Development of Ciprofloxacin-Loaded Electrospun Yarns of Application Interest as Antimicrobial Surgical Suture Materials
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
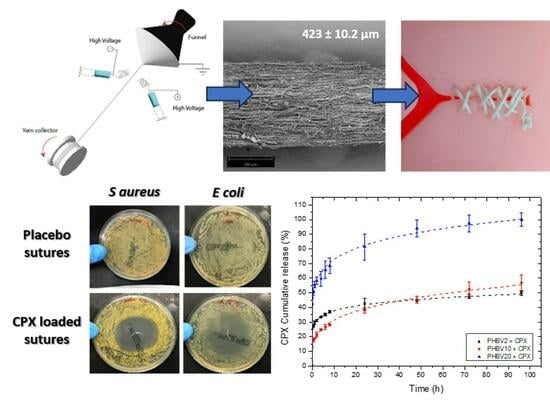
2.2. Electrospun Fibre Yarn
2.3. Yarn Characterization
2.3.1. Morphology
2.3.2. Wide-Angle X-ray Scattering (WAXS)
2.3.3. Attenuated Total Reflection—Fourier Transform Infrared Spectroscopy (ATR-FTIR)
2.3.4. Thermal Analysis
2.3.5. Mechanical Tests
2.3.6. In Vitro Drug Release: Kinetics Study
2.3.7. Antimicrobial Activity
2.3.8. Statistical Analysis
3. Results
3.1. Morphological Characterization of Yarn Sutures
3.2. Thermal, Crystallinity and Molecular Characterization
3.3. Process Loading Efficiency and In Vitro Release
3.4. Antimicrobial Activity
3.5. Mechanical Properties
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wu, S.; Dong, T.; Li, Y.; Sun, M.; Qi, Y.; Liu, J.; Kuss, M.A.; Chen, S.; Duan, B. State-of-the-art review of advanced electrospun nanofiber yarn-based textiles for biomedical applications. Appl. Mater. Today 2022, 27, 101473. [Google Scholar] [CrossRef]
- Xu, L.; Liu, Y.; Zhou, W.; Yu, D. Electrospun Medical Sutures for Wound Healing: A Review. Polymers 2022, 14, 1637. [Google Scholar] [CrossRef]
- Arora, A.; Aggarwal, G.; Chander, J.; Maman, P.; Nagpal, M. Drug eluting sutures: A recent update. J. Appl. Pharm. Sci. 2019, 9, 111–123. [Google Scholar] [CrossRef]
- Weldon, C.B.; Tsui, J.H.; Shankarappa, S.A.; Nguyen, V.T.; Ma, M.; Anderson, D.G.; Kohane, D.S. Electrospun drug-eluting sutures for local anesthesia. J. Control. Release 2012, 161, 903–909. [Google Scholar] [CrossRef]
- García-Vargas, M.; González-Chomón, C.; Magariños, B.; Concheiro, A.; Alvarez-Lorenzo, C.; Bucio, E. Acrylic polymer-grafted polypropylene sutures for covalent immobilization or reversible adsorption of vancomycin. Int. J. Pharm. 2014, 461, 286–295. [Google Scholar] [CrossRef]
- Wang, X.; Liu, P.; Wu, Q.; Zheng, Z.; Xie, M.; Chen, G.; Yu, J.; Wang, X.; Li, G.; Kaplan, D. Sustainable Antibacterial and Anti-Inflammatory Silk Suture with Surface Modification of Combined-Therapy Drugs for Surgical Site Infection. ACS Appl. Mater. Interfaces 2022, 14, 11177–11191. [Google Scholar] [CrossRef] [PubMed]
- Günday, C.; Anand, S.; Gencer, H.B.; Munafò, S.; Moroni, L.; Fusco, A.; Donnarumma, G.; Ricci, C.; Hatir, P.C.; Türeli, N.G.; et al. Ciprofloxacin-loaded polymeric nanoparticles incorporated electrospun fibers for drug delivery in tissue engineering applications. Drug Deliv. Transl. Res. 2020, 10, 706–720. [Google Scholar] [CrossRef] [PubMed]
- Catanzano, O.; Acierno, S.; Russo, P.; Cervasio, M.; Del Basso De Caro, M.; Bolognese, A.; Sammartino, G.; Califano, L.; Marenzi, G.; Calignano, A.; et al. Melt-spun bioactive sutures containing nanohybrids for local delivery of anti-inflammatory drugs. Mater. Sci. Eng. C 2014, 43, 300–309. [Google Scholar] [CrossRef] [PubMed]
- Deng, X.; Qasim, M.; Ali, A. Engineering and polymeric composition of drug-eluting suture: A review. J. Biomed. Mater. Res. Part A 2021, 109, 2065–2081. [Google Scholar] [CrossRef]
- Champeau, M.; Thomassin, J.M.; Tassaing, T.; Jérôme, C. Current manufacturing processes of drug-eluting sutures. Expert. Opin. Drug Deliv. 2017, 14, 1293–1303. [Google Scholar] [CrossRef]
- Wang, L.; Chen, D.; Sun, J. Layer-by-layer deposition of polymeric microgel films on surgical sutures for loading and release of ibuprofen. Langmuir 2009, 25, 7990–7994. [Google Scholar] [CrossRef]
- Valarezo, E.; Tammaro, L.; Malagón, O.; González, S.; Armijos, C.; Vittoria, V. Fabrication and characterization of poly(lactic acid)/poly(ε-caprolactone) blend electrospun fibers loaded with amoxicillin for tunable delivering. J. Nanosci. Nanotechnol. 2015, 15, 4706–4712. [Google Scholar] [CrossRef]
- Li, J.; Pan, H.; Ye, Q.; Shi, C.; Zhang, X.; Pan, W. Carvedilol-loaded polyvinylpyrrolidone electrospun nanofiber film for sublingual delivery. J. Drug Deliv. Sci. Technol. 2020, 58, 101726. [Google Scholar] [CrossRef]
- Khan, Z.; Kafiah, F.; Zahid Shafi, H.; Nufaiei, F.; Ahmed Furquan, S.; Matin, A. Morphology, Mechanical Properties and Surface Characteristics of Electrospun Polyacrylonitrile (PAN) Nanofiber Mats. Int. J. Adv. Eng. Nano Technol. 2015, 2, 15–22. [Google Scholar]
- Cirillo, V. Design of Bicomponent Electrospun Conduits for Peripheral Nerve Regeneration; University of Naples Federico II: Napoli, Italy, 2013. [Google Scholar]
- Yan, T.; Shi, Y.; Zhuang, H.; Lin, Y.; Lu, D.; Cao, S.; Zhu, L. Electrospinning mechanism of nanofiber yarn and its multiscale wrapping yarn. Polymers 2021, 13, 3189. [Google Scholar] [CrossRef]
- Kashiwabuchi, F.; Parikh, K.S.; Omiadze, R.; Zhang, S.; Luo, L.; Patel, H.V.; Xu, Q.; Ensign, L.M.; Hanes, J.; Mcdonnell, P.J. Development of Absorbable, Antibiotic-Eluting Sutures for Ophthalmic Surgery. Transl. Vis. Sci. Technol. 2017, 6, 1. [Google Scholar] [CrossRef] [PubMed]
- Haghighat, F.; Ravandi, S.A.H. Mechanical properties and in vitro degradation of PLGA suture manufactured via electrospinning. Fibers Polym. 2014, 15, 71–77. [Google Scholar] [CrossRef]
- Dart, A.J.; Dart, C.M. 7.38 Suture Material: Conventional and Stimuli Responsive. In Comprehensive Biomaterials II; Elsevier: Amsterdam, The Netherlands, 2017; pp. 746–771. [Google Scholar] [CrossRef]
- He, Y.; Hu, Z.; Ren, M.; Ding, C.; Chen, P.; Gu, Q.; Wu, Q. Evaluation of PHBHHx and PHBV/PLA fibers used as medical sutures. J. Mater. Sci. Mater. Med. 2014, 25, 561–571. [Google Scholar] [CrossRef] [PubMed]
- Shishatskaya, E.I.; Volova, T.G.; Puzyr, A.P.; Mogilnaya, O.A.; Efremov, S.N. Tissue response to the implantation of biodegradable polyhydroxyalkanoate sutures. J. Mater. Sci. Mater. Med. 2004, 15, 719–728. [Google Scholar] [CrossRef] [PubMed]
- Volova, T.; Shishatskaya, E.; Sevastianov, V.; Efremov, S.; Mogilnaya, O. Results of biomedical investigations of PHB and PHB/PHV fibers. Biochem. Eng. J. 2003, 16, 125–133. [Google Scholar] [CrossRef]
- Melendez-rodriguez, B.; Reis, M.A.M.; Carvalheira, M.; Sammon, C.; Cabedo, L.; Torres-giner, S.; Lagaron, J.M. Development and Characterization of Electrospun Biopapers of Poly(3-hydroxybutyrate-co-3-hydroxyvalerate) Derived from Cheese Whey with Varying 3-Hydroxyvalerate Contents. Biomacromolecules 2021, 22, 2935–2953. [Google Scholar] [CrossRef]
- Savenkova, L.; Gercberga, Z.; Bibers, I.; Kalnin, M. Effect of 3-hydroxy valerate content on some physical and mechanical properties of polyhydroxyalkanoates produced by Azotobacter chroococcum. Process Biochem. 2000, 36, 445–450. [Google Scholar] [CrossRef]
- Choudhury, A.; Das, S.; Dhangar, S.; Kapasiya, S.; Kanango, A. Development and characterization buccoadhesive film of ciprofloxacin hydrochloride. Int. J. PharmTech Res. 2010, 2, 1050–1057. [Google Scholar]
- Melendez-Rodriguez, B.; Castro-Mayorga, J.L.; Reis, M.A.M.; Sammon, C.; Cabedo, L.; Torres-Giner, S.; Lagaron, J.M. Preparation and Characterization of Electrospun Food Biopackaging Films of Poly(3-hydroxybutyrate-co-3-hydroxyvalerate) Derived From Fruit Pulp Biowaste. Front. Sustain. Food Syst. 2018, 2, 38. [Google Scholar] [CrossRef]
- Mathematical models of drug release. In Strategies to Modify the Drug Release from Pharmaceutical Systems; Elsevier: Amsterdam, The Netherlands, 2015; pp. 63–86. ISBN 9780081000922.
- Figueroa-Lopez, K.J.; Andrade-Mahecha, M.M.; Torres-Vargas, O.L. Spice oleoresins containing antimicrobial agents improve the potential use of bio-composite films based on gelatin. Food Packag. Shelf Life 2018, 17, 50–56. [Google Scholar] [CrossRef]
- Li, H.; Zhang, Z.; Godakanda, V.U.; Chiu, Y.J.; Angkawinitwong, U.; Patel, K.; Stapleton, P.G.; de Silva, R.M.N.; de Silva, K.M.N.; Zhu, L.M.; et al. The effect of collection substrate on electrospun ciprofloxacin-loaded poly(vinylpyrrolidone) and ethyl cellulose nanofibers as potential wound dressing materials. Mater. Sci. Eng. C 2019, 104, 109917. [Google Scholar] [CrossRef] [PubMed]
- Okoye, E.; Okolie, T. Development and in vitro characterization of ciprofloxacin loaded polymeric films for wound dressing. Int. J. Heal. Allied Sci. 2015, 4, 234. [Google Scholar] [CrossRef]
- Sobhani, Z.; Samani, S.M.; Montaseri, H.; Khezri, E. Nanoparticles of chitosan loaded ciprofloxacin: Fabrication and antimicrobial activity. Adv. Pharm. Bull. 2017, 7, 427–432. [Google Scholar] [CrossRef] [PubMed]
- Karimi, K.; Pallagi, E.; Szabó-Révész, P.; Csóka, I.; Ambrus, R. Development of a microparticle-based dry powder inhalation formulation of ciprofloxacin hydrochloride applying the quality by design approach. Drug Des. Devel. Ther. 2016, 10, 3331–3343. [Google Scholar] [CrossRef] [PubMed]
- Hanafy, A.F. In-vitro bioequivalence, physicochemical and economic benefits study for marketed innovator and generic ciprofloxacin hydrochloride tablets in Saudi Arabia. J. Appl. Pharm. Sci. 2016, 6, 63–68. [Google Scholar] [CrossRef]
- Rovira, F.; Mas, L.C.; Lorena, J.; Mayorga, C. Antimicrobial nanocomposites and electrospun coatings based on poly(3-hydroxybutyrate-co-3-hydroxyvalerate) and copper oxide nanoparticles for active packaging and coating applications. J. Appl. Polym. Sci. 2018, 135, 45673. [Google Scholar] [CrossRef]
- Pavlova, E.R.; Bagrov, D.V.; Kopitsyna, M.N.; Shchelokov, D.A.; Bonartsev, A.P.; Zharkova, I.I.; Mahina, T.K.; Myshkina, V.L.; Bonartseva, G.A.; Shaitan, K.V.; et al. Poly(hydroxybutyrate-co-hydroxyvalerate) and bovine serum albumin blend prepared by electrospinning. J. Appl. Polym. Sci. 2017, 134, 45090. [Google Scholar] [CrossRef]
- Lemes, A.P.; Talim, R.; Gomes, R.C.; Gomes, R.C. Preparation and Characterization of Maleic Anhydride Grafted Poly(Hydroxybutirate-CO-Hydroxyvalerate)—PHBV-g-MA. Mater. Res. 2016, 19, 229–235. [Google Scholar] [CrossRef]
- Figueroa-Lopez, K.J.; Torres-Giner, S.; Enescu, D.; Cabedo, L.; Cerqueira, M.A.; Pastrana, L.M.; Lagaron, J.M. Electrospun active biopapers of food waste derived poly(3-hydroxybutyrate-co-3-hydroxyvalerate) with short-term and long-term antimicrobial performance. Nanomaterials 2020, 10, 506. [Google Scholar] [CrossRef] [PubMed]
- Castro-Mayorga, J.L.; Fabra, M.J.; Lagaron, J.M. Stabilized nanosilver based antimicrobial poly(3-hydroxybutyrate-co-3-hydroxyvalerate) nanocomposites of interest in active food packaging. Innov. Food Sci. Emerg. Technol. 2016, 33, 524–533. [Google Scholar] [CrossRef]
- Aytac, Z.; Ipek, S.; Erol, I.; Durgun, E.; Uyar, T. Fast-dissolving electrospun gelatin nanofibers encapsulating ciprofloxacin/cyclodextrin inclusion complex. Colloids Surf. B Biointerfaces 2019, 178, 129–136. [Google Scholar] [CrossRef] [PubMed]
- Furukawa, T.; Sato, H.; Murakami, R.; Zhang, J.; Duan, Y.X.; Noda, I.; Ochiai, S.; Ozaki, Y. Structure, dispersibility, and crystallinity of poly(hydroxybutyrate)/poly(L-lactic acid) blends studied by FT-IR microspectroscopy and differential scanning calorimetry. Macromolecules 2005, 38, 6445–6454. [Google Scholar] [CrossRef]
- Wu, Q.; Li, Z.; Hong, H.; Yin, K.; Tie, L. Adsorption and intercalation of ciprofloxacin on montmorillonite. Appl. Clay Sci. 2010, 50, 204–211. [Google Scholar] [CrossRef]
- Li, H.; Williams, G.R.; Wu, J.; Wang, H.; Sun, X.; Zhu, L.M. Poly(N-isopropylacrylamide)/poly(L-lactic acid-co-ɛ-caprolactone) fibers loaded with ciprofloxacin as wound dressing materials. Mater. Sci. Eng. C 2017, 79, 245–254. [Google Scholar] [CrossRef]
- Nunes, S.P.; Culfaz-Emecen, P.Z.; Ramon, G.Z.; Visser, T.; Koops, G.H.; Jin, W.; Ulbricht, M. Thinking the future of membranes: Perspectives for advanced and new membrane materials and manufacturing processes. J. Memb. Sci. 2020, 598, 117761. [Google Scholar] [CrossRef]
- Teno, J.; Pardo-Figuerez, M.; Figueroa-Lopez, K.J.; Prieto, C.; Lagaron, J.M. Development of Multilayer Ciprofloxacin Hydrochloride Electrospun Patches for Buccal Drug Delivery. J. Funct. Biomater. 2022, 13, 170. [Google Scholar] [CrossRef]
- Liu, S.; Yu, J.; Li, H.; Wang, K.; Wu, G.; Li, F.; Zhang, M. Controllable Drug Release Behavior of Polylactic Acid (PLA) Surgical Suture Coating with. Polymers 2020, 12, 288. [Google Scholar] [CrossRef]
- Kamal, R.; Razzaq, A.; Shah, K.A.; Khan, Z.U.; Khan, N.U.; Menaa, F.; Iqbal, H.; Cui, J. Evaluation of cephalexin-loaded PHBV nanofibers for MRSA-infected diabetic foot ulcers treatment. J. Drug Deliv. Sci. Technol. 2022, 71, 103349. [Google Scholar] [CrossRef]
- Wei, L.; Wu, S.; Shi, W.; Aldrich, A.L.; Kielian, T.; Carlson, M.A.; Sun, R.; Qin, X.; Duan, B. Large-Scale and Rapid Preparation of Nanofibrous Meshes and Their Application for Drug-Loaded Multilayer Mucoadhesive Patch Fabrication for Mouth Ulcer Treatment. ACS Appl. Mater. Interfaces 2019, 11, 28740–28751. [Google Scholar] [CrossRef] [PubMed]
- Odermatt, E.K.; Funk, L.; Bargon, R.; Martin, D.P.; Rizk, S.; Williams, S.F. MonoMax suture: A new long-term absorbable monofilament suture made from poly-4-hydroxybutyrate. Int. J. Polym. Sci. 2012, 2012, 216137. [Google Scholar] [CrossRef]
- Williams, S.F.; Rizk, S.; Martin, D.P. Poly-4-hydroxybutyrate (P4HB): A new generation of resorbable medical devices for tissue repair and regeneration. Biomed. Tech. 2013, 58, 439–452. [Google Scholar] [CrossRef] [PubMed]
- Albertsmeier, M.; Seiler, C.M.; Fischer, L.; Baumann, P.; Hüsing, J.; Seidlmayer, C.; Franck, A.; Jauch, K.W.; Knaebel, H.P.; Büchler, M.W. Evaluation of the safety and efficacy of MonoMax® suture material for abdominal wall closure after primary midline laparotomy—A controlled prospective multicentre trial: ISSAAC [NCT005725079]. Langenbeck’s Arch. Surg. 2012, 397, 363–371. [Google Scholar] [CrossRef] [PubMed]
- Zhao, H.; Cui, Z.; Sun, X.; Turng, L.S.; Peng, X. Morphology and properties of injection molded solid and microcellular polylactic acid/polyhydroxybutyrate-valerate (PLA/PHBV) blends. Ind. Eng. Chem. Res. 2013, 52, 2569–2581. [Google Scholar] [CrossRef]
- Padmakumar, S.; Joseph, J.; Neppalli, M.H.; Mathew, S.E.; Nair, S.V.; Shankarappa, S.A.; Menon, D. Electrospun Polymeric Core-sheath Yarns as Drug Eluting Surgical Sutures. ACS Appl. Mater. Interfaces 2016, 8, 6925–6934. [Google Scholar] [CrossRef] [PubMed]
- Shishatskaya, E.I.; Volova, T.G.; Gordeev, S.A.; Puzyr, A.P. Degradation of P(3HB) and P(3HB-co-3HV) in biological media. J. Biomater. Sci. Polym. Ed. 2005, 16, 643–657. [Google Scholar] [CrossRef]
- Amobonye, A.; Bhagwat, P.; Singh, S.; Pillai, S. Plastic Biodegradation: Frontline Microbes and Their Enzymes; Elsevier B.V.: Amsterdam, The Netherlands, 2021; Volume 759, ISBN 0000000310. [Google Scholar]
- Dilkes-Hoffman, L.S.; Lant, P.A.; Laycock, B.; Pratt, S. The rate of biodegradation of PHA bioplastics in the marine environment: A meta-study. Mar. Pollut. Bull. 2019, 142, 15–24. [Google Scholar] [CrossRef] [PubMed]






| Sample | Flow-Rate (mL/h) | Voltage V+/V− (kV) | Funnel to Yarn Collector Distance (cm) | Needle Distance to Funnel (cm) | Funnel Speed (rpm) | Yarn Collector (rpm) |
|---|---|---|---|---|---|---|
| PHBV2 | 12 | 10/−10 | 26.5 | 34 | 300 | 5 |
| PHBV2 + CPX | 10 | 13/−13 | 26.5 | 35.5 | 300 | 5 |
| PHBV10 | 5 | 18/−18 | 25.5 | 34 | 300 | 5 |
| PHBV10 + CPX | 5 | 18/−18 | 28.5 | 34 | 300 | 5 |
| PHBV20 | 3 | 15/−15 | 24.5 | 34 | 300 | 5 |
| PHBV20 + CPX | 5 | 15/−15 | 26.5 | 35.5 | 300 | 5 |
| Sample | CPX Loading in the Yarns (%) |
|---|---|
| PHBV2 + CPX | 97.33 ± 2.42 |
| PHBV10 + CPX | 95.50 ± 7.50 |
| PHBV20 + CPX | 96.54 ± 4.43 |
| Korsmeyer-Peppas | |||
|---|---|---|---|
| Sample | K | n | r2 |
| PHBV2 + CPX | 28.88 | 0.12 | 0.99 |
| PHBV10 + CPX | 17.68 | 0.25 | 0.99 |
| PHBV20 + CPX | 52.56 | 0.15 | 0.99 |
| Sample | E (MPa) | σb (MPa) | εb (%) |
|---|---|---|---|
| PHBV2 | 660 ± 131 | 8.4 ± 1.8 | 14.3 ± 1.6 |
| PHBV2 + CPX | 996 ± 224 | 14.5 ± 3.2 | 11.0 ±0.5 |
| PHBV10 | 575± 281 | 10.1± 2.6 | 14.8 ± 5.1 |
| PHBV10 + CPX | 1099 ± 204 | 13.6 ± 2.0 | 14.7 ± 9.1 |
| PHBV20 | 438 ± 126 | 8.5 ± 2.4 | 10.6 ± 3.2 |
| PHBV20 + CPX | 731 ± 334 | 11.1 ± 1.8 | 16.5 ± 6.3 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Teno, J.; Pardo-Figuerez, M.; Evtoski, Z.; Prieto, C.; Cabedo, L.; Lagaron, J.M. Development of Ciprofloxacin-Loaded Electrospun Yarns of Application Interest as Antimicrobial Surgical Suture Materials. Pharmaceutics 2024, 16, 220. https://doi.org/10.3390/pharmaceutics16020220
Teno J, Pardo-Figuerez M, Evtoski Z, Prieto C, Cabedo L, Lagaron JM. Development of Ciprofloxacin-Loaded Electrospun Yarns of Application Interest as Antimicrobial Surgical Suture Materials. Pharmaceutics. 2024; 16(2):220. https://doi.org/10.3390/pharmaceutics16020220
Chicago/Turabian StyleTeno, Jorge, Maria Pardo-Figuerez, Zoran Evtoski, Cristina Prieto, Luis Cabedo, and Jose M. Lagaron. 2024. "Development of Ciprofloxacin-Loaded Electrospun Yarns of Application Interest as Antimicrobial Surgical Suture Materials" Pharmaceutics 16, no. 2: 220. https://doi.org/10.3390/pharmaceutics16020220
APA StyleTeno, J., Pardo-Figuerez, M., Evtoski, Z., Prieto, C., Cabedo, L., & Lagaron, J. M. (2024). Development of Ciprofloxacin-Loaded Electrospun Yarns of Application Interest as Antimicrobial Surgical Suture Materials. Pharmaceutics, 16(2), 220. https://doi.org/10.3390/pharmaceutics16020220