Rosemary Essential Oil-Loaded Lipid Nanoparticles: In Vivo Topical Activity from Gel Vehicles
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Lipid Nanoparticles Preparation
2.3. Nanoparticles Characterization
2.3.1. Transmission Electron Microscopy
2.3.2. Size and Zeta Potential Measurements
2.3.3. Stability Tests
2.4. Gel Preparation
2.5. In Vivo Evaluation of Gel Formulations
3. Results and Discussion
3.1. Lipid Nanoparticles Characterization
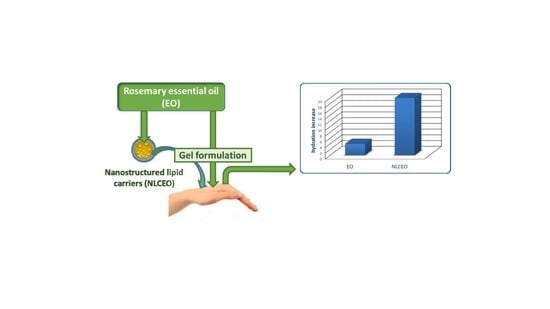
3.2. In Vivo Evaluation of Gel Formulations
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Saad, N.Y.; Muller, C.D.; Lobstein, A. Major bioactivities and mechanism of action of essential oils and their components. Flavour. Fragr. J. 2013, 28, 269–279. [Google Scholar] [CrossRef]
- Adorjan, B.; Buchbauer, G. Biological properties of essential oils: An updated review. Flavour. Fragr. J. 2010, 25, 407–426. [Google Scholar] [CrossRef]
- Bakkali, F.; Averbeck, S.; Averbeck, D.; Idaomar, M. Biological effects of essential oils—A review. Food Chem. Toxicol. 2008, 46, 446–475. [Google Scholar] [CrossRef] [PubMed]
- Franz, C.M. Essential oil research: Past, present and future. Flavour. Fragr. J. 2010, 25, 112–113. [Google Scholar] [CrossRef]
- Anthony, K.P.; Deolu-Sobogun, S.A.; Saleh, M.A. Comprehensive assessment of antioxidant activity of essential oils. J. Food Sci. 2012, 77, C839–C843. [Google Scholar] [CrossRef] [PubMed]
- Edris, A.E. Pharmaceutical and therapeutic potentials of essential oils and their individual volatile constituents: A review. Phytother. Res. 2007, 21, 308–323. [Google Scholar] [CrossRef] [PubMed]
- Raut, J.S.; Karuppayil, S.M. A status review on the medicinal properties of essential oils. Ind. Crops Prod. 2014, 62, 250–264. [Google Scholar] [CrossRef]
- Schweiggert, U.; Carle, R.; Schieber, A. Conventional and alternative processes for spice production—A review. Trends Food Sci. Technol. 2007, 18, 260–268. [Google Scholar] [CrossRef]
- Christensson, J.B.; Forsstrӧm, P.; Wennberg, A.M.; Karlberg, A.T.; Matura, M. Air oxidation increases skin irritation from fragrance terpenes. Contact Dermat. 2009, 60, 32–40. [Google Scholar] [CrossRef] [PubMed]
- Divkovic, M.; Pease, C.K.; Gerberick, G.F.; Basketter, D.A. Hapten-protein binding: From theory to practical application in the in vitro prediction of skin sensitization. Contact Dermat. 2005, 53, 189–200. [Google Scholar] [CrossRef] [PubMed]
- Bilia, A.R.; Guccione, C.; Isacchi, B.; Righeschi, C.; Firenzuoli, F.; Bergonzi, M.C. Essential oils loaded in nanosystems: A developing strategy for a successful therapeutic approach. Evid. Based Complement. Altern. Med. 2014, 2014, 651593. [Google Scholar] [CrossRef] [PubMed]
- Müller, R.H.; Mäder, K.; Gohla, S. Solid lipid nanoparticles (SLN) for controlled drug delivery—A review of the state of the art. Eur. J. Pharm. Biopharm. 2000, 50, 161–177. [Google Scholar] [CrossRef]
- Nair, R.; Arun Kumar, K.S.; Vishnu Priya, K.; Sevukarajan, M. Recent advances in solid lipid nanoparticle based drug delivery systems. J. Biomed. Sci. Res. 2011, 3, 368–384. [Google Scholar]
- Singhal, G.B.; Patel, R.P.; Prajapati, B.G.; Patel, N.A. Solid lipid nanoparticles and nano lipid carriers: As novel solid lipid based drug carrier. IRJP 2011, 2, 40–52. [Google Scholar]
- Souto, E.B.; Müller, R.H. Lipid nanoparticles: Effect on bioavailability and pharmacokinetic changes. Handb. Exp. Pharmacol. 2010, 197, 115–141. [Google Scholar] [CrossRef]
- Schäfer-Korting, M.; Mehnert, W.; Korting, H.C. Lipid nanoparticles for improved topical application of drugs for skin diseases. Adv. Drug Deliv. Rev. 2007, 59, 427–443. [Google Scholar] [CrossRef] [PubMed]
- Montenegro, L. Lipid-based nanoparticles as carriers for dermal delivery of antioxidants. Curr. Drug Metab. 2017, 18, 469–480. [Google Scholar] [CrossRef]
- Shi, F.; Zhao, J.H.; Liu, Y.; Wang, Z.; Zhang, Y.T.; Feng, N.P. Preparation and characterization of solid lipid nanoparticles loaded with frankincense and myrrh oil. Int. J. Nanomed. 2012, 7, 2033–2043. [Google Scholar] [CrossRef]
- Moghimipour, E.; Ramezani, Z.; Handali, S. Solid lipid nanoparticles as a delivery system for Zataria multiflora essential oil: Formulation and characterization. Curr. Drug Deliv. 2013, 10, 151–157. [Google Scholar] [CrossRef] [PubMed]
- Alhaj, N.A.; Shamsudin, M.N.; Alipiah, N.M.; Zamri, H.F.; Bustamam, A.; Ibrahim, S.; Abdullah, R. Characterization of Nigella sativa L. essential oil-loaded solid lipid nanoparticles. Am. J. Pharmacol. Toxicol. 2010, 5, 52–57. [Google Scholar] [CrossRef]
- Lai, F.; Sinico, C.; de Logu, A.; Zaru, M.; Müller, R.H.; Fadda, A.M. SLN as a topical delivery system for Artemisia arborescens essential oil: In vitro antiviral activity and skin permeation study. Int. J. Nanomed. 2007, 2, 419–425. [Google Scholar]
- Gad, A.S.; Sayd, A.F. Antioxidant Properties of Rosemary and Its Potential Uses as Natural Antioxidant in Dairy Products—A Review. Food Nutr. Sci. 2015, 6, 179–193. [Google Scholar] [CrossRef]
- Ngo, S.N.; Williams, D.B.; Head, R.J. Rosemary and cancer prevention: Preclinical perspectives. Crit. Rev. Food Sci. Nutr. 2011, 51, 946–954. [Google Scholar] [CrossRef] [PubMed]
- Lo, A.H.; Liang, Y.C.; Lin-Shiau, S.Y.; Ho, C.T.; Lin, J.K. Carnosol, an antioxidant in rosemary, suppresses inducible nitric oxide synthase through down-regulating nuclear factor-kappaB in mouse macrophages. Carcinogenesis 2002, 23, 983–991. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Y.; Wu, N.; Fu, Y.J.; Wang, W.; Luo, M.; Zhao, C.J.; Zu, Y.G.; Liu, X.L. Chemical composition and antimicrobial activity of the essential oil of Rosemary. Environ. Toxicol. Pharmacol. 2011, 32, 63–68. [Google Scholar] [CrossRef] [PubMed]
- Calabrese, V.; Scapagnini, G.; Catalano, C.; Dinotta, F.; Geraci, D.; Morganti, P. Biochemical studies of a natural antioxidant isolated from rosemary and its application in cosmetic dermatology. Int. J. Tissue React. 2000, 22, 5–13. [Google Scholar] [PubMed]
- Montenegro, L.; Campisi, A.; Sarpietro, M.G.; Carbone, C.; Acquaviva, R.; Raciti, G.; Puglisi, G. In vitro evaluation of idebenone-loaded solid lipid nanoparticles for drug delivery to the brain. Drug Dev. Ind. Pharm. 2011, 37, 737–746. [Google Scholar] [CrossRef] [PubMed]
- Montenegro, L.; Trapani, A.; Latrofa, A.; Puglisi, G. In vitro evaluation on a model of blood brain barrier of idebenone-loaded solid lipid nanoparticles. J. Nanosci. Nanotechnol. 2012, 12, 330–337. [Google Scholar] [CrossRef] [PubMed]
- Pardeike, J.; Hommoss, A.; Müller, R.H. Lipid nanoparticles (SLN, NLC) in cosmetic and pharmaceutical dermal products. Int. J. Pharm. 2009, 366, 170–184. [Google Scholar] [CrossRef] [PubMed]
- Montenegro, L.; Sinico, C.; Castangia, I.; Carbone, C.; Puglisi, G. Idebenone-loaded solid lipid nanoparticles for drug delivery to the skin: In vitro evaluation. Int. J. Pharm. 2012, 434, 169–174. [Google Scholar] [CrossRef] [PubMed]
- Bahari, L.A.S.; Hamishehkar, H. The impact of variables on particle size of solid lipid nanoparticles and nanostructured lipid carriers; a comparative literature review. Adv. Pharm. Bull. 2016, 6, 143–151. [Google Scholar] [CrossRef] [PubMed]
- Fernandes, C.P.; Mascarenhas, M.P.; Zibetti, F.M.; Lima, B.G.; Oliveira, R.P.R.F.; Rocha, L.; Falcão, D.Q. HLB value, an important parameter for the development of essential oil phytopharmaceuticals. Braz. J. Pharmacogn. 2013, 23, 108–114. [Google Scholar] [CrossRef]
- Arai, H.; Shinoda, K. The effect of mixing of oils and of nonionic surfactants on the phase inversion temperatures of emulsions. J. Colloid Interface Sci. 1967, 25, 396–400. [Google Scholar] [CrossRef]
- Souto, E.B.; Almeida, A.J.; Müller, R.H. Lipid nanoparticles (SLN®, NLC®) for cutaneous drug delivery: Structure, protection and skin effects. J. Biomed. Nanotechnol. 2007, 3, 317–331. [Google Scholar] [CrossRef]
- Katz, L.M.; Dewan, K.; Bronaugh, R.L. Nanotechnology in cosmetics. Food Chem. Toxicol. 2015, 85, 127–137. [Google Scholar] [CrossRef] [PubMed]
- Montenegro, L.; Lai, F.; Offerta, A.; Sarpietro, M.G.; Micicchè, L.; Maccioni, A.M.; Valenti, D.; Fadda, A.M. From nanoemulsions to nanostructured lipid carriers: A relevant development in dermal delivery of drugs and cosmetics. J. Drug Del. Sci. Technol. 2016, 32, 100–112. [Google Scholar] [CrossRef]
- Zhai, H.; Maibach, H.I. Effects of skin occlusion on percutaneous absorption: An overview. Skin Pharmacol. Appl. Skin Physiol. 2001, 14, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Wissing, S.A.; Müller, R.H. The influence of solid lipid nanoparticles on skin hydration and viscoelasticity-in vivo study. Eur. J. Pharm. Biopharm. 2003, 56, 67–72. [Google Scholar] [CrossRef]
- El-Gammal, R.E. Antioxidative activity of nanoparticles of rosemary. Int. J. ChemTech Res. 2016, 9, 844–854. [Google Scholar]
- Lacatusu, I.; Badea, N.; Murariu, A.; Nichita, C.; Bojin, D.; Meghea, A. Antioxidant capacity of lipid nanoparticles loaded with rosemary extract. Mol. Cryst. Liq. Cryst. 2010, 523, 260–272. [Google Scholar] [CrossRef]






| Code | Ingredients | |||
|---|---|---|---|---|
| Oleth-20 | GO | CP | EO | |
| A | 9.0 | 5.0 | 7.0 | -- |
| BEO1 | 9.0 | 5.0 | 6.0 | 1.0 |
| BEO2 | 9.0 | 5.0 | 5.0 | 2.0 |
| BEO3 | 9.0 | 5.0 | 4.0 | 3.0 |
| BEO4 | 9.0 | 5.0 | 3.0 | 4.0 |
| CEO1 | 9.0 | 5.0 | 7.0 | 1.0 |
| CEO2 | 9.0 | 5.0 | 7.0 | 2.0 |
| CEO3 | 9.0 | 5.0 | 7.0 | 3.0 |
| Code | Ingredient | ||||||
|---|---|---|---|---|---|---|---|
| Carbopol | TEA | EO | Solubilisant | SLN A | NLC CEO3 | Water | |
| A | 0.8 | 0.8 | -- | -- | -- | -- | 98.4 |
| B | 0.8 | 0.8 | 1.5 | 4.5 | -- | -- | 92.4 |
| B1 | 0.8 | 0.8 | 3.0 | 9.0 | -- | -- | 86.4 |
| C | 0.8 | 0.8 | -- | -- | 98.4 | -- | -- |
| D | 0.8 | 0.8 | -- | -- | -- | 49.2 | 49.2 |
| D1 | 0.8 | 0.8 | -- | -- | -- | 98.4 | -- |
| Code | Z-Ave (nm) | PDI | ζ potential ± S.D. (mV) | PIT (°C) |
|---|---|---|---|---|
| A | 36.5 ± 1.45 | 0.208 ± 0.010 | −1.55 ± 0.27 | 72 |
| BEO1 | 26.9 ± 1.23 | 0.341 ± 0.021 | −1.76 ± 0.38 | 58 |
| BEO2 | 27.6 ± 1.01 | 0.368 ± 0.018 | −2.05 ± 0.37 | 50 |
| BEO3 | 141.9 ± 5.43 | 0.495 ± 0.022 | −1.99 ± 0.39 | 45 |
| BEO4 | 171.7 ± 4.66 | 0.336 ± 0.019 | −1.78 ± 0.45 | 40 |
| CEO1 | 34.8 ± 1.10 | 0.367 ± 0.017 | −2.19 ± 0.44 | 70 |
| CEO2 | 31.6 ± 1.92 | 0.325 ± 0.020 | −1.89 ± 0.58 | 65 |
| CEO3 | 28.1 ± 1.02 | 0.171 ± 0.011 | −1.93 ± 0.66 | 57 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Montenegro, L.; Pasquinucci, L.; Zappalà, A.; Chiechio, S.; Turnaturi, R.; Parenti, C. Rosemary Essential Oil-Loaded Lipid Nanoparticles: In Vivo Topical Activity from Gel Vehicles. Pharmaceutics 2017, 9, 48. https://doi.org/10.3390/pharmaceutics9040048
Montenegro L, Pasquinucci L, Zappalà A, Chiechio S, Turnaturi R, Parenti C. Rosemary Essential Oil-Loaded Lipid Nanoparticles: In Vivo Topical Activity from Gel Vehicles. Pharmaceutics. 2017; 9(4):48. https://doi.org/10.3390/pharmaceutics9040048
Chicago/Turabian StyleMontenegro, Lucia, Lorella Pasquinucci, Agata Zappalà, Santina Chiechio, Rita Turnaturi, and Carmela Parenti. 2017. "Rosemary Essential Oil-Loaded Lipid Nanoparticles: In Vivo Topical Activity from Gel Vehicles" Pharmaceutics 9, no. 4: 48. https://doi.org/10.3390/pharmaceutics9040048
APA StyleMontenegro, L., Pasquinucci, L., Zappalà, A., Chiechio, S., Turnaturi, R., & Parenti, C. (2017). Rosemary Essential Oil-Loaded Lipid Nanoparticles: In Vivo Topical Activity from Gel Vehicles. Pharmaceutics, 9(4), 48. https://doi.org/10.3390/pharmaceutics9040048