Microbiota and Mitochondrial Sex-Dependent Imbalance in Fibromyalgia: A Pilot Descriptive Study
Abstract
:1. Introduction
2. Materials and Methods
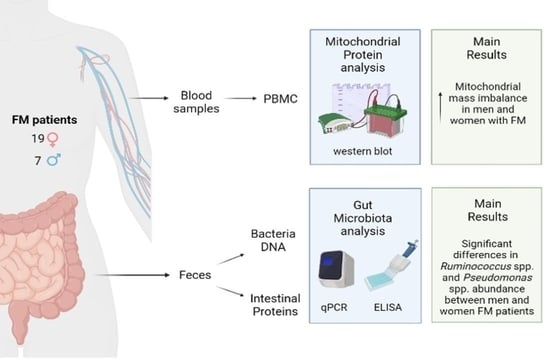
2.1. Patients
2.2. Health Questionnaires
2.3. Determination of Mitochondrial Mass and Total Antioxidant Capacity
2.4. Microbiota Analysis
3. Results
3.1. Demographic Profile of Patients
3.2. Mitochondrial Mass and Total Antioxidant Capacity
3.3. Microbiota Composition
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Longley, K. Fibromyalgia: Aetiology, Diagnosis, Symptoms and Management. Br. J. Nurs. 2006, 15, 729–733. [Google Scholar] [CrossRef] [PubMed]
- Marques, A.P.; Santo, A.D.S.D.E.; Berssaneti, A.A.; Matsutani, L.A.; Yuan, S.L.K. Prevalence of Fibromyalgia: Literature Review Update. Rev. Bras. Reumatol. 2017, 57, 356–363. [Google Scholar] [CrossRef]
- Wolfe, F.; Clauw, D.J.; Fitzcharles, M.A.; Goldenberg, D.L.; Katz, R.S.; Mease, P.; Russell, A.S.; Russell, I.J.; Winfield, J.B.; Yunus, M.B. The American College of Rheumatology Preliminary Diagnostic Criteria for Fibromyalgia and Measurement of Symptom Severity. Arthritis Care Res. 2010, 62, 600–610. [Google Scholar] [CrossRef] [PubMed]
- Sarzi-Puttini, P.; Giorgi, V.; Marotto, D.; Atzeni, F. Fibromyalgia: An Update on Clinical Characteristics, Aetiopathogenesis and Treatment. Nat. Rev. Rheumatol. 2020, 16, 645–660. [Google Scholar] [CrossRef]
- Hackshaw, K.V. The Search for Biomarkers in Fibromyalgia. Diagnostics 2021, 11, 156. [Google Scholar] [CrossRef]
- Gerdle, B.; Ghafouri, B.; Lund, E.; Bengtsson, A.; Lundberg, P.; van Ettinger-Veenstra, H.; Leinhard, O.D.; Forsgren, M.F. Evidence of Mitochondrial Dysfunction in Fibromyalgia: Deviating Muscle Energy Metabolism Detected Using Microdialysis and Magnetic Resonance. J. Clin. Med. 2020, 9, 3527. [Google Scholar] [CrossRef]
- Jung, Y.H.; Kim, H.; Lee, D.; Lee, J.Y.; Moon, J.Y.; Choi, S.H.; Kang, D.H. Dysfunctional Energy Metabolisms in Fibromyalgia Compared with Healthy Subjects. Mol. Pain. 2021, 17, 17448069211012833. [Google Scholar] [CrossRef]
- Huttenhower, C.; Gevers, D.; Knight, R.; Abubucker, S.; Badger, J.H.; Chinwalla, A.T.; Creasy, H.H.; Earl, A.M.; Fitzgerald, M.G.; Fulton, R.S.; et al. Structure, Function and Diversity of the Healthy Human Microbiome. Nature 2012, 486, 207. [Google Scholar] [CrossRef]
- Spor, A.; Koren, O.; Ley, R. Unravelling the Effects of the Environment and Host Genotype on the Gut Microbiome. Nat. Rev. Microbiol. 2011, 9, 279–290. [Google Scholar] [CrossRef]
- Larsen, N.; Vogensen, F.K.; van den Berg, F.W.J.; Nielsen, D.S.; Andreasen, A.S.; Pedersen, B.K.; Al-Soud, W.A.; Sørensen, S.J.; Hansen, L.H.; Jakobsen, M. Gut Microbiota in Human Adults with Type 2 Diabetes Differs from Non-Diabetic Adults. PLoS ONE 2010, 5, e9085. [Google Scholar] [CrossRef]
- Vatanen, T.; Kostic, A.D.; D’Hennezel, E.; Siljander, H.; Franzosa, E.A.; Yassour, M.; Kolde, R.; Vlamakis, H.; Arthur, T.D.; Hämäläinen, A.M.; et al. Variation in Microbiome LPS Immunogenicity Contributes to Autoimmunity in Humans. Cell 2016, 165, 842–853. [Google Scholar] [CrossRef]
- Koeth, R.A.; Wang, Z.; Levison, B.S.; Buffa, J.A.; Org, E.; Sheehy, B.T.; Britt, E.B.; Fu, X.; Wu, Y.; Li, L.; et al. Intestinal Microbiota Metabolism of L-Carnitine, a Nutrient in Red Meat, Promotes Atherosclerosis. Nat. Med. 2013, 19, 576–585. [Google Scholar] [CrossRef]
- Tilg, H. A Gut Feeling about Thrombosis. N. Engl. J. Med. 2016, 374, 2494–2496. [Google Scholar] [CrossRef]
- Jangi, S.; Gandhi, R.; Cox, L.M.; Li, N.; von Glehn, F.; Yan, R.; Patel, B.; Mazzola, M.A.; Liu, S.; Glanz, B.L.; et al. Alterations of the Human Gut Microbiome in Multiple Sclerosis. Nat. Commun. 2016, 7, 12015. [Google Scholar] [CrossRef]
- Chen, J.; Wright, K.; Davis, J.M.; Jeraldo, P.; Marietta, E.V.; Murray, J.; Nelson, H.; Matteson, E.L.; Taneja, V. An Expansion of Rare Lineage Intestinal Microbes Characterizes Rheumatoid Arthritis. Genome Med. 2016, 8, 43. [Google Scholar] [CrossRef]
- Collado, A.; Gomez, E.; Coscolla, R.; Sunyol, R.; Solé, E.; Rivera, J.; Altarriba, E.; Carbonell, J.; Castells, X. Work, Family and Social Environment in Patients with Fibromyalgia in Spain: An Epidemiological Study: EPIFFAC Study. BMC Health Serv. Res. 2014, 14, 513. [Google Scholar] [CrossRef]
- Minerbi, A.; Gonzalez, E.; Brereton, N.J.B.; Anjarkouchian, A.; Dewar, K.; Fitzcharles, M.A.; Chevalier, S.; Shir, Y. Altered Microbiome Composition in Individuals with Fibromyalgia. Pain 2019, 160, 2589–2602. [Google Scholar] [CrossRef]
- Albayrak, B.; Süsgün, S.; Küçükakkaş, O.; Akbaş, F.; Yabaci, A.; Özçelik, S. Investigating of Relation Between Fibromyalgia Syndrome and Intestinal Microbiota. Mikrobiyol. Bul. 2021, 55, 146–160. [Google Scholar] [CrossRef]
- Clos-Garcia, M.; Andrés-Marin, N.; Fernández-Eulate, G.; Abecia, L.; Lavín, J.L.; van Liempd, S.; Cabrera, D.; Royo, F.; Valero, A.; Errazquin, N.; et al. Gut Microbiome and Serum Metabolome Analyses Identify Molecular Biomarkers and Altered Glutamate Metabolism in Fibromyalgia. EBioMedicine 2019, 46, 499–511. [Google Scholar] [CrossRef]
- Baldi, S.; Pagliai, G.; Dinu, M.; di Gloria, L.; Nannini, G.; Curini, L.; Pallecchi, M.; Russo, E.; Niccolai, E.; Danza, G.; et al. Effect of Ancient Khorasan Wheat on Gut Microbiota, Inflammation, and Short-Chain Fatty Acid Production in Patients with Fibromyalgia. World J. Gastroenterol. 2022, 28, 1965–1980. [Google Scholar] [CrossRef]
- D’Erchia, A.M.; Atlante, A.; Gadaleta, G.; Pavesi, G.; Chiara, M.; de Virgilio, C.; Manzari, C.; Mastropasqua, F.; Prazzoli, G.M.; Picardi, E.; et al. Tissue-Specific MtDNA Abundance from Exome Data and Its Correlation with Mitochondrial Transcription, Mass and Respiratory Activity. Mitochondrion 2015, 20, 13–21. [Google Scholar] [CrossRef] [PubMed]
- Siracusa, R.; di Paola, R.; Cuzzocrea, S.; Impellizzeri, D. Fibromyalgia: Pathogenesis, Mechanisms, Diagnosis and Treatment Options Update. Int. J. Mol. Sci. 2021, 22, 3891. [Google Scholar] [CrossRef] [PubMed]
- Cordero, M.D.; Díaz-Parrado, E.; Carrión, A.M.; Alfonsi, S.; Sánchez-Alcazar, J.A.; Bullón, P.; Battino, M.; de Miguel, M. Is Inflammation a Mitochondrial Dysfunction-Dependent Event in Fibromyalgia? Antioxid Redox Signal 2013, 18, 800–807. [Google Scholar] [CrossRef] [PubMed]
- Danda, S.; Thomas, B.; Paramasivam, G.; Thomas, R.; Mathew, J.; Danda, D. A Descriptive Pilot Study of Mitochondrial Mutations & Clinical Phenotype in Fibromyalgia Syndrome. Indian J. Med. Res. 2019, 149, 47–50. [Google Scholar] [CrossRef] [PubMed]
- Cordero, M.D.; de Miguel, M.; Carmona-López, I.; Bonal, P.; Campa, F.; Moreno-Fernández, A.M. Oxidative Stress and Mitochondrial Dysfunction in Fibromyalgia. Neuroendocrinol. Lett. 2010, 31, 169–173. [Google Scholar]
- Cordero, M.D.; de Miguel, M.; Moreno Fernández, A.M.; Carmona López, I.M.; Garrido Maraver, J.; Cotán, D.; Gómez Izquierdo, L.; Bonal, P.; Campa, F.; Bullon, P.; et al. Mitochondrial Dysfunction and Mitophagy Activation in Blood Mononuclear Cells of Fibromyalgia Patients: Implications in the Pathogenesis of the Disease. Arthritis Res. Ther. 2010, 12, R17. [Google Scholar] [CrossRef]
- Meeus, M.; Nijs, J.; Hermans, L.; Goubert, D.; Calders, P. The Role of Mitochondrial Dysfunctions Due to Oxidative and Nitrosative Stress in the Chronic Pain or Chronic Fatigue Syndromes and Fibromyalgia Patients: Peripheral and Central Mechanisms as Therapeutic Targets? Expert Opin. Ther. Targets 2013, 17, 1081–1089. [Google Scholar] [CrossRef]
- Martínez-Lara, A.; Moreno-Fernández, A.M.; Jiménez-Guerrero, M.; Díaz-López, C.; De-Miguel, M.; Cotán, D.; Sánchez-Alcázar, J.A. Mitochondrial Imbalance as a New Approach to the Study of Fibromyalgia. Open Access Rheumatol. 2020, 12, 175–185. [Google Scholar] [CrossRef]
- Fatima, G.; Das, S.K.; Mahdi, A.A. Some Oxidative and Antioxidative Parameters and Their Relationship with Clinical Symptoms in Women with Fibromyalgia Syndrome. Int. J. Rheum. Dis. 2017, 20, 39–45. [Google Scholar] [CrossRef]
- Sánchez-Domínguez, B.; Bullón, P.; Román-Malo, L.; Marín-Aguilar, F.; Alcocer-Gómez, E.; Carrión, A.M.; Sánchez-Alcazar, J.A.; Cordero, M.D. Oxidative Stress, Mitochondrial Dysfunction and, Inflammation Common Events in Skin of Patients with Fibromyalgia. Mitochondrion 2015, 21, 69–75. [Google Scholar] [CrossRef]
- Jackson, D.N.; Theiss, A.L. Gut Bacteria Signaling to Mitochondria in Intestinal Inflammation and Cancer. Gut Microbes 2020, 11, 285–304. [Google Scholar] [CrossRef]
- Serrano-Serra, J.P.; Montero-Vilchez, T.; Buendia-Eisman, A.; Arias-Santiago, S. Epidermal Barrier Function and Skin Homeostasis in Skin with Permanent and Adhesive Tattoos: A Cross-Sectional Study. J. Clin. Med. 2021, 10, 888. [Google Scholar] [CrossRef]
- Aranaz, M.; Costas-Rodríguez, M.; Lobo, L.; García, M.; González-Iglesias, H.; Pereiro, R.; Vanhaecke, F. Homeostatic Alterations Related to Total Antioxidant Capacity, Elemental Concentrations and Isotopic Compositions in Aqueous Humor of Glaucoma Patients. Anal. Bioanal. Chem. 2022, 414, 515–524. [Google Scholar] [CrossRef]
- Walter, J.; Hertel, C.; Tannock, G.W.; Lis, C.M.; Munro, K.; Hammes, W.P. Detection of Lactobacillus, Pediococcus, Leuconostoc, and Weissella Species in Human Feces by Using Group-Specific PCR Primers and Denaturing Gradient Gel Electrophoresis. Appl. Environ. Microbiol. 2001, 67, 2578–2585. [Google Scholar] [CrossRef]
- Bartosch, S.; Fite, A.; Macfarlane, G.T.; McMurdo, M.E.T. Characterization of Bacterial Communities in Feces from Healthy Elderly Volunteers and Hospitalized Elderly Patients by Using Real-Time PCR and Effects of Antibiotic Treatment on the Fecal Microbiota. Appl. Environ. Microbiol. 2004, 70, 3575–3581. [Google Scholar] [CrossRef]
- Rekha, R.; Rizvi, M.A.; Jaishree, P. Designing and Validation of Genus-Specific Primers for Human Gut Flora Study. Electron. J. Biotechnol. 2006, 9, 505–511. [Google Scholar] [CrossRef]
- Derrien, M. Mucin Utilisation and Host Interactions of the Novel Intestinal Microbe Akkermansia Muciniphila; Wageningen University: Wageningen, The Netherlands, 2007. [Google Scholar]
- Guo, X.; Xia, X.; Tang, R.; Zhou, J.; Zhao, H.; Wang, K. Development of a Real-Time PCR Method for Firmicutes and Bacteroidetes in Faeces and Its Application to Quantify Intestinal Population of Obese and Lean Pigs. Lett. Appl. Microbiol. 2008, 47, 367–373. [Google Scholar] [CrossRef]
- Lauritsen, J.G.; Hansen, M.L.; Bech, P.K.; Jelsbak, L.; Gram, L.; Strube, M.L. Identification and Differentiation of Pseudomonas Species in Field Samples Using an RpoD Amplicon Sequencing Methodology. mSystems 2021, 6, e0070421. [Google Scholar] [CrossRef]
- Magne, F.; Gotteland, M.; Gauthier, L.; Zazueta, A.; Pesoa, S.; Navarrete, P.; Balamurugan, R. The Firmicutes/Bacteroidetes Ratio: A Relevant Marker of Gut Dysbiosis in Obese Patients? Nutrients 2020, 12, 1474. [Google Scholar] [CrossRef]
- Hjorth, M.F.; Blædel, T.; Bendtsen, L.Q.; Lorenzen, J.K.; Holm, J.B.; Kiilerich, P.; Roager, H.M.; Kristiansen, K.; Larsen, L.H.; Astrup, A. Prevotella-to-Bacteroides Ratio Predicts Body Weight and Fat Loss Success on 24-Week Diets Varying in Macronutrient Composition and Dietary Fiber: Results from a Post-Hoc Analysis. Int. J. Obes. 2018, 43, 149–157. [Google Scholar] [CrossRef]
- Aminov, R.I.; Walker, A.W.; Duncan, S.H.; Harmsen, H.J.M.; Welling, G.W.; Flint, H.J. Molecular Diversity, Cultivation, and Improved Detection by Fluorescent in Situ Hybridization of a Dominant Group of Human Gut Bacteria Related to Roseburia Spp. or Eubacterium Rectale. Appl. Environ. Microbiol. 2006, 72, 6371–6376. [Google Scholar] [CrossRef] [PubMed]
- Martínez, M.P.; Sánchez, A.I.; Prados, G.; Lami, M.J.; Villar, B.; Miró, E. Fibromyalgia as a Heterogeneous Condition: Subgroups of Patients Based on Physical Symptoms and Cognitive-Affective Variables Related to Pain. Span J. Psychol. 2021, 24, e33. [Google Scholar] [CrossRef] [PubMed]
- Castro-Marrero, J.; Cordero, M.D.; Sáez-Francas, N.; Jimenez-Gutierrez, C.; Aguilar-Montilla, F.J.; Aliste, L.; Alegre-Martin, J. Could Mitochondrial Dysfunction Be a Differentiating Marker between Chronic Fatigue Syndrome and Fibromyalgia? Antioxid. Redox Signal. 2013, 19, 1855–1860. [Google Scholar] [CrossRef]
- Flatters, S.J.L. The Contribution of Mitochondria to Sensory Processing and Pain. Prog. Mol. Biol. Transl. Sci. 2015, 131, 119–146. [Google Scholar] [CrossRef] [PubMed]
- Marchev, A.S.; Dimitrova, P.A.; Burns, A.J.; Kostov, R.V.; Dinkova-Kostova, A.T.; Georgiev, M.I. Oxidative Stress and Chronic Inflammation in Osteoarthritis: Can NRF2 Counteract These Partners in Crime? Ann. N. Y. Acad. Sci. 2017, 1401, 114–135. [Google Scholar] [CrossRef]
- Shim, H.S.; Bae, C.; Wang, J.; Lee, K.H.; Hankerd, K.M.; Kim, H.K.; Chung, J.M.; La, J.H. Peripheral and Central Oxidative Stress in Chemotherapy-Induced Neuropathic Pain. Mol. Pain. 2019, 15, 1744806919840098. [Google Scholar] [CrossRef]
- Dai, C.Q.; Guo, Y.; Chu, X.Y. Neuropathic Pain: The Dysfunction of Drp1, Mitochondria, and ROS Homeostasis. Neurotox Res. 2020, 38, 553–563. [Google Scholar] [CrossRef]
- dos Santos, J.M.; Lacerda, A.C.R.; Ribeiro, V.G.C.; Figueiredo, P.H.S.; Fonseca, S.F.; da Lage, V.K.S.; Costa, H.S.; Lima, V.P.; Sañudo, B.; Bernardo-Filho, M.; et al. Oxidative Stress Biomarkers and Quality of Life Are Contributing Factors of Muscle Pain and Lean Body Mass in Patients with Fibromyalgia. Biology 2022, 11, 935. [Google Scholar] [CrossRef]
- Yamamoto, T.; Yamada, A.; Watanabe, M.; Yoshimura, Y.; Yamazaki, N.; Yoshimura, Y.; Yamauchi, T.; Kataoka, M.; Nagata, T.; Terada, H.; et al. VDAC1, Having a Shorter N-Terminus than VDAC2 but Showing the Same Migration in an SDS-Polyacrylamide Gel, Is the Predominant Form Expressed in Mitochondria of Various Tissues. J. Proteome Res. 2006, 5, 3336–3344. [Google Scholar] [CrossRef]
- Tanida, I.; Ueno, T.; Kominami, E. LC3 and Autophagy. Methods Mol. Biol. 2008, 445, 77–88. [Google Scholar] [CrossRef]
- Huang, R.; Liu, W. Identifying an Essential Role of Nuclear LC3 for Autophagy. Autophagy 2015, 11, 852–853. [Google Scholar] [CrossRef]
- Lyakhovich, A.; Graifer, D. Mitochondria-Mediated Oxidative Stress: Old Target for New Drugs. Curr. Med. Chem. 2015, 22, 3040–3053. [Google Scholar] [CrossRef]
- Vezza, T.; Abad-Jiménez, Z.; Marti-Cabrera, M.; Rocha, M.; Víctor, V.M. Microbiota-Mitochondria Inter-Talk: A Potential Therapeutic Strategy in Obesity and Type 2 Diabetes. Antioxidants 2020, 9, 848. [Google Scholar] [CrossRef]
- Kramer, P. Mitochondria-Microbiota Interaction in Neurodegeneration. Front. Aging Neurosci. 2021, 13, 776936. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhang, J.; Duan, L. The Role of Microbiota-Mitochondria Crosstalk in Pathogenesis and Therapy of Intestinal Diseases. Pharmacol. Res. 2022, 186, 106530. [Google Scholar] [CrossRef]
- Russo, R.; Cristiano, C.; Avagliano, C.; de Caro, C.; la Rana, G.; Raso, G.M.; Canani, R.B.; Meli, R.; Calignano, A. Gut-Brain Axis: Role of Lipids in the Regulation of Inflammation, Pain and CNS Diseases. Curr. Med. Chem. 2018, 25, 3930–3952. [Google Scholar] [CrossRef]
- Auteri, M.; Zizzo, M.G.; Serio, R. GABA and GABA Receptors in the Gastrointestinal Tract: From Motility to Inflammation. Pharmacol. Res. 2015, 93, 11–21. [Google Scholar] [CrossRef]
- Otaru, N.; Ye, K.; Mujezinovic, D.; Berchtold, L.; Constancias, F.; Cornejo, F.A.; Krzystek, A.; de Wouters, T.; Braegger, C.; Lacroix, C.; et al. GABA Production by Human Intestinal Bacteroides Spp.: Prevalence, Regulation, and Role in Acid Stress Tolerance. Front. Microbiol. 2021, 12, 656895. [Google Scholar] [CrossRef]
- Guo, R.; Chen, L.H.; Xing, C.; Liu, T. Pain Regulation by Gut Microbiota: Molecular Mechanisms and Therapeutic Potential. Br. J. Anaesth 2019, 123, 637–654. [Google Scholar] [CrossRef]
- Koliada, A.; Syzenko, G.; Moseiko, V.; Budovska, L.; Puchkov, K.; Perederiy, V.; Gavalko, Y.; Dorofeyev, A.; Romanenko, M.; Tkach, S.; et al. Association between Body Mass Index and Firmicutes/Bacteroidetes Ratio in an Adult Ukrainian Population. BMC Microbiol. 2017, 17, 120. [Google Scholar] [CrossRef]
- Qadir, R.M.; Assafi, M.S. The Association between Body Mass Index and the Oral Firmicutes and Bacteroidetes Profiles of Healthy Individuals. Malays. Fam. Physician 2021, 16, 36–43. [Google Scholar] [CrossRef] [PubMed]
- Bahar-Tokman, H.; Demirci, M.; Keskin, F.E.; Cagatay, P.; Taner, Z.; Ozturk-Bakar, Y.; Ozyazar, M.; Kiraz, N.; Kocazeybek, B.S. Firmicutes/Bacteroidetes Ratio in the Gut Microbiota and IL-1β, IL-6, IL-8, TLR2, TLR4, TLR5 Gene Expressions in Type 2 Diabetes. Clin. Lab. 2022, 68, 1903–1910. [Google Scholar] [CrossRef] [PubMed]
- Nkosi, B.V.Z.; Padayachee, T.; Gront, D.; Nelson, D.R.; Syed, K. Contrasting Health Effects of Bacteroidetes and Firmicutes Lies in Their Genomes: Analysis of P450s, Ferredoxins, and Secondary Metabolite Clusters. Int. J. Mol. Sci. 2022, 23, 5057. [Google Scholar] [CrossRef]
- Maseda, D.; Ricciotti, E. NSAID-Gut Microbiota Interactions. Front. Pharmacol. 2020, 11, 1153. [Google Scholar] [CrossRef] [PubMed]
- Zádori, Z.S.; Király, K.; Al-Khrasani, M.; Gyires, K. Interactions between NSAIDs, opioids and the gut microbiota—Future perspectives in the management of inflammation and pain. Pharmacol. Ther. 2023, 241, 108327. [Google Scholar] [CrossRef]
- Craven, M.; Egan, C.E.; Dowd, S.E.; McDonough, S.P.; Dogan, B.; Denkers, E.Y.; Bowman, D.; Scherl, E.J.; Simpson, K.W. Inflammation drives dysbiosis and bacterial invasion in murine models of ileal Crohn's disease. PLoS ONE 2012, 7, e41594. [Google Scholar] [CrossRef]
- Lázár, B.; László, S.B.; Hutka, B.; Tóth, A.S.; Mohammadzadeh, A.; Berekméri, E.; Ágg, B.; Balogh, M.; Sajtos, V.; Király, K.; et al. A comprehensive time course and correlation analysis of indomethacin-induced inflammation, bile acid alterations and dysbiosis in the rat small intestine. Biochem. Pharmacol. 2021, 190, 114590. [Google Scholar] [CrossRef]
- Maseda, D.; Zackular, J.P.; Trindade, B.; Kirk, L.; Roxas, J.L.; Rogers, L.M.; Washington, M.K.; Du, L.; Koyama, T.; Viswanathan, V.K.; et al. Nonsteroidal Anti-inflammatory Drugs Alter the Microbiota and Exacerbate Clostridium difficile Colitis while Dysregulating the Inflammatory Response. mBio 2019, 10, e02282-18. [Google Scholar] [CrossRef]
- Terán-Ventura, E.; Aguilera, M.; Vergara, P.; Martínez, V. Specific changes of gut commensal microbiota and TLRs during indomethacin-induced acute intestinal inflammation in rats. J. Crohn’s Colitis 2014, 8, 1043–1054. [Google Scholar] [CrossRef]
- Colucci, R.; Pellegrini, C.; Fornai, M.; Tirotta, E.; Antonioli, L.; Renzulli, C.; Ghelardi, E.; Piccoli, E.; Gentile, D.; Benvenuti, L.; et al. Pathophysiology of NSAID-Associated Intestinal Lesions in the Rat: Luminal Bacteria and Mucosal Inflammation as Targets for Prevention. Front. Pharmacol. 2018, 9, 1340. [Google Scholar] [CrossRef]
- Lu, Z.; Lu, Y.; Wang, X.; Wang, F.; Zhang, Y. Activation of intestinal GR-FXR and PPARα-UGT signaling exacerbates ibuprofen-induced enteropathy in mice. Arch. Toxicol. 2018, 92, 1249–1265. [Google Scholar] [CrossRef]
- Xu, Z.; Jiang, W.; Huang, W.; Lin, Y.; Chan, F.K.L.; Ng, S.C. Gut microbiota in patients with obesity and metabolic disorders—a systematic review. Genes Nutr. 2022, 17, 2. [Google Scholar] [CrossRef]
- Henke, M.T.; Kenny, D.J.; Cassilly, C.D.; Vlamakis, H.; Xavier, R.J.; Clardy, J. Ruminococcus Gnavus, a Member of the Human Gut Microbiome Associated with Crohn’s Disease, Produces an Inflammatory Polysaccharide. Proc. Natl. Acad. Sci. USA 2019, 116, 12672–12677. [Google Scholar] [CrossRef]
- Yunus, M.B.; Inanici, F.; Aldag, J.C.; Mangold, R.F. Fibromyalgia in Men: Comparison of Clinical Features with Women. J. Rheumatol. 2000, 27, 485–490. [Google Scholar]
- Cao, Y.; Jiang, C.; Jia, Y.; Xu, D.; Yu, Y. Letrozole and the Traditional Chinese Medicine, Shaofu Zhuyu Decoction, Reduce Endometriotic Disease Progression in Rats: A Potential Role for Gut Microbiota. Evid. Based Complement. Alternat. Med. 2020, 2020, 3687498. [Google Scholar] [CrossRef]
- Greenbaum, H.; Weil, C.; Chodick, G.; Shalev, V.; Eisenberg, V.H. Evidence for an Association between Endometriosis, Fibromyalgia, and Autoimmune Diseases. Am. J. Reprod. Immunol. 2019, 81, e13095. [Google Scholar] [CrossRef]
- Meng, J.; Banerjee, S.; Zhang, L.; Sindberg, G.; Moidunny, S.; Li, B.; Robbins, D.J.; Girotra, M.; Segura, B.; Ramakrishnan, S.; et al. Opioids Impair Intestinal Epithelial Repair in HIV-Infected Humanized Mice. Front. Immunol. 2020, 10, 2999. [Google Scholar] [CrossRef]
- Ervin, S.M.; Li, H.; Lim, L.; Roberts, L.R.; Liang, X.; Mani, S.; Redinbo, M.R. Gut Microbial β-Glucuronidases Reactivate Estrogens as Components of the Estrobolome That Reactivate Estrogens. J. Biol. Chem. 2019, 294, 18586. [Google Scholar] [CrossRef]
- Koca, T.; Koçyiğit, B.; Seyithanoğlu, M.; Berk, E. The Importance of G-Protein Coupled Estrogen Receptor in Patients With Fibromyalgia. Arch. Rheumatol. 2019, 34, 419. [Google Scholar] [CrossRef]
- Hu, Y.; Qing, Y.; Chen, J.; Liu, C.; Lu, J.; Wang, Q.; Zhen, S.; Zhou, H.; Huang, L.; Zhang, R. Prevalence, Risk Factors, and Molecular Epidemiology of Intestinal Carbapenem-Resistant Pseudomonas Aeruginosa. Microbiol. Spectr. 2021, 9, e01344-21. [Google Scholar] [CrossRef]
- Maes, M.; Mihaylova, I.; Leunis, J.C. Increased Serum IgA and IgM against LPS of Enterobacteria in Chronic Fatigue Syndrome (CFS): Indication for the Involvement of Gram-Negative Enterobacteria in the Etiology of CFS and for the Presence of an Increased Gut-Intestinal Permeability. J. Affect Disord. 2007, 99, 237–240. [Google Scholar] [CrossRef] [PubMed]
- Pabst, O.; Slack, E. IgA and the Intestinal Microbiota: The Importance of Being Specific. Mucosal Immunol. 2019, 13, 12–21. [Google Scholar] [CrossRef] [PubMed]




| Bacteria | Primer Pair | References |
|---|---|---|
| Akkermansia muciniphila | AkkF, AkkR | [10,34,35,36,37,38,39] |
| Bacteroides spp. | BacF 1, BacR 1 | |
| Bacteroidetes spp. | BatsF, BatsR | |
| Eubacterium spp. | EuF A, EuR A | |
| Firmicutes spp. | FirmF, FirmR | |
| Prevotella spp. | PrevF 2, PrevR 2 | |
| Pseudomonas spp. | PseF A, PseR A | |
| Roseburia spp. | RosF A, RosR A | |
| Ruminococcus spp. | RumF 3, RumR 3 | |
| All bacteria | AllF A, AllR A |
| Variable | Outcome | Male (n = 7) | Female (n = 18) | p-Value |
|---|---|---|---|---|
| Age | 45.43 (±8.96) | 50.28 (±10.21) | 0.26 | |
| Body Mass Index (BMI) | 26.26 (±3.31) | 24.97 (3.07) | 0.39 | |
| IFM questionnaire score | 51.80 (±15.87) | 62.17 (±35.37) | 0.06 | |
| Nutrition | Not controlled | 7 | 18 | n.a. * |
| Under control | 0 | 0 | ||
| Physical exercise | None | 3 | 7 | n.a. * |
| Yes | 4 | 11 | ||
| Psychological therapy | None | 4 | 7 | n.a. * |
| Yes | 3 | 11 | ||
| Drugs 1 | NSAIDs | 4 | 14 | n.a. * |
| Antidepressants | 4 | 10 | ||
| Neuropathic drugs | 1 | 7 | ||
| Analgesics | 5 | 15 | ||
| Antibiotics 2 | 2 | 2 | ||
| Probiotics 2 | 2 | 5 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ramírez-Tejero, J.A.; Durán-González, E.; Martínez-Lara, A.; Lucena del Amo, L.; Sepúlveda, I.; Huancas-Díaz, A.; Carvajal, M.; Cotán, D. Microbiota and Mitochondrial Sex-Dependent Imbalance in Fibromyalgia: A Pilot Descriptive Study. Neurol. Int. 2023, 15, 868-880. https://doi.org/10.3390/neurolint15030055
Ramírez-Tejero JA, Durán-González E, Martínez-Lara A, Lucena del Amo L, Sepúlveda I, Huancas-Díaz A, Carvajal M, Cotán D. Microbiota and Mitochondrial Sex-Dependent Imbalance in Fibromyalgia: A Pilot Descriptive Study. Neurology International. 2023; 15(3):868-880. https://doi.org/10.3390/neurolint15030055
Chicago/Turabian StyleRamírez-Tejero, Jorge A., Elena Durán-González, Antonio Martínez-Lara, Laura Lucena del Amo, Isabel Sepúlveda, Andrés Huancas-Díaz, Marco Carvajal, and David Cotán. 2023. "Microbiota and Mitochondrial Sex-Dependent Imbalance in Fibromyalgia: A Pilot Descriptive Study" Neurology International 15, no. 3: 868-880. https://doi.org/10.3390/neurolint15030055
APA StyleRamírez-Tejero, J. A., Durán-González, E., Martínez-Lara, A., Lucena del Amo, L., Sepúlveda, I., Huancas-Díaz, A., Carvajal, M., & Cotán, D. (2023). Microbiota and Mitochondrial Sex-Dependent Imbalance in Fibromyalgia: A Pilot Descriptive Study. Neurology International, 15(3), 868-880. https://doi.org/10.3390/neurolint15030055