Relationship between Undernutrition and Anemia in Patients with Ulcerative Colitis
Abstract
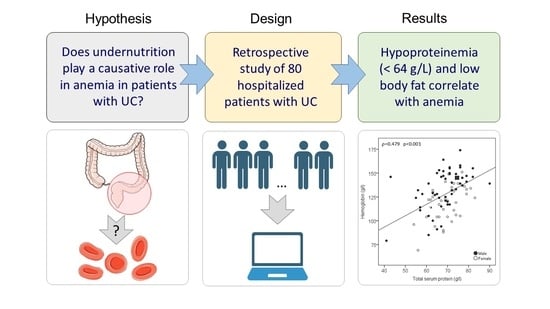
:1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Patients and Data Collection
2.3. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Chang, J.T. Pathophysiology of Inflammatory Bowel Diseases. N. Engl. J. Med. 2020, 383, 2652–2664. [Google Scholar] [CrossRef] [PubMed]
- Neurath, M.F. Targeting immune cell circuits and trafficking in inflammatory bowel disease. Nat. Immunol. 2019, 20, 970–979. [Google Scholar] [CrossRef] [PubMed]
- Guagnozzi, D.; Lucendo, A.J. Anemia in inflammatory bowel disease: A neglected issue with relevant effects. World J. Gastroenterol. 2014, 20, 3542–3551. [Google Scholar] [CrossRef] [PubMed]
- Bergamaschi, G.; Castiglione, F.; D’Incà, R.; Astegiano, M.; Fries, W.; Milla, M.; Ciacci, C.; Rizzello, F.; Saibeni, S.; Ciccocioppo, R.; et al. Prevalence, Pathogenesis and Management of Anemia in Inflammatory Bowel Disease: An IG-IBD Multicenter, Prospective, and Observational Study. Inflamm. Bowel Dis. 2022, izac054. [Google Scholar] [CrossRef]
- Filmann, N.; Rey, J.; Schneeweiss, S.; Ardizzone, S.; Bager, P.; Bergamaschi, G.; Koutroubakis, I.; Lindgren, S.; de la Morena, F.; Moum, B.; et al. Prevalence of Anemia in Inflammatory Bowel Diseases in European Countries: A Systematic Review and Individual Patient Data Meta-analysis. Inflamm. Bowel Dis. 2014, 20, 936–945. [Google Scholar] [CrossRef] [PubMed]
- Wells, C.W.; Lewis, S.; Barton, J.R.; Corbett, S. Effects of Changes in Hemoglobin Level on Quality of Life and Cognitive Function in Inflammatory Bowel Disease Patients. Inflamm. Bowel Dis. 2006, 12, 123–130. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lopez, A.; Cacoub, P.; Macdougall, I.C.; Peyrin-Biroulet, L. Iron deficiency anaemia. Lancet 2016, 387, 907–916. [Google Scholar] [CrossRef] [PubMed]
- Cucino, C.; Sonnenberg, A. Cause of Death in Patients with Inflammatory Bowel Disease. Inflamm. Bowel Dis. 2001, 7, 250–255. [Google Scholar] [CrossRef]
- Kulnigg, S.; Gasche, C. Systematic review: Managing anaemia in Crohn’s disease. Aliment. Pharmacol. Ther. 2006, 24, 1507–1523. [Google Scholar] [CrossRef]
- Resal, T.; Farkas, K.; Molnar, T. Iron Deficiency Anemia in Inflammatory Bowel Disease: What Do We Know? Front. Med. 2021, 8, 686778. [Google Scholar] [CrossRef]
- Maas, L.A.; Krishna, M.; Parian, A.M. Ironing It All Out: A Comprehensive Review of Iron Deficiency Anemia in Inflammatory Bowel Disease Patients. Dig. Dis. Sci. 2022. [Google Scholar] [CrossRef] [PubMed]
- Weiss, G.; Ganz, T.; Goodnough, L.T. Anemia of inflammation. Blood 2019, 133, 40–50. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Poulia, K.A.; Klek, S.; Doundoulakis, I.; Bouras, E.; Karayiannis, D.; Baschali, A.; Passakiotou, M.; Chourdakis, M. The two most popular malnutrition screening tools in the light of the new ESPEN consensus definition of the diagnostic criteria for malnutrition. Clin. Nutr. 2017, 36, 1130–1135. [Google Scholar] [CrossRef] [PubMed]
- Kondrup, J.; Rasmussen, H.H.; Hamberg, O.; Stanga, Z.; Ad Hoc ESPEN Working Group. Nutritional risk screening (NRS 2002): A new method based on an analysis of controlled clinical trials. Clin. Nutr. 2003, 22, 321–336. [Google Scholar] [CrossRef]
- Cederholm, T.; Jensen, G.L.; Correia, M.I.T.D.; Gonzalez, M.C.; Fukushima, R.; Higashiguchi, T.; Baptista, G.; Barazzoni, R.; Blaauw, R.; Coats, A.; et al. GLIM criteria for the diagnosis of malnutrition–A consensus report from the global clinical nutrition community. Clin. Nutr. 2019, 38, 1–9. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lin, A.; Micic, D. Nutrition Considerations in Inflammatory Bowel Disease. Nutr. Clin. Pract. 2021, 36, 298–311. [Google Scholar] [CrossRef]
- Sandozai, M.K.; Haquani, A.H.; Rajeshvari, V.; Kaur, J. Kwashiorkor. A clinico-haematological study. Br. Med. J. 1963, 2, 93–96. [Google Scholar] [CrossRef]
- Body Fat Mass and Percentage. Available online: http://tanita.eu/tanita-academy/understanding-your-measurements (accessed on 28 October 2022).
- Dube, P.E.; Punit, S.; Polk, D.B. Redeeming an old foe: Protective as well as pathophysiological roles for tumor necrosis factor in inflammatory bowel disease. Am. J. Physiol. Gastrointest. Liver Physiol. 2015, 308, G161–G170. [Google Scholar] [CrossRef] [Green Version]
- Mavropoulou, E.; Mechie, N.C.; Knoop, R.; Petzold, G.; Ellenrieder, V.; Kunsch, S.; Pilavakis, Y.; Amanzada, A. Association of serum interleukin–6 and soluble interleukin–2–receptor levels with disease activity status in patients with inflammatory bowel disease: A prospective observational study. PLoS ONE 2020, 15, e0233811. [Google Scholar] [CrossRef]
- Nicholson, J.P.; Wolmarans, M.R.; Park, G.R. The role of albumin in critical illness. Br. J. Anaesth. 2000, 85, 599–610. [Google Scholar] [CrossRef]
- Don, B.R.; Kaysen, G. Poor Nutritional Status and Inflammation: Serum Albumin: Relationship to Inflammation and Nutrition. Semin. Dial. 2004, 17, 432–437. [Google Scholar] [CrossRef] [PubMed]
- Lambert, K.; Pappas, D.; Miglioretto, C.; Javadpour, A.; Reveley, H.; Frank, L.; Grimm, M.C.; Samocha-Bonet, D.; Hold, G.L. Systematic review with meta-analysis: Dietary intake in adults with inflammatory bowel disease. Aliment. Pharmacol. Ther. 2021, 54, 742–754. [Google Scholar] [CrossRef] [PubMed]
- Singh, S.; Arthur, S.; Sundaram, U. Mechanisms of Regulation of Transporters of Amino Acid Absorption in Inflammatory Bowel Diseases. Compr. Physiol. 2020, 10, 673–686. [Google Scholar] [CrossRef]
- Bryant, R.V.; Trott, M.J.; Bartholomeusz, F.D.; Andrews, J.M. Systematic review: Body composition in adults with inflammatory bowel disease. Aliment. Pharmacol. Ther. 2013, 38, 213–225. [Google Scholar] [CrossRef] [PubMed]
- Gold, S.L.; Rabinowitz, L.G.; Manning, L.; Keefer, L.; Rivera-Carrero, W.; Stanley, S.; Sherman, A.; Castillo, A.; Tse, S.; Hyne, A.; et al. High Prevalence of Malnutrition and Micronutrient Deficiencies in Patients with Inflammatory Bowel Disease Early in Disease Course. Inflamm. Bowel Dis. 2022, izac102. [Google Scholar] [CrossRef] [PubMed]
- Caballero, T.; Nogueras, F.; Medina, M.T.; Caracuel, M.D.; de Sola, C.; Martínez-Salmerón, F.J.; Rodrigo, M.; García del Moral, R. Intraepithelial and lamina propria leucocyte subsets in inflammatory bowel disease: An immunohistochemical study of colon and rectal biopsy specimens. J. Clin. Pathol. 1995, 48, 743–748. [Google Scholar] [CrossRef] [Green Version]
- Leppkes, M.; Roulis, M.; Neurath, M.F.; Kollias, G.; Becker, C. Pleiotropic functions of TNF-α in the regulation of the intestinal epithelial response to inflammation. Int. Immunol. 2014, 26, 509–515. [Google Scholar] [CrossRef] [Green Version]
- Heller, F.; Florian, P.; Bojarski, C.; Richter, J.; Christ, M.; Hillenbrand, B.; Mankertz, J.; Gitter, A.H.; Burgel, N.; Fromm, M.; et al. Interleukin-13 Is the Key Effector Th2 Cytokine in Ulcerative Colitis That Affects Epithelial Tight Junctions, Apoptosis, and Cell Restitution. Gastroenterology 2005, 129, 550–564. [Google Scholar] [CrossRef]
- Sasaki, M.; Johtatsu, T.; Kurihara, M.; Iwakawa, H.; Tanaka, T.; Bamba, S.; Tsujikawa, T.; Fujiyama, Y.; Andoh, A. Energy expenditure in Japanese patients with severe or moderate ulcerative colitis. J. Clin. Biochem. Nutr. 2010, 47, 32–36. [Google Scholar] [CrossRef] [Green Version]
- Nardone, O.M.; de Sire, R.; Petito, V.; Testa, A.; Villani, G.; Scaldaferri, F.; Castiglione, F. Inflammatory Bowel Diseases and Sarcopenia: The Role of Inflammation and Gut Microbiota in the Development of Muscle Failure. Front. Immunol. 2021, 12, 694217. [Google Scholar] [CrossRef]
- Dhaliwal, A.; Quinlan, J.I.; Overthrow, K.; Greig, C.; Lord, J.M.; Armstrong, M.J.; Cooper, S.C. Sarcopenia in Inflammatory Bowel Disease: A Narrative Overview. Nutrients 2021, 13, 656. [Google Scholar] [CrossRef] [PubMed]
- Polyzos, S.A.; Margioris, A.N. Sarcopenic obesity. Hormones 2018, 17, 321–331. [Google Scholar] [CrossRef] [PubMed]
- Bennett, B.D.; Solar, G.P.; Yuan, J.Q.; Mathias, J.; Thomas, G.R.; Matthews, W. A role for leptin and its cognate receptor in hematopoiesis. Curr. Biol. 1996, 6, 1170–1180. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Axelsson, J.; Qureshi, A.R.; Heimbürger, O.; Lindholm, B.; Stenvinkel, P.; Barany, P. Body Fat Mass and Serum Leptin Levels Influence Epoetin Sensitivity in Patients with ESRD. Am. J. Kidney Dis. 2005, 46, 628–634. [Google Scholar] [CrossRef] [PubMed]
- Considine, R.V.; Sinha, M.K.; Heiman, M.L.; Kriauciunas, A.; Stephens, T.W.; Nyce, M.R.; Ohannesian, J.P.; Marco, C.C.; McKee, L.J.; Bauer, T.L.; et al. Serum Immunoreactive-Leptin Concentrations in Normal-Weight and Obese Humans. N. Engl. J. Med. 1996, 334, 292–295. [Google Scholar] [CrossRef] [PubMed]
- Maccio, A.; Madeddu, C.; Gramignano, G.; Mulas, C.; Tanca, L.; Cherchi, M.C.; Floris, C.; Omoto, I.; Barracca, A.; Ganz, T. The role of inflammation, iron, and nutritional status in cancer-related anemia: Results of a large, prospective, observational study. Haematologica 2015, 100, 124–132. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Moschonis, G.; Chrousos, G.P.; Lionis, C.; Mougios, V.; Manios, Y. Association of total body and visceral fat mass with iron deficiency in preadolescents: The Healthy Growth Study. Br. J. Nutr. 2012, 108, 710–719. [Google Scholar] [CrossRef] [Green Version]
- González-Domínguez, Á.; Visiedo-García, F.M.; Domínguez-Riscart, J.; González-Domínguez, R.; Mateos, R.M.; Lechuga-Sancho, A.M. Iron Metabolism in Obesity and Metabolic Syndrome. Int. J. Mol. Sci. 2020, 21, 5529. [Google Scholar] [CrossRef]
- Chung, B.; Matak, P.; McKie, A.T.; Sharp, P. Leptin Increases the Expression of the Iron Regulatory Hormone Hepcidin in HuH7 Human Hepatoma Cells. J. Nutr. 2007, 137, 2366–2370. [Google Scholar] [CrossRef]


| Variables | n (%) | Median (Q1; Q3) |
|---|---|---|
| All | 80 (100.0) | |
| Nutritional status | ||
| BMI, kg/m2 | 22.1 (19.6; 25.3) | |
| <18.5 kg/m2 | 12 (15.0) | |
| ≥18.5 kg/m2 | 68 (85.0) | |
| Fat mass loss | ||
| Yes | 8 (10.0) | |
| No | 72 (90.0) | |
| Total serum protein, g/L | 69 (64; 74) | |
| <64 g/L | 16 (20.0) | |
| ≥64 g/L | 64 (80.0) | |
| Hemoglobin, g/dL | 129.5 (114.0; 145.0) | |
| Anemia | ||
| Yes | 32 (40.0) | |
| No | 48 (60.0) | |
| Demographic characteristics | ||
| Gender | ||
| Male | 51 (63.7) | |
| Female | 29 (36.3) | |
| Age | 34.5 (27.0; 50.8) | |
| Disease-associated characteristics | ||
| Acute disease | ||
| Yes (S1–S3) | 69 (86.3) | |
| No (S0) | 11 (13.8) | |
| Extent of gut involvement | ||
| Proctitis and left-sided colitis | 40 (50.0) | |
| Pancolitis | 40 (50.0) | |
| Immunosuppressive therapy | ||
| Yes | 20 (25.0) | |
| No | 60 (75.0) | |
| Quantity of relapses from disease beginning | 5 (3; 8) |
| Patient Characteristics | Anemia, n (%) | p Value | |
|---|---|---|---|
| Yes | No | ||
| All | 32 (40.0) | 48 (60.0) | |
| BMI level | 0.206 | ||
| <18.5 kg/m2 | 7 (8.8) | 5 (6.3) | |
| ≥18.5 kg/m2 | 25 (31.3) | 43 (53.8) | |
| Fat mass losses | 0.054 | ||
| Yes | 6 (7.5) | 2 (2.5) | |
| No | 46 (57.5) | 26 (32.5) | |
| Total serum protein | 0.009 | ||
| <64 g/L | 11 (13.8) | 5 (6.3) | |
| ≥64 g/L | 21 (26.3) | 43 (53.8) | |
| Gender | 0.255 | ||
| Male | 18 (22.5) | 33 (41.3) | |
| Female | 14 (17.5) | 15 (18.8) | |
| Acute disease | 0.511 | ||
| Yes (S1–S3) | 29 (36.3) | 40 (50.0) | |
| No (S0) | 8 (10.0) | 3 (3.8) | |
| Extent of gut involvement | 0.361 | ||
| Proctitis and left-sided colitis | 14 (17.5) | 26 (32.5) | |
| Pancolitis | 18 (22.5) | 22 (27.5) | |
| Immunosuppressive therapy | 0.598 | ||
| Yes | 11 (13.8) | 9 (11.3) | |
| No | 23 (28.7) | 37 (46.3) | |
| Patient Characteristics | Crude | Adjusted * | ||||
|---|---|---|---|---|---|---|
| Model 1 | Model 2 | |||||
| OR (95% CI) | p Value | OR (95% CI) | p Value | OR (95% CI) | p Value | |
| BMI level | 0.168 | 0.259 | - | - | ||
| <18.5 kg/m2 | 2.4 (0.7; 8.4) | 2.2 (0.6; 8.5) | ||||
| ≥18.5 kg/m2 | reference | reference | ||||
| Fat mass loss | 0.050 | - | - | 0.037 | ||
| Yes | 5.3 (0.1; 28.2) | 8.5 (1.1; 63.6) | ||||
| No | reference | reference | ||||
| Total serum protein | 0.012 | 0.010 | 0.009 | |||
| <64 g/L | 4.5 (1.4; 14.6) | 5.1 (1.5; 17.8) | 5.6 (1.5; 20.7) | |||
| ≥64 g/L | reference | reference | reference | |||
| Acute disease | 0.360 | 0.269 | 0.153 | |||
| Yes (S1–S3) | 1.9 (0.5; 7.9) | 2.6 (0.5; 14.1) | 3.6 (0.6; 20.9) | |||
| No (S0) | reference | reference | reference | |||
| Extent of gut involvement | 0.362 | 0.601 | 0.730 | |||
| Proctitis and left-sided colitis | reference | reference | reference | |||
| Pancolitis | 1.5 (0.6; 3.7) | 1.3 (0.4; 4.0) | 1.2 (0.4; 4.5) | |||
| Immuno-suppressive therapy | 0.599 | 0.697 | 0.744 | |||
| Yes | 1.3 (0.5; 3.7) | 1.3 (0.4; 4.4) | 1.2 (0.3; 4.5) | |||
| No | reference | reference | reference | |||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Uspenskiy, Y.P.; Ivanov, S.V.; Krasichkov, A.S.; Galagudza, M.M.; Fominykh, Y.A. Relationship between Undernutrition and Anemia in Patients with Ulcerative Colitis. Gastroenterol. Insights 2023, 14, 27-36. https://doi.org/10.3390/gastroent14010003
Uspenskiy YP, Ivanov SV, Krasichkov AS, Galagudza MM, Fominykh YA. Relationship between Undernutrition and Anemia in Patients with Ulcerative Colitis. Gastroenterology Insights. 2023; 14(1):27-36. https://doi.org/10.3390/gastroent14010003
Chicago/Turabian StyleUspenskiy, Yury P., Sergei V. Ivanov, Alexander S. Krasichkov, Michael M. Galagudza, and Yulia A. Fominykh. 2023. "Relationship between Undernutrition and Anemia in Patients with Ulcerative Colitis" Gastroenterology Insights 14, no. 1: 27-36. https://doi.org/10.3390/gastroent14010003
APA StyleUspenskiy, Y. P., Ivanov, S. V., Krasichkov, A. S., Galagudza, M. M., & Fominykh, Y. A. (2023). Relationship between Undernutrition and Anemia in Patients with Ulcerative Colitis. Gastroenterology Insights, 14(1), 27-36. https://doi.org/10.3390/gastroent14010003