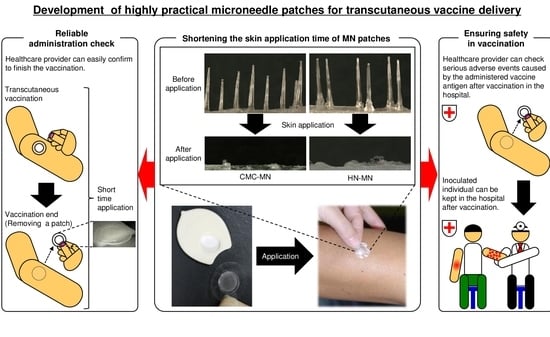
Development of Novel Faster-Dissolving Microneedle Patches for Transcutaneous Vaccine Delivery
Abstract
:1. Introduction
2. Materials and Methods
2.1. Animals
2.2. Fabrication of Faster-Dissolving MN Patches and Measurement of Fracture Force for MN
2.3. Analysis of MN-Dissolution Kinetics after Puncturing the Skin
2.4. In Vivo Fluorescence Imaging for Antigen Deposition Assessment
2.5. Vaccination and Measurement of OVA-Specific IgG Titers
2.6. Fluorescence Imaging for MN Insertion into Excised Human Skin
2.7. Transepidermal Water Loss (TEWL) Measurement and Human Safety Study after Placebo MN Patch Applications in Clinical Research
2.8. Statistical Analysis
3. Results
3.1. Pharmaceutical Characteristics and MN-Dissolution Kinetics of Faster-Dissolving MN Patches
3.2. Localization and Deposition of Antigen Delivery by the MN Patches
3.3. In Vivo Antigen-Specific Immune Response Induction using OVA-Loaded MN Patches
3.4. Antigen Delivery, Dissolution Kinetics of MNs, Skin Puncturability, and Safety of Faster-Dissolving MN Patches in Humans
4. Discussion
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Lee, M.-Y.; Shin, M.-C.; Yang, V.C. Transcutaneous antigen delivery system. BMB Rep. 2013, 46, 17–24. [Google Scholar] [CrossRef] [PubMed]
- Matsuo, K.; Hirobe, S.; Okada, N.; Nakagawa, S. Frontiers of transcutaneous vaccination systems: Novel technologies and devices for vaccine delivery. Vaccine 2013, 31, 2403–2415. [Google Scholar] [CrossRef] [PubMed]
- Matsuo, K.; Yokota, Y.; Zhai, Y.; Quan, Y.-S.; Kamiyama, F.; Mukai, Y.; Okada, N.; Nakagawa, S. A low-invasive and effective transcutaneous immunization system using a novel dissolving microneedle array for soluble and particulate antigens. J. Control. Release 2012, 161, 10–17. [Google Scholar] [CrossRef] [PubMed]
- Matsuo, K.; Hirobe, S.; Yokota, Y.; Ayabe, Y.; Seto, M.; Quan, Y.-S.; Kamiyama, F.; Tougan, T.; Horii, T.; Mukai, Y.; et al. Transcutaneous immunization using dissolving microneedle array protects against tetanus, diphtheria, malaria, and influenza. J. Control. Release 2012, 160, 495–501. [Google Scholar] [CrossRef] [PubMed]
- Sullivan, S.P.; Koutsonanos, D.G.; Martin, M.P.; Lee, J.W.; Zarnitsyn, V.; Choi, S.-O.; Murthy, N.; Compans, R.W.; Skountzou, I.; Prausnitz, M.R. Dissolving polymer microneedle patches for influenza vaccination. Nat. Med. 2010, 16, 915–920. [Google Scholar] [CrossRef] [PubMed]
- DeMuth, P.C.; Li, A.V.; Abbink, P.; Liu, J.; Li, H.; Stanley, K.A.; Smith, K.M.; Lavine, C.L.; Seaman, M.S.; Kramer, J.A.; et al. Vaccine delivery with microneedle skin patches in nonhuman primates. Nat. Biotechnol. 2013, 31, 1082–1085. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bachy, V.; Hervouet, C.; Becker, P.D.; Chorro, L.; Carlin, L.M.; Herath, S.; Papagatsias, T.; Barbaroux, J.-B.; Oh, S.-J.; Benlahrech, A.; et al. Langerin negative dendritic cells promote potent CD8+ T-cell priming by skin delivery of live adenovirus vaccine microneedle arrays. Proc. Natl. Acad. Sci. USA 2013, 110, 3041–3046. [Google Scholar] [CrossRef] [PubMed]
- Hirobe, S.; Okada, N.; Nakagawa, S. Transcutaneous vaccines—Current and emerging strategies. Expert Opin. Drug Deliv. 2013, 10, 485–498. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.-C.; Park, J.-H.; Prausnitz, M.R. Microneedles for drug and vaccine delivery. Adv. Drug Deliv. Rev. 2012, 64, 1547–1568. [Google Scholar] [CrossRef] [PubMed]
- Maaden, K.; Jiskoot, W.; Bouwstra, J. Microneedle technologies for (trans)dermal drug and vaccine delivery. J. Control. Release 2012, 161, 645–655. [Google Scholar] [CrossRef] [PubMed]
- Rejinold, N.S.; Shin, J.-H.; Seok, H.Y.; Kim, Y.-C. Biomedical applications of microneedles in therapeutics: Recent advancements and implications in drug delivery. Expert Opin. Drug Deliv. 2016, 13, 109–131. [Google Scholar] [CrossRef] [PubMed]
- Tuan-Mahmood, M.-T.; McCrudden, M.T.C.; Torrisi, B.M.; McAlister, E.; Garland, M.J.; Singh, T.R.R.; Donnelly, R.F. Microneedles for intradermal and transdermal delivery. Eur. J. Pharm. Sci. 2013, 50, 623–637. [Google Scholar] [CrossRef] [PubMed]
- Donnelly, R.F.; McCrudden, M.T.C.; Zaid Alkilani, A.; Larraneta, E.; McAlister, E.; Courtenay, A.J.; Kearney, M.-C.; Singh, T.R.R.; McCarthy, H.O.; Kett, V.L.; et al. Hydrogel-forming microneedles prepared from “super swelling” polymers combined with lyophilised wafers for transdermal delivery. PLoS ONE 2014, 9, e111547. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hirobe, S.; Azukizawa, H.; Matsuo, K.; Zhai, Y.; Quan, Y.-S.; Kamiyama, F.; Suzuki, H.; Katayama, I.; Okada, N.; Nakagawa, S. Development and clinical study of a self-dissolving microneedle patch for transcutaneous immunization device. Pharm. Res. 2013, 30, 2664–2674. [Google Scholar] [CrossRef] [PubMed]
- Hirobe, S.; Azukizawa, H.; Hanafusa, T.; Matsuo, K.; Quan, Y.-S.; Kamiyama, F.; Katayama, I.; Okada, N.; Nakagawa, S. Clinical study and stability assessment of a novel transcutaneous influenza vaccination using a dissolving microneedle patch. Biomaterial 2015, 57, 50–58. [Google Scholar] [CrossRef] [PubMed]
- Nokleby, H. Vaccination and anaphylaxis. Curr. Allergy Asthma Rep. 2006, 6, 9–13. [Google Scholar] [CrossRef] [PubMed]
- Loizidou, E.Z.; Williams, N.A.; Barrow, D.A.; Eaton, M.J.; McCrory, J.; Evans, S.L.; Allender, C.J. Structural characterisation and transdermal delivery studies on sugar microneedles: Experimental and finite element modelling analyses. Eur. J. Pharm. Biopharm. 2015, 89, 224–231. [Google Scholar] [CrossRef] [PubMed]
- Raphael, A.P.; Crichton, M.L.; Falconer, R.J.; Meliga, S.; Chen, X.; Fernando, G.J.P.; Huang, H.; Kendall, M.A.F. Formulations for microprojection/microneedle vaccine delivery: Structure, strength and release profiles. J. Control. Release 2016, 225, 40–52. [Google Scholar] [CrossRef] [PubMed]
- Raphael, A.P.; Prow, T.W.; Crichton, M.L.; Chen, X.; Fernando, G.J.P.; Kendall, M.A.F. Targeted, needle-free vaccinations in skin using multilayered, densely-packed dissolving microprojection arrays. Small 2010, 6, 1785–1793. [Google Scholar] [CrossRef] [PubMed]
- Lahiji, S.F.; Dangol, M.; Jung, H. A patches dissolving microneedle delivery system enabling rapid and efficient transdermal drug delivery. Sci. Rep. 2015, 5, 7914. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.W.; Park, J.-H.; Prausnitz, M.R. Dissolving microneedles for transdermal drug delivery. Biomaterials 2008, 29, 2113–2124. [Google Scholar] [CrossRef] [PubMed]
- Yan, L.; Raphael, A.P.; Zhu, X.; Wang, B.; Chen, W.; Tang, T.; Deng, Y.; Sant, H.J.; Zhu, G.; Choy, K.W.; et al. Nanocomposite-strengthened dissolving microneedles for improved transdermal delivery to human skin. Adv. Healthcare Mater. 2014, 3, 555–564. [Google Scholar] [CrossRef] [PubMed]
- Hiraishi, Y.; Nakagawa, T.; Quan, Y.-S.; Kamiyama, F.; Hirobe, S.; Okada, N.; Nakagawa, S. Performance and characteristics evaluation of a sodium hyaluronate-based microneedle patch for a transcutaneous drug delivery system. Int. J. Pharm. 2013, 441, 570–579. [Google Scholar] [CrossRef] [PubMed]
- Ono, A.; Azukizawa, H.; Ito, S.; Nakamura, Y.; Asada, H.; Quan, Y.-C.; Kamiyama, F.; Katayama, I.; Hirobe, S.; Okada, N. Development of novel double-decker microneedle patches for transcutaneous vaccine delivery. Int. J. Pharm. (under revision).
- Sivamani, R.K.; Stoeber, B.; Liepmann, D.; Maibach, H.I. Microneedle penetration and injection past the stratum corneum in humans. J. Dermatolog. Treat. 2009, 20, 156–159. [Google Scholar] [CrossRef] [PubMed]





| Score | Reactions |
|---|---|
| − | Negative reaction |
| +? | Doubtful reaction; faint erythema only |
| + | Weak (non-vesicular) positive reaction; erythema, infiltration and possibly papules |
| ++ | Strong (vesicular) positive reaction; erythema, infiltration, papules, vesicles |
| +++ | Extreme positive reaction; bullous reaction |
| IR | Irritant reaction |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ono, A.; Ito, S.; Sakagami, S.; Asada, H.; Saito, M.; Quan, Y.-S.; Kamiyama, F.; Hirobe, S.; Okada, N. Development of Novel Faster-Dissolving Microneedle Patches for Transcutaneous Vaccine Delivery. Pharmaceutics 2017, 9, 27. https://doi.org/10.3390/pharmaceutics9030027
Ono A, Ito S, Sakagami S, Asada H, Saito M, Quan Y-S, Kamiyama F, Hirobe S, Okada N. Development of Novel Faster-Dissolving Microneedle Patches for Transcutaneous Vaccine Delivery. Pharmaceutics. 2017; 9(3):27. https://doi.org/10.3390/pharmaceutics9030027
Chicago/Turabian StyleOno, Akihiko, Sayami Ito, Shun Sakagami, Hideo Asada, Mio Saito, Ying-Shu Quan, Fumio Kamiyama, Sachiko Hirobe, and Naoki Okada. 2017. "Development of Novel Faster-Dissolving Microneedle Patches for Transcutaneous Vaccine Delivery" Pharmaceutics 9, no. 3: 27. https://doi.org/10.3390/pharmaceutics9030027
APA StyleOno, A., Ito, S., Sakagami, S., Asada, H., Saito, M., Quan, Y. -S., Kamiyama, F., Hirobe, S., & Okada, N. (2017). Development of Novel Faster-Dissolving Microneedle Patches for Transcutaneous Vaccine Delivery. Pharmaceutics, 9(3), 27. https://doi.org/10.3390/pharmaceutics9030027