Scholar] [CrossRef]
- Mu, J.; Lin, J.; Huang, P.; Chen, X. Development of endogenous enzyme-responsive nanomaterials for theranostics. Chem. Soc. Rev. 2018, 47, 5554–5573. [Google Scholar] [CrossRef] [PubMed]
- Qi, J.; Ou, H.; Liu, Q.; Ding, D. Gathering brings strength: How organic aggregates boost disease phototheranostics. Aggregate 2021, 2, 95–113. [Google Scholar] [CrossRef]
- Zhang, N.Y.; Hu, X.J.; An, H.W.; Liang, J.X.; Wang, H. Programmable design and self-assembly of peptide conjugated AIEgens for biomedical applications. Biomaterials 2022, 287, 121655. [Google Scholar] [CrossRef] [PubMed]
- Qi, G.B.; Gao, Y.J.; Wang, L.; Wang, H. Self-Assembled Peptide-Based Nanomaterials for Biomedical Imaging and Therapy. Adv. Mater. 2018, 30, e1703444. [Google Scholar] [CrossRef]
- Gao, J.; Zhan, J.; Yang, Z. Enzyme-Instructed Self-Assembly (EISA) and Hydrogelation of Peptides. Adv. Mater. 2020, 32, e1805798. [Google Scholar] [CrossRef]
- Sun, M.; Wang, C.; Lv, M.; Fan, Z.; Du, J. Intracellular Self-Assembly of Peptides to Induce Apoptosis against Drug-Resistant Melanoma. J. Am. Chem. Soc. 2022, 144, 7337–7345. [Google Scholar] [CrossRef]
- Sun, S.; Liang, H.-W.; Wang, H.; Zou, Q. Light-Triggered Self-Assembly of Peptide Nanoparticles into Nanofibers in Living Cells through Molecular Conformation Changes and H-Bond Interactions. ACS Nano 2022, 16, 18978–18989. [Google Scholar] [CrossRef]
- Wu, C.; Li, Y.; Cheng, Z.; Wang, P.; Ma, Z.; Liu, K.; Cheng, Y.; Zhou, Y.; Lin, X.; Shao, X.; et al. Cell-penetrating riboflavin conjugate for antitumor photodynamic therapy. Chin. Chem. Lett. 2022, 33, 4339–4344. [Google Scholar] [CrossRef]
- Wei, D.; Huang, Y.; Wang, B.; Ma, L.; Karges, J.; Xiao, H. Photo-Reduction with NIR Light of Nucleus-Targeting PtIV Nanoparticles for Combined Tumor-Targeted Chemotherapy and Photodynamic Immunotherapy. Angew. Chem. Int. Ed. 2022, 61, e202201486. [Google Scholar] [CrossRef]
- Tian, Y.; Cheng, Q.; Dang, H.; Qian, H.; Teng, C.; Xie, K.; Yan, L. Amino modified iodinated BODIPY photosensitizer for highly efficient NIR imaging-guided photodynamic therapy with ultralow dose. Dye. Pigment. 2021, 194, 109611. [Google Scholar] [CrossRef]
- Lin, H.; Zhou, R.; Yu, T.; He, J. An acid-targeting peptide can be used as a carrier for photodynamic therapy (PDT). Mater. Today Commun. 2022, 31, 103659. [Google Scholar] [CrossRef]
- Cheng, H.; Fan, G.-L.; Fan, J.-H.; Yuan, P.; Deng, F.-A.; Qiu, X.-Z.; Yu, X.-Y.; Li, S.-Y. Epigenetics-inspired photosensitizer modification for plasma membrane-targeted photodynamic tumor therapy. Biomaterials 2019, 224, 119497. [Google Scholar] [CrossRef]
- Cheng, H.; Zheng, R.R.; Fan, G.L.; Fan, J.H.; Zhao, L.P.; Jiang, X.Y.; Yang, B.; Yu, X.Y.; Li, S.Y.; Zhang, X.Z. Mitochondria and plasma membrane dual-targeted chimeric peptide for single-agent synergistic photodynamic therapy. Biomaterials 2019, 188, 1–11. [Google Scholar] [CrossRef]
- Zhang, C.; Gao, F.; Wu, W.; Qiu, W.X.; Zhang, L.; Li, R.; Zhuang, Z.N.; Yu, W.; Cheng, H.; Zhang, X.Z. Enzyme-Driven Membrane-Targeted Chimeric Peptide for Enhanced Tumor Photodynamic Immunotherapy. ACS Nano 2019, 13, 11249–11262. [Google Scholar] [CrossRef] [PubMed]
- Dhillon, S.K.; Porter, S.L.; Rizk, N.; Sheng, Y.; McKaig, T.; Burnett, K.; White, B.; Nesbitt, H.; Matin, R.N.; McHale, A.P.; et al. Rose Bengal-Amphiphilic Peptide Conjugate for Enhanced Photodynamic Therapy of Malignant Melanoma. J. Med. Chem. 2020, 63, 1328–1336. [Google Scholar] [CrossRef] [PubMed]
- Ihsanullah, K.M.; Kumar, B.N.; Zhao, Y.; Muhammad, H.; Liu, Y.; Wang, L.; Liu, H.; Jiang, W. Stepwise-activatable hypoxia triggered nanocarrier-based photodynamic therapy for effective synergistic bioreductive chemotherapy. Biomaterials 2020, 245, 119982. [Google Scholar] [CrossRef] [PubMed]
- Kang, M.; Zhang, Z.; Xu, W.; Wen, H.; Zhu, W.; Wu, Q.; Wu, H.; Gong, J.; Wang, Z.; Wang, D.; et al. Good Steel Used in the Blade: Well-Tailored Type-I Photosensitizers with Aggregation-Induced Emission Characteristics for Precise Nuclear Targeting Photodynamic Therapy. Adv. Sci. 2021, 8, 2100524. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Yin, Y.; Yang, L.; Lu, B.; Yang, Z.; Wang, W.; Li, R. Nucleus-Targeted Photosensitizer Nanoparticles for Photothermal and Photodynamic Therapy of Breast Carcinoma. Int. J. Nanomed. 2021, 16, 1473–1485. [Google Scholar] [CrossRef]
- Cheng, H.; Fan, J.-H.; Zhao, L.-P.; Fan, G.-L.; Zheng, R.-R.; Qiu, X.-Z.; Yu, X.-Y.; Li, S.-Y.; Zhang, X.-Z. Chimeric peptide engineered exosomes for dual-stage light guided plasma membrane and nucleus targeted photodynamic therapy. Biomaterials 2019, 211, 14–24. [Google Scholar] [CrossRef]
- Krysko, D.V.; Garg, A.D.; Kaczmarek, A.; Krysko, O.; Agostinis, P.; Vandenabeele, P. Immunogenic Cell Death and DAMPs in Cancer Therapy. Nat. Rev. Cancer 2012, 12, 860–875. [Google Scholar] [CrossRef]
- Li, W.; Yang, J.; Luo, L.; Jiang, M.; Qin, B.; Yin, H.; Zhu, C.; Yuan, X.; Zhang, J.; Luo, Z.; et al. Targeting Photodynamic and Photothermal Therapy to the Endoplasmic Reticulum Enhances Immunogenic Cancer Cell Death. Nat. Commun. 2019, 10, 3349. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Gao, H.; Liu, R.; Chen, C.; Zeng, S.; Liu, Q.; Ding, D. Endoplasmic reticulum targeted AIE bioprobe as a highly efficient inducer of immunogenic cell death. Sci. China Chem. 2020, 63, 1428–1434. [Google Scholar] [CrossRef]
- Chen, P.-L.; Huang, P.-Y.; Chen, J.-Y.; Shi, Q.-Y.; Zhu, Y.-Y.; Chen, Y.; Liu, L.-H.; Zhang, X.-Z. A self-delivery chimeric peptide for high efficient cell membrane-targeting low-temperature photothermal/photodynamic combinational therapy and metastasis suppression of tumor. Biomaterials 2022, 286, 121593. [Google Scholar] [CrossRef]
- Zheng, D.; Liu, J.; Xie, L.; Wang, Y.; Ding, Y.; Peng, R.; Cui, M.; Wang, L.; Zhang, Y.; Zhang, C.; et al. Enzyme-instructed and mitochondria-targeting peptide self-assembly to efficiently induce immunogenic cell death. Acta Pharm. Sin. B 2022, 12, 2740–2750. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Chang, R.; Xing, R.; Yan, X. Bioactive Peptide Nanodrugs Based on Supramolecular Assembly for Boosting Immunogenic Cell Death-Induced Cancer Immunotherapy. Small Methods 2023, 7, 2201708. [Google Scholar] [CrossRef]
- Wang, T.; Gao, Z.; Zhang, Y.; Hong, Y.; Tang, Y.; Shan, K.; Kong, X.; Wang, Z.; Shi, Y.; Ding, D. A supramolecular self-assembled nanomaterial for synergistic therapy of immunosuppressive tumor. J. Control. Release 2022, 351, 272–283. [Google Scholar] [CrossRef] [PubMed]
- Youssef, Z.; Yesmurzayeva, N.; Larue, L.; Jouan-Hureaux, V.; Colombeau, L.; Arnoux, P.; Acherar, S.; Vanderesse, R.; Frochot, C. New Targeted Gold Nanorods for the Treatment of Glioblastoma by Photodynamic Therapy. J. Clin. Med. 2019, 8, 2205. [Google Scholar] [CrossRef]
- Dai, G.; Chu, J.C.H.; Chan, C.K.W.; Choi, C.H.J.; Ng, D.K.P. Reactive oxygen species-responsive polydopamine nanoparticles for targeted and synergistic chemo and photodynamic anticancer therapy. Nanoscale 2021, 13, 15899–15915. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.-J.; Lee, H.-I.; Kim, J.-K.; Kim, C.-H.; Kim, Y.-J. Peptide 18-4/chlorin e6-conjugated polyhedral oligomeric silsesquioxane nanoparticles for targeted photodynamic therapy of breast cancer. Colloids Surf. B 2020, 189, 110829. [Google Scholar] [CrossRef] [PubMed]
- Panikar, S.S.; Ramírez-García, G.; Banu, N.; Vallejo-Cardona, A.A.; Lugo-Fabres, P.; Camacho-Villegas, T.A.; Salas, P.; De la Rosa, E. Ligand-targeted Theranostic Liposomes combining methylene blue attached upconversion nanoparticles for NIR activated bioimaging and photodynamic therapy against HER-2 positive breast cancer. J. Lumin. 2021, 237, 118143. [Google Scholar] [CrossRef]
- Xue, E.Y.; Wong, R.C.H.; Wong, C.T.T.; Fong, W.-P.; Ng, D.K.P. Synthesis and biological evaluation of an epidermal growth factor receptor-targeted peptide-conjugated phthalocyanine-based photosensitiser. RSC Adv. 2019, 9, 20652–20662. [Google Scholar] [CrossRef] [PubMed]
- Yu, L.; Wang, Q.; Wong, R.C.H.; Zhao, S.; Ng, D.K.P.; Lo, P.-C. Synthesis and biological evaluation of phthalocyanine-peptide conjugate for EGFR-targeted photodynamic therapy and bioimaging. Dyes Pigm. 2019, 163, 197–203. [Google Scholar] [CrossRef]
- Chu, J.C.H.; Fong, W.-P.; Wong, C.T.T.; Ng, D.K.P. Facile Synthesis of Cyclic Peptide–Phthalocyanine Conjugates for Epidermal Growth Factor Receptor-Targeted Photodynamic Therapy. J. Med. Chem. 2021, 64, 2064–2076. [Google Scholar] [CrossRef]
- Yan, S.; Tang, D.; Hong, Z.; Wang, J.; Yao, H.; Lu, L.; Yi, H.; Fu, S.; Zheng, C.; He, G.; et al. CD133 peptide-conjugated pyropheophorbide-a as a novel photosensitizer for targeted photodynamic therapy in colorectal cancer stem cells. Biomater. Sci. 2021, 9, 2020–2031. [Google Scholar] [CrossRef]
- Zahmatkeshan, M.; Gheybi, F.; Rezayat, S.M.; Jaafari, M.R. Improved drug delivery and therapeutic efficacy of PEgylated liposomal doxorubicin by targeting anti-HER2 peptide in murine breast tumor model. Eur. J. Pharm. Sci. 2016, 86, 125–135. [Google Scholar] [CrossRef]
- Panikar, S.S.; Ramírez-García, G.; Vallejo-Cardona, A.A.; Banu, N.; Patrón-Soberano, O.A.; Cialla-May, D.; Camacho-Villegas, T.A.; de la Rosa, E. Novel anti-HER2 peptide-conjugated theranostic nanoliposomes combining NaYF4:Yb,Er nanoparticles for NIR-activated bioimaging and chemo-photodynamic therapy against breast cancer. Nanoscale 2019, 11, 20598–20613. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.-L.; Chou, Y.-T.; Su, B.-K.; Wu, C.-C.; Wang, C.-H.; Chang, K.-H.; Ho, J.-A.A.; Chou, P.-T. Comprehensive Thione-Derived Perylene Diimides and Their Bio-Conjugation for Simultaneous Imaging, Tracking, and Targeted Photodynamic Therapy. J. Am. Chem. Soc. 2022, 144, 17249–17260. [Google Scholar] [CrossRef] [PubMed]
- Park, J.Y.; Shin, Y.; Won, W.R.; Lim, C.; Kim, J.C.; Kang, K.; Husni, P.; Lee, E.S.; Youn, Y.S.; Oh, K.T. Development of AE147 Peptide-Conjugated Nanocarriers for Targeting uPAR-Overexpressing Cancer Cells. Int. J. Nanomed. 2021, 16, 5437–5449. [Google Scholar] [CrossRef]
- Pethő, L.; Murányi, J.; Pénzes, K.; Gurbi, B.; Brauswetter, D.; Halmos, G.; Csík, G.; Mező, G. Suitability of GnRH Receptors for Targeted Photodynamic Therapy in Head and Neck Cancers. Int. J. Mol. Sci. 2019, 20, 5027. [Google Scholar] [CrossRef]
- Zhang, L.; Wang, P.; Zhou, X.-Q.; Bretin, L.; Zeng, X.; Husiev, Y.; Polanco, E.A.; Zhao, G.; Wijaya, L.S.; Biver, T.; et al. Cyclic Ruthenium-Peptide Conjugates as Integrin-Targeting Phototherapeutic Prodrugs for the Treatment of Brain Tumors. J. Am. Chem. Soc. 2023, 145, 14963–14980. [Google Scholar] [CrossRef] [PubMed]
- Ibrahimova, V.; González-Delgado, J.A.; Levêque, M.; Torres, T.; Garanger, E.; Lecommandoux, S. Photooxidation Responsive Elastin-Like Polypeptide Conjugates for Photodynamic Therapy Application. Bioconjug. Chem. 2021, 32, 1719–1728. [Google Scholar] [CrossRef] [PubMed]
- Le, D.H.T.; Ibrahimova, V.; van den Wildenberg, S.A.H.; Wu, H.; Fonseca, A.; Torres, T.; Garanger, E.; Leenders, W.P.J.; Brock, R.; Lecommandoux, S.; et al. Light-Responsive Elastin-Like Peptide-Based Targeted Nanoparticles for Enhanced Spheroid Penetration. Angew. Chem. Int. Ed. 2023, 62, e202300511. [Google Scholar] [CrossRef] [PubMed]
- Zhang, P.; Gao, Z.; Cui, J.; Hao, J. Dual-Stimuli-Responsive Polypeptide Nanoparticles for Photothermal and Photodynamic Therapy. ACS Appl. Bio Mater. 2019, 3, 561–569. [Google Scholar] [CrossRef] [PubMed]
- Shrestha, S.; Wu, J.; Sah, B.; Vanasse, A.; Cooper, L.N.; Ma, L.; Li, G.; Zheng, H.; Chen, W.; Antosh, M.P. X-ray induced photodynamic therapy with copper-cysteamine nanoparticles in mice tumors. Proc. Natl. Acad. Sci. USA 2019, 116, 16823–16828. [Google Scholar] [CrossRef] [PubMed]
- Ballance, W.C.; Qin, E.C.; Chung, H.J.; Gillette, M.U.; Kong, H. Reactive oxygen species-responsive drug delivery systems for the treatment of neurodegenerative diseases. Biomaterials 2019, 217, 119292. [Google Scholar] [CrossRef] [PubMed]
- Ding, Y.; Wang, C.; Ma, Y.; Zhu, L.; Lu, B.; Wang, Y.; Wang, J.; Chen, T.; Dong, C.-M.; Yao, Y. pH/ROS dual-responsive supramolecular polypeptide prodrug nanomedicine based on host-guest recognition for cancer therapy. Acta Biomater. 2022, 143, 381–391. [Google Scholar] [CrossRef] [PubMed]
- Lu, S.; Lei, X.; Ren, H.; Zheng, S.; Qiang, J.; Zhang, Z.; Chen, Y.; Wei, T.; Wang, F.; Chen, X. PEGylated Dimeric BODIPY Photosensitizers as Nanocarriers for Combined Chemotherapy and Cathepsin B-Activated Photodynamic Therapy in 3D Tumor Spheroids. ACS Appl. Bio Mater. 2020, 3, 3835–3845. [Google Scholar] [CrossRef]
- Hu, C.; Zhuang, W.; Yu, T.; Chen, L.; Liang, Z.; Li, G.; Wang, Y. Multi-stimuli responsive polymeric prodrug micelles for combined chemotherapy and photodynamic therapy. J. Mater. Chem. B 2020, 8, 5267–5279. [Google Scholar] [CrossRef]
- Qi, G.; Liu, X.; Shi, L.; Wu, M.; Liu, J.; Liu, B. Enzyme-Mediated Intracellular Polymerization of AIEgens for Light-Up Tumor Localization and Theranostics. Adv. Mater. 2021, 34, 2106885. [Google Scholar] [CrossRef]
- Zhang, J.; Xu, M.; Mu, Y.; Li, J.; Foda, M.F.; Zhang, W.; Han, K.; Han, H. Reasonably retard O2 consumption through a photoactivity conversion nanocomposite for oxygenated photodynamic therapy. Biomaterials 2019, 218, 119312. [Google Scholar] [CrossRef]
- Zhang, W.; Cai, K.; Li, X.; Zhang, J.; Ma, Z.; Foda, M.F.; Mu, Y.; Dai, X.; Han, H. Au Hollow Nanorods-Chimeric Peptide Nanocarrier for NIR-II Photothermal Therapy and Real-time Apoptosis Imaging for Tumor Theranostics. Theranostics 2019, 9, 4971–4981. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Mu, Y.; Xu, M.; Foda, M.F.; Han, H. Sequential assembled chimeric peptide for precise synergistic phototherapy and photoacoustic imaging of tumor apoptosis. Chem. Eng. J. 2022, 427, 130775. [Google Scholar] [CrossRef]
- Fan, Y.; Li, P.; Hu, B.; Liu, T.; Huang, Z.; Shan, C.; Cao, J.; Cheng, B.; Liu, W.; Tang, Y. A Smart Photosensitizer–Cerium Oxide Nanoprobe for Highly Selective and Efficient Photodynamic Therapy. Inorg. Chem. 2019, 58, 7295–7302. [Google Scholar] [CrossRef] [PubMed]
- Su, Z.; Xiao, Z.; Huang, J.; Wang, Y.; An, Y.; Xiao, H.; Peng, Y.; Pang, P.; Han, S.; Zhu, K.; et al. Dual-Sensitive PEG-Sheddable Nanodrug Hierarchically Incorporating PD-L1 Antibody and Zinc Phthalocyanine for Improved Immuno-Photodynamic Therapy. ACS Appl. Mater. Interfaces 2021, 13, 12845–12856. [Google Scholar] [CrossRef] [PubMed]
- Shu, M.; Tang, J.; Chen, L.; Zeng, Q.; Li, C.; Xiao, S.; Jiang, Z.; Liu, J. Tumor microenvironment triple-responsive nanoparticles enable enhanced tumor penetration and synergetic chemo-photodynamic therapy. Biomaterials 2021, 268, 120574. [Google Scholar] [CrossRef]
- Wang, J.; Yang, B.; Lv, C.; Chen, T.; Sun, L.; Sun, L.; Hao, J.; Ding, F.; Wang, T.; Jiang, J.; et al. Amino porphyrin-peptide assemblies induce ribosome damage and cancer stem cell inhibition for an enhanced photodynamic therapy. Biomaterials 2022, 289, 121812. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Wang, A.; Ren, P.; Yan, X.; Bai, S. One-step co-assembly method to fabricate photosensitive peptide nanoparticles for two-photon photodynamic therapy. Chem. Commun. 2019, 55, 3191–3194. [Google Scholar] [CrossRef] [PubMed]
- Hou, Y.; Kuang, Y.; Jiang, Q.; Zhou, S.; Yu, J.; He, Z.; Sun, J. Arginine-peptide complex-based assemblies to combat tumor hypoxia for enhanced photodynamic therapeutic effect. Nano Res. 2022, 15, 5183–5192. [Google Scholar] [CrossRef]
- Xu, Y.; Teng, C.; Dang, H.; Yin, D.; Yan, L. Highly bright stable organic radicals encapsulated by amphiphilic polypeptide for efficient near-infrared phototheranostics. Talanta 2024, 266, 124948. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Liu, Y.; Liang, R.; Hong, G.; An, J.; Peng, X.; Zheng, W.-H.; Song, F. Self-assembly of amphiphilic peptides to construct activatable nanophotosensitizers for theranostic photodynamic therapy. Chin. Chem. Lett. 2021, 32, 3903–3906. [Google Scholar] [CrossRef]
- Liu, Z.; Li, J.; Chen, W.; Liu, L.; Yu, F. Light and sound to trigger the Pandora’s box against breast cancer: A combination strategy of sonodynamic, photodynamic and photothermal therapies. Biomaterials 2020, 232, 119685. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Wong, R.C.H.; Yan, X.; Ng, D.K.P.; Lo, P.-C. Self-Assembled Nanophotosensitizing Systems with Zinc(II) Phthalocyanine-Peptide Conjugates as Building Blocks for Targeted Chemo-Photodynamic Therapy. ACS Appl. Bio Mater. 2020, 3, 5463–5473. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.; Jiao, Q.; Wang, C.; Zheng, Y.; Pan, X.; Zhong, W.; Xu, K. Nanofibrillar peptide hydrogels for self-delivery of lonidamine and synergistic photodynamic therapy. Acta Biomater. 2023, 155, 139–153. [Google Scholar] [CrossRef]
- Gao, Q.; Huang, D.; Deng, Y.; Yu, W.; Jin, Q.; Ji, J.; Fu, G. Chlorin e6 (Ce6)-loaded supramolecular polypeptide micelles with enhanced photodynamic therapy effect against Pseudomonas aeruginosa. Chem. Eng. J. 2021, 417, 129334. [Google Scholar] [CrossRef]
- Zheng, X.; Pan, D.; Chen, X.; Wu, L.; Chen, M.; Wang, W.; Zhang, H.; Gong, Q.; Gu, Z.; Luo, K. Self-Stabilized Supramolecular Assemblies Constructed from PEGylated Dendritic Peptide Conjugate for Augmenting Tumor Retention and Therapy. Adv. Sci. 2021, 8, 2102741. [Google Scholar] [CrossRef]
- Zhu, H.; Wang, H.; Shi, B.; Shangguan, L.; Tong, W.; Yu, G.; Mao, Z.; Huang, F. Supramolecular peptide constructed by molecular Lego allowing programmable self-assembly for photodynamic therapy. Nat. Commun. 2019, 10, 2412. [Google Scholar] [CrossRef]
- Li, S.; Zhao, L.; Chang, R.; Xing, R.; Yan, X. Spatiotemporally Coupled Photoactivity of Phthalocyanine–Peptide Conjugate Self-Assemblies for Adaptive Tumor Theranostics. Chem. Eur. J. 2019, 25, 13429–13435. [Google Scholar] [CrossRef]
- Zou, Q.; Chang, R.; Xing, R.; Yuan, C.; Yan, X. Injectable self-assembled bola-dipeptide hydrogels for sustained photodynamic prodrug delivery and enhanced tumor therapy. J. Control Release 2020, 319, 344–351. [Google Scholar] [CrossRef] [PubMed]
- Jiang, L.; Chen, D.; Jin, Z.; Xia, C.; Xu, Q.; Fan, M.; Dai, Y.; Liu, J.; Li, Y.; He, Q. Light-triggered nitric oxide release and structure transformation of peptide for enhanced intratumoral retention and sensitized photodynamic therapy. Bioact. Mater. 2022, 12, 303–313. [Google Scholar] [CrossRef] [PubMed]
- Liu, R.; Yu, M.; Yang, X.; Umeshappa, C.S.; Hu, C.; Yu, W.; Qin, L.; Huang, Y.; Gao, H. Linear Chimeric Triblock Molecules Self-Assembled Micelles with Controllably Transformable Property to Enhance Tumor Retention for Chemo-Photodynamic Therapy of Breast Cancer. Adv. Funct. Mater. 2019, 29, 1808462. [Google Scholar] [CrossRef]
- Cheng, Z.; Cheng, Y.; Chen, Q.; Li, M.; Wang, J.; Liu, H.; Li, M.; Ning, Y.; Yu, Z.; Wang, Y.; et al. Self-assembly of pentapeptides into morphology-adaptable nanomedicines for enhanced combinatorial chemo-photodynamic therapy. Nano Today 2020, 33, 100878. [Google Scholar] [CrossRef]
- Li, M.; Ning, Y.; Chen, J.; Duan, X.; Song, N.; Ding, D.; Su, X.; Yu, Z. Proline Isomerization-Regulated Tumor Microenvironment-Adaptable Self-Assembly of Peptides for Enhanced Therapeutic Efficacy. Nano Lett. 2019, 19, 7965–7976. [Google Scholar] [CrossRef] [PubMed]
- Sun, B.; Chang, R.; Cao, S.; Yuan, C.; Zhao, L.; Yang, H.; Li, J.; Yan, X.; van Hest, J.C.M. Acid-Activatable Transmorphic Peptide-Based Nanomaterials for Photodynamic Therapy. Angew. Chem. Int. Ed. 2020, 59, 20582–20588. [Google Scholar] [CrossRef]
- Zhong, C.; Zhang, L.; Yu, L.; Huang, J.; Huang, S.; Yao, Y. A review for antimicrobial peptides with anticancer properties: Re-purposing of potential anticancer agents. BIO Integr. 2021, 1, 156–167. [Google Scholar] [CrossRef]
- Moret, F.; Gobbo, M.; Reddi, E. Conjugation of photosensitisers to antimicrobial peptides increases the efficiency of photodynamic therapy in cancer cells. Photochem. Photobiol. Sci. 2015, 14, 1238–1250. [Google Scholar] [CrossRef]
- Song, M.; Liu, G.; Liu, Y.; Cheng, Z.; Lin, H.; Liu, J.; Wu, Z.; Xue, J.; Hong, W.; Huang, M.; et al. Using porphyrins as albumin-binding molecules to enhance antitumor efficacies and reduce systemic toxicities of antimicrobial peptides. Eur. J. Med. Chem. 2021, 217, 113382. [Google Scholar] [CrossRef]
- Xu, Y.; Yao, Y.; Wang, L.; Chen, H.; Tan, N. Hyaluronic Acid Coated Liposomes Co-Delivery of Natural Cyclic Peptide RA-XII and Mitochondrial Targeted Photosensitizer for Highly Selective Precise Combined Treatment of Colon Cancer. Int. J. Nanomed. 2021, 16, 4929–4942. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Hu, Y.; Sun, Y.; Wan, C.; Zhang, Z.; Dai, X.; Lin, Z.; He, Q.; Yang, Z.; Huang, P.; et al. Co-delivery of Bee Venom Melittin and a Photosensitizer with an Organic–Inorganic Hybrid Nanocarrier for Photodynamic Therapy and Immunotherapy. ACS Nano 2019, 13, 12638–12652. [Google Scholar] [CrossRef] [PubMed]
- Wang, N.; Zhou, Y.; Xu, Y.; Ren, X.; Zhou, S.; Shang, Q.; Jiang, Y.; Luan, Y. Molecular engineering of anti-PD-L1 peptide and photosensitizer for immune checkpoint blockade photodynamic-immunotherapy. Chem. Eng. J. 2020, 400, 125995. [Google Scholar] [CrossRef]
- Qiu, Z.; Lu, Z.; Huang, J.; Zhong, Y.; Yan, N.; Kong, R.; Cheng, H. Self-reinforced photodynamic immunostimulator to downregulate and block PD-L1 for metastatic breast cancer treatment. Biomaterials 2023, 303, 122392. [Google Scholar] [CrossRef] [PubMed]
- Qi, J.; Jia, S.; Kang, X.; Wu, X.; Hong, Y.; Shan, K.; Kong, X.; Wang, Z.; Ding, D. Semiconducting Polymer Nanoparticles with Surface-Mimicking Protein Secondary Structure as Lysosome-Targeting Chimaeras for Self-Synergistic Cancer Immunotherapy. Adv. Mater. 2022, 34, 2203309. [Google Scholar] [CrossRef] [PubMed]
- Dai, J.; Hu, J.J.; Dong, X.; Chen, B.; Dong, X.; Liu, R.; Xia, F.; Lou, X. Deep Downregulation of PD-L1 by Caged Peptide-Conjugated AIEgen/miR-140 Nanoparticles for Enhanced Immunotherapy. Angew. Chem. Int. Ed. 2022, 61, e202117798. [Google Scholar] [CrossRef]
- Zhang, R.; Chen, X.; Cheng, Y.; Chen, Z.; Li, X.; Deng, Y. Recent advances of nanomaterials for intervention in Parkinson’s disease in the context of anti-inflammation. Coord. Chem. Rev. 2024, 502, 215616. [Google Scholar] [CrossRef]
- Wang, H.; Li, Q.; Alam, P.; Bai, H.; Bhalla, V.; Bryce, M.R.; Cao, M.; Chen, C.; Chen, S.; Chen, X.; et al. Aggregation-Induced Emission (AIE), Life and Health. ACS Nano 2023, 17, 14347–14405. [Google Scholar] [CrossRef]










| Peptide Sequence (Name) | Targeting Receptor | Types of Cancer Cells | References |
|---|---|---|---|
| QRHKPRE (QRH) | Epidermal growth factor receptor (EGFR) | Lung cancer, colon cancer, breast cancer, kidney cancer, head and neck cancer, glioma, etc. | [67,74] |
| YHWYGYTPQNVI (GE11) | [75] | ||
| CMYIEALDKYAC (N.A.) | [76] | ||
| WxEAAYQrFL (peptide 18-4) | Keratin 1 (KRT1) | breast cancer | [72] |
| LQNAPRS (N.A.) | CD133 | Colorectal cancer | [77] |
| anti-HER2 peptide | Human epidermal growth factor receptor 2 (HER2) | HER-2 positive breast cancer | [74,78,79] |
| cyclo-[2-NaI-Gly-d-Tyr-Arg-Arg] (FC131) | CXCR4, a cell-surface chemokine receptor | Breast cancer, ovarian cancer, lung cancer, colorectal cancer, primary brain tumors, etc. | [80] |
| KSD-cha-FskYLWSSK(AE147) | Urokinase-type plasminogen activator receptor (uPAR) | Aggressive cancer cells such as breast, prostate, glioma, colorectal, endometrial, bladder, liver, and melanoma cancer | [81] |
| KDKPPR (N.A.) | NRP-1 | Glioma, acute myeloid leukemia, pancreatic cancer, lung cancer, ovarian cancer, gastrointestinal tumors, melanoma, etc. | [66] |
| EHWSYGLRPG (N.A.) | Gonadotropin-releasing hormone receptor (GnRH-R) | Head and neck squamous cell carcinomas | [82] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, Q.; Ming, R.; Huang, L.; Zhang, R. Versatile Peptide-Based Nanosystems for Photodynamic Therapy. Pharmaceutics 2024, 16, 218. https://doi.org/10.3390/pharmaceutics16020218
Li Q, Ming R, Huang L, Zhang R. Versatile Peptide-Based Nanosystems for Photodynamic Therapy. Pharmaceutics. 2024; 16(2):218. https://doi.org/10.3390/pharmaceutics16020218
Chicago/Turabian StyleLi, Qiuyan, Ruiqi Ming, Lili Huang, and Ruoyu Zhang. 2024. "Versatile Peptide-Based Nanosystems for Photodynamic Therapy" Pharmaceutics 16, no. 2: 218. https://doi.org/10.3390/pharmaceutics16020218
APA StyleLi, Q., Ming, R., Huang, L., & Zhang, R. (2024). Versatile Peptide-Based Nanosystems for Photodynamic Therapy. Pharmaceutics, 16(2), 218. https://doi.org/10.3390/pharmaceutics16020218