Preparation and Characterization of Carbamazepine Cocrystal in Polymer Solution
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Sample Preparation
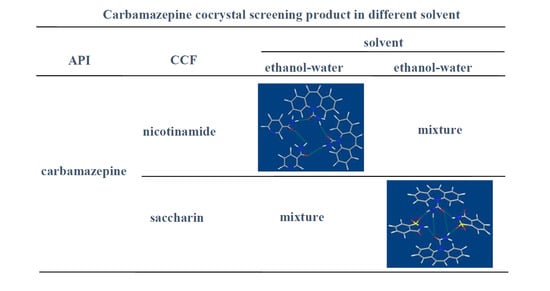
2.2.1. Preparation of CBZ-NIC, CBZ-SAC Cocrystal in Ethanol-Water Solvent Mixture
2.2.2. Preparation of CBZ-NIC, CBZ-SAC Cocrystal in PVP Solution
2.3. Methods
2.3.1. Solubility Test
2.3.2. Fourier Transform Infrared Spectroscopy (FTIR)
2.3.3. Differential Scanning Calorimetry (DSC)
2.3.4. Powder X-ray Diffraction (PXRD)
3. Results and Discussion
3.1. Solubility Test
3.2. FTIR Analysis
3.3. DSC Analysis
3.4. PXRD Analysis
3.5. Molecular Structures of CBZ Cocrystals
3.6. Discussion of the Effects of PVP on CBZ Cocrystal Preparation
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Aakeröy, C.B.; Salmon, D.J. Building co-crystals with molecular sense and supramolecular sensibility. Cryst. Eng. Commun. 2005, 7, 439–448. [Google Scholar] [CrossRef]
- Qiao, N.; Li, M.; Schlindwein, W.; Malek, N.; Davies, A.; Trappitt, G. Pharmaceutical cocrystals: An overview. Int. J. Pharm. 2011, 419, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Vishweshwar, P.; McMahon, J.A.; Bis, J.A.; Zaworotko, M.J. Pharmaceutical co-crystals. J. Pharm. Sci. 2006, 95, 499–516. [Google Scholar] [CrossRef] [PubMed]
- Shan, N.; Zaworotko, M.J. The role of cocrystals in pharmaceutical science. Drug Discov. Today 2008, 13, 440–446. [Google Scholar] [CrossRef] [PubMed]
- Blagden, N.; De Matas, M.; Gavan, P.T.; York, P. Crystal engineering of active pharmaceutical ingredients to improve solubility and dissolution rates. Adv. Drug Deliv. Rev. 2007, 59, 617–630. [Google Scholar] [CrossRef] [PubMed]
- Schultheiss, N.; Newman, A. Pharmaceutical Cocrystals and Their Physicochemical Properties. Cryst. Growth Des. 2009, 9, 2950–2967. [Google Scholar] [CrossRef] [PubMed]
- McNamara, D.P.; Childs, S.L.; Giordano, J.; Iarriccio, A.; Cassidy, J.; Shet, M.S.; Mannion, R.; O’donnell, E.; Park, A. Use of a glutaric acid cocrystal to improve oral bioavailability of a low solubility API. Pharm. Res. 2006, 23, 1888–1897. [Google Scholar] [CrossRef] [PubMed]
- Good, D.J.; Rodríguez-Hornedo, N.R. Solubility Advantage of Pharmaceutical Cocrystals. Cryst. Growth Des. 2009, 9, 2252–2264. [Google Scholar] [CrossRef]
- Qiao, N.; Wang, K.; Schlindwein, W.; Davies, A.; Li, M. In situ monitoring of carbamazepine–nicotinamide cocrystal intrinsic dissolution behaviour. Eur. J. Pharm. Biopharm. 2013, 83, 415–426. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Qiao, N.; Wang, K. Influence of Sodium Lauryl Sulfate and Tween 80 on Carbamazepine–Nicotinamide Cocrystal Solubility and Dissolution Behaviour. Pharmaceutics 2013, 5, 508–524. [Google Scholar] [CrossRef] [PubMed]
- Rahman, Z.; Samy, R.; Sayeed, V.A.; Khan, M.A. Physicochemical and mechanical properties of carbamazepine cocrystals with saccharin. Pharm. Dev. Technol. 2012, 17, 457–465. [Google Scholar] [CrossRef] [PubMed]
- Porter, W.W., III; Elie, S.C.; Matzger, A.J. Polymorphism in Carbamazepine Cocrystals. Cryst. Growth Des. 2008, 8, 14–16. [Google Scholar] [CrossRef] [PubMed]
- Chieng, N.; Hubert, M.; Saville, D.; Rades, T.; Aaltonen, J. Formation Kinetics and Stability of Carbamazepine–Nicotinamide Cocrystals Prepared by Mechanical Activation. Cryst. Growth Des. 2009, 9, 2377–2386. [Google Scholar] [CrossRef]
- Seefeldt, K.; Miller, J.; Alvarez-Nunez, F.; Rodriguez-Hornedo, N. Crystallization pathways and kinetics of carbamazepine-nicotinamide cocrystals from the amorphous state by in situ thermomicroscopy, spectroscopy and calorimetry studies. J. Pharm. Sci. 2007, 96, 1147–1158. [Google Scholar] [CrossRef] [PubMed]
- Friščić, T.; Jones, W. Recent Advances in Understanding the Mechanism of Cocrystal Formation via Grinding. Cryst. Growth Des. 2009, 9, 1621–1637. [Google Scholar] [CrossRef]
- Abd Hashib, S.; Anuar, N.; Jamburi, N.; Ahmad, N.F.; Abd Rahim, S. Screening for Ibuprofen-Sachharin Co-Crystal Formation in Wet Milling. Appl. Mech. Mater. 2015, 754–755, 1002–1006. [Google Scholar] [CrossRef]
- Trask, A.V.; Motherwell, W.D.S.; Jones, W. Solvent-drop grinding: Green polymorph control of cocrystallisation. Chem. Commun. 2004, 890–891. [Google Scholar] [CrossRef] [PubMed]
- Wang, I.C.; Lee, M.J.; Sim, S.J.; Kim, W.S.; Chun, N.H.; Choi, G.J. Anti-solvent co-crystallization of carbamazepine and saccharin. Int. J. Pharm. 2013, 450, 311–322. [Google Scholar] [CrossRef] [PubMed]
- Nishimaru, M.; Kudo, S.; Takiyama, H. Cocrystal production method reducing deposition risk of undesired single component crystals in anti-solvent cocrystallization. J. Ind. Eng. Chem. 2016, 36, 40–43. [Google Scholar] [CrossRef]
- Childs, S.L.; Rodríguez-Hornedo, N.; Reddy, L.S.; Jayasankar, A.; Maheshwari, C.; McCausland, L.; Shipplett, R.; Stahly, B.C. Screening strategies based on solubility and solution composition generate pharmaceutically acceptable cocrystals of carbamazepine. Cryst. Eng. Comm. 2008, 10, 856–864. [Google Scholar] [CrossRef]
- Rodríguez-Hornedo, N.; Nehm, S.J.; Seefeldt, K.F.; Pagan-Torres, Y.; Falkiewicz, C.J. Reaction crystallization of pharmaceutical molecular complexes. Mol. Pharm. 2006, 3, 362–367. [Google Scholar] [CrossRef] [PubMed]
- Shen, Z.; Xie, C.; Du, W.; Yin, Q. Preparation of Ibuprofen-Nicotinamide Cocrystal and Its Solubility Measurement. Chem. Ind. Eng. 2014, 31, 38–42. [Google Scholar] [CrossRef]
- Rager, T.; Hilfiker, R. Cocrystal Formation from Solvent Mixtures. Cryst. Growth Des. 2010, 10, 3237–3241. [Google Scholar] [CrossRef]
- Gu, C.H.; Young, V.; Grant, D.J.W. Polymorph screening: Influence of solvents on the rate of solvent-mediated polymorphic transformation. J. Pharm. Sci. 2001, 90, 1878–1890. [Google Scholar] [CrossRef] [PubMed]
- Di Profio, G.; Grosso, V.; Caridi, A.; Caliandro, R.; Guagliardi, A.; Chita, G.; Curcio, E.; Drioli, E. Direct production of carbamazepine–saccharin cocrystals from water/ethanol solvent mixtures by membrane-based crystallization technology. Cryst. Eng. Comm. 2011, 13, 5670–5673. [Google Scholar] [CrossRef]
- Guo, M.; Wang, K.; Hamill, N.; Lorimer, K.; Li, M. Investigating the Influence of Polymers on Supersaturated Flufenamic Acid Cocrystal Solutions. Mol. Pharm. 2016, 13. [Google Scholar] [CrossRef] [PubMed]
- Qiu, S.; Lai, J.; Guo, M.; Wang, K.; Lai, X.; Desai, U.; Juma, N.; Li, M. Role of polymers in solution and tablet based carbamazepine cocrystal formulations. Cryst. Eng. Comm 2016, 18, 2664–2678. [Google Scholar] [CrossRef]
- Huang, N.; Rodríguez-Hornedo, N. Engineering cocrystal solubility, stability, and pH(max) by micellar solubilization. J. Pharm. Sci. 2011, 100, 5219–5234. [Google Scholar] [CrossRef] [PubMed]
- Gift, A.D.; Luner, P.E.; Luedeman, L.; Taylor, L.S. Influence of polymeric excipients on crystal hydrate formation kinetics in aqueous slurries. J. Pharm. Sci. 2008, 97, 5198–5211. [Google Scholar] [CrossRef] [PubMed]
- Higuchi, T.; Kuramoto, R. Study of possible complex formation between macromolecules and certain pharmaceuticals. II. Polyvinylpyrrolidone with p-aminobenzoic acid, aminopyrine, benzoic acid, salicylic acid, p-hydroxybenzoic acid, m-hydroxybenzoic acid, citric acid, and phenobarbit. J. Am. Pharm. Assoc. 1954, 43, 398–401. [Google Scholar] [CrossRef]
- Karavas, E.; Ktistis, G.; Xenakis, A.; Georgarakis, E. Effect of hydrogen bonding interactions on the release mechanism of felodipine from nanodispersions with polyvinylpyrrolidone. Eur. J. Pharm. Biopharm. 2006, 63, 103–114. [Google Scholar] [CrossRef] [PubMed]
- Van den Mooter, G.; Wuyts, M.; Blaton, N.; Busson, R.; Grobet, P.; Augustijns, P.; Kinget, R. Physical stabilisation of amorphous ketoconazole in solid dispersions with polyvinylpyrrolidone K25. Eur. J. Pharm. Biopharm. 2001, 12, 261–269. [Google Scholar] [CrossRef]
- D’Souza, A.J.; Schowen, R.L.; Topp, E.M. Polyvinylpyrrolidone-drug conjugate: Synthesis and release mechanism. J. Control. Release 2004, 94, 91–100. [Google Scholar] [CrossRef] [PubMed]
- Gupta, P.; Kakumanu, V.K.; Bansal, A.K. Stability and solubility of celecoxib-PVP amorphous dispersions: A molecular perspective. Pharm. Res. 2004, 21, 1762–1769. [Google Scholar] [CrossRef] [PubMed]
- Daimay, L.V. Handbook of Infrared and Raman Characteristic Frequencies of Organic Molecules; Academic Press: Boston, MA, USA, 1991. [Google Scholar]
- Li, R.; Mao, H.; Gong, J. Preparation and Characterisation Study of Carbamazpine-Saccharin Cocrystal. 2011. Available online: http://www.paper.edu.cn (accessed on 23 November 2011).
- Liu, X.; Lu, M.; Guo, Z.; Huang, L.; Feng, X.; Wu, C. Improving the chemical stability of amorphous solid dispersion with cocrystal technique by hot melt extrusion. Pharm. Res. 2012, 29, 806–817. [Google Scholar] [CrossRef] [PubMed]
- Berry, D.J.; Seaton, C.C.; Clegg, W.; Harrington, R.W.; Coles, S.J.; Horton, P.N.; Hursthouse, M.B.; Storey, R.; Jones, W.; Friscic, T.; et al. Applying hot-stage microscopy to co-crystal screening: A study of nicotinamide with seven active pharmaceutical ingredients. Cryst. Growth Des. 2008, 8, 1697–1712. [Google Scholar] [CrossRef]
- Fleischman, S.G.; Kuduva, S.S.; McMahon, J.A.; Moulton, B.; Bailey Walsh, R.D.; Rodríguez-Hornedo, N.; Zaworotko, M.J. Crystal Engineering of the Composition of Pharmaceutical Phases: Multiple-Component Crystalline Solids Involving Carbamazepine. Cryst. Growth Des. 2003, 3, 909–919. [Google Scholar] [CrossRef]
- Zerlia, T.; Marini, A.; Berbenni, V.; Massarotti, V.; Giordano, F.; La Manna, A.; Bettinetti, G.P.; Margheritis, C. Solid State Interaction study on the system polyvinylpyrrolidone-XL/trimethoprim. Solid State Ion. 1989, 32, 613–624. [Google Scholar] [CrossRef]
- Lin, S.P.; Hou, Y.C.; Liao, T.Y.; Tsai, S.Y. Enhancing the bioavailability of magnolol in rabbits using melting solid dispersion with polyvinylpyrrolidone. Drug Dev. Ind. Pharm. 2014, 40, 330–337. [Google Scholar] [CrossRef] [PubMed]
- Guo, S.R.; Guo, L. Effects of PVP K30 on Aqueous Solubility and Dissolution Properti of Daidzein. J. Chin. Pharm. Sci. 2004, 13, 42–48. [Google Scholar] [CrossRef]
- Shete, A.; Murthy, S.; Thorat, B.; Yadav, A.; Sajane, S.; Sakhare, S.; Doijad, R. Studies on effect of hydrophilic polymers on physicochemical properties of itraconazole cocrystals. Future J. Pharm. Sci. 2017. [Google Scholar] [CrossRef]









| API/CCF | Water | Ethanol | Ethanol-Water | Ethanol-Water + PVP |
|---|---|---|---|---|
| CBZ | 0.00001 | 0.00500 | 0.00011 | 0.00014 |
| NIC | 0.09242 | 0.17656 | 0.01961 | 0.02963 |
| SAC | 0.00032 | 0.00931 | 0.00127 | 0.00141 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, H.; Zhu, Y.; Qiao, N.; Chen, Y.; Gao, L. Preparation and Characterization of Carbamazepine Cocrystal in Polymer Solution. Pharmaceutics 2017, 9, 54. https://doi.org/10.3390/pharmaceutics9040054
Zhang H, Zhu Y, Qiao N, Chen Y, Gao L. Preparation and Characterization of Carbamazepine Cocrystal in Polymer Solution. Pharmaceutics. 2017; 9(4):54. https://doi.org/10.3390/pharmaceutics9040054
Chicago/Turabian StyleZhang, Hao, Ying Zhu, Ning Qiao, Yang Chen, and Linghuan Gao. 2017. "Preparation and Characterization of Carbamazepine Cocrystal in Polymer Solution" Pharmaceutics 9, no. 4: 54. https://doi.org/10.3390/pharmaceutics9040054
APA StyleZhang, H., Zhu, Y., Qiao, N., Chen, Y., & Gao, L. (2017). Preparation and Characterization of Carbamazepine Cocrystal in Polymer Solution. Pharmaceutics, 9(4), 54. https://doi.org/10.3390/pharmaceutics9040054