Short-Term Response of the Soil Microbial Abundances and Enzyme Activities to Experimental Warming in a Boreal Peatland in Northeast China
Abstract
:1. Introduction
2. Materials and Methods
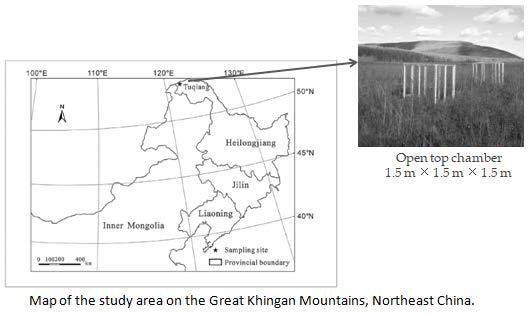
2.1. Study Sites and Experimental Design
2.2. Chemical Analyses
2.3. Real-time PCR Assay of Functional Genes
2.4. Soil Enzyme Activities Analysis
2.5. Statistical Analyses
3. Results
3.1. Soil Carbon and Nitrogen Contents, Soil Moisture, and Soil pH
3.2. Soil Microbial Abundance
3.3. Enzyme Activities
4. Discussion
4.1. Response of Soil C and N Contents to Experimental Warming
4.2. Response of Soil Carbon and Nitrogen Cycling Genes Abundance to Experimental Warming
4.3. Response of Soil Enzyme Activities to Experimental Warming
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Oliverio, A.M.; Bradford, M.A.; Fierer, N. Identifying the microbial taxa that consistently respond to soil warming across time and space. Glob. Chang. Biol. 2017, 23, 2117–2129. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.Z.; Xue, K.; Xie, J.P.; Deng, Y.; Wu, L.Y.; Cheng, X.L.; Fei, S.F.; Deng, S.P.; He, Z.L.; Van Nostrand, J.D.; et al. Microbial mediation of carbon-cycle feedbacks to climate warming. Nat. Clim. Chang. 2012, 2, 106–110. [Google Scholar] [CrossRef]
- Waghmode, T.R.; Chen, S.M.; Li, J.Z.; Sun, R.B.; Liu, B.B.; Hu, C.S. Response of nitrifier and denitrifier abundance and microbial community structure to experimental warming in an agricultural ecosystem. Front. Microbiol. 2018, 9, 474. [Google Scholar] [CrossRef] [PubMed]
- Sheik, C.S.; Beasley, W.H.; Elshahed, M.S.; Zhou, X.H.; Luo, Y.Q.; Krumholz, L.R. Effect of warming and drought on grassland microbial communities. ISME J. 2011, 5, 1692–1700. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, Z.M.; Yang, S.H.; Van Nostrand, J.D.; Zhou, J.Z.; Fang, W.; Qi, Q.; Liu, Y.R.; Wullschleger, S.D.; Liang, L.Y.; Graham, D.E.; et al. Microbial community and functional gene changes in Arctic Tundra soils in a microcosm warming experiment. Front. Microbiol. 2017, 8, 1741. [Google Scholar] [CrossRef]
- Zogg, G.P.; Zak, D.R.; Ringelberg, D.B.; MacDonald, N.W.; Pregitzer, K.S.; White, D.C. Compositional and functional shifts in microbial communities due to soil warming. Soil Sci. Soc. Am. J. 1997, 61, 475–481. [Google Scholar] [CrossRef]
- Bradford, M.A.; Davies, C.A.; Frey, S.D.; Maddox, T.R.; Melillo, J.M.; Mohan, J.E.; Reynolds, J.F.; Treseder, K.K.; Wallenstein, M.D. Thermal adaptation of soil microbial respiration to elevated temperature. Ecol. Lett. 2008, 11, 1316–1327. [Google Scholar] [CrossRef] [Green Version]
- Chen, J.; Luo, Y.; Garcia-Palacios, P.; Cao, J.; Dacal, M.; Zhou, X.; Li, J.; Xia, J.; Niu, S.; Yang, H.; et al. Differential responses of carbon-degrading enzyme activities to warming: Implications for soil respiration. Glob. Chang. Biol. 2018, 24, 4816–4826. [Google Scholar] [CrossRef]
- Chen, J.; Luo, Y.; Xia, J.; Jiang, L.; Zhou, X.; Lu, M.; Liang, J.; Shi, Z.; Shelton, S.; Cao, J. Stronger warming effects on microbial abundances in colder regions. Sci. Rep. 2015, 5, 18032. [Google Scholar] [CrossRef] [Green Version]
- Kim, S.Y.; Freeman, C.; Fenner, N.; Kang, H. Functional and structural responses of bacterial and methanogen communities to 3-year warming incubation in different depths of peat mire. Appl. Soil Ecol. 2012, 57, 23–30. [Google Scholar] [CrossRef]
- Peltoniemi, K.; Laiho, R.; Juottonen, H.; Bodrossy, L.; Kell, D.K.; Minkkinen, K.; Makiranta, P.; Mehtatalo, L.; Penttila, T.; Siljanen, H.M.P.; et al. Responses of methanogenic and methanotrophic communities to warming in varying moisture regimes of two boreal fens. Soil Biol. Biochem. 2016, 97, 144–156. [Google Scholar] [CrossRef]
- Xue, K.; Yuan, M.M.; Shi, Z.J.; Qin, Y.J.; Deng, Y.; Cheng, L.; Wu, L.Y.; He, Z.L.; Van Nostrand, J.D.; Bracho, R.; et al. Tundra soil carbon is vulnerable to rapid microbial decomposition under climate warming. Nat. Clim. Chang. 2016, 6, 595–600. [Google Scholar] [CrossRef]
- Yergeau, E.; Bokhorst, S.; Kang, S.; Zhou, J.Z.; Greer, C.W.; Aerts, R.; Kowalchuk, G.A. Shifts in soil microorganisms in response to warming are consistent across a range of Antarctic environments. ISME J. 2012, 6, 692–702. [Google Scholar] [CrossRef] [PubMed]
- Kotroczó, Z.; Veres, Z.; Biró, B.; Tóth, J.A.; Fekete, I. Influence of temperature and organic matter content on soil respiration in a deciduous oak forest. Eurasian Soil Sci. 2014, 3, 303–310. [Google Scholar] [CrossRef]
- Peng, S.S.; Piao, S.L.; Wang, T.; Sun, J.Y.; Shen, Z.H. Temperature sensitivity of soil respiration in different ecosystems in China. Soil Biol. Biochem. 2009, 41, 1008–1014. [Google Scholar] [CrossRef]
- Supramaniam, Y.; Chong, C.W.; Silvaraj, S.; Tan, I.K.P. Effect of short term variation in temperature and water content on the bacterial community in a tropical soil. Appl. Soil Ecol. 2016, 107, 279–289. [Google Scholar] [CrossRef]
- Fekete, I.; Lajtha, K.; Kotroczo, Z.; Varbiro, G.; Varga, C.; Toth, J.A.; Demeter, I.; Veperdi, G.; Berki, I. Long-term effects of climate change on carbon storage and tree species composition in a dry deciduous forest. Glob. Chang. Biol. 2017, 23, 3154–3168. [Google Scholar] [CrossRef] [PubMed]
- Jung, J.; Yeom, J.; Han, J.; Kim, J.; Park, W. Seasonal changes in nitrogen-cycle gene abundances and in bacterial communities in acidic forest soils. J. Microbiol. 2012, 50, 365–373. [Google Scholar] [CrossRef]
- Rotthauwe, J.H.; Witzel, K.P.; Liesack, W. The ammonia monooxygenase structural gene amoA as a functional marker: Molecular fine-scale analysis of natural ammonia-oxidizing populations. Appl. Environ. Microb. 1997, 63, 4704–4712. [Google Scholar]
- Pierre, S.; Hewson, I.; Sparks, J.P.; Litton, C.M.; Giardina, C.; Groffman, P.M.; Fahey, T.J. Ammonia oxidizer populations vary with nitrogen cycling across a tropical montane mean annual temperature gradient. Ecology 2017, 98, 1896–1907. [Google Scholar] [CrossRef]
- Espenberg, M.; Truu, M.; Mander, U.; Kasak, K.; Nõlvak, H.; Ligi, T.; Oopkaup, K.; Maddison, M.; Truu, J. Differences in microbial community structure and nitrogen cycling in natural and drained tropical peatland soils. Sci. Rep. 2018, 8, 4742. [Google Scholar] [CrossRef] [PubMed]
- Penton, C.R.; Louis, D.S.; Pham, A.; Cole, J.R.; Wu, L.Y.; Luo, Y.Q.; Schuur, E.A.G.; Zhou, J.Z.; Tiedje, J.M. Denitrifying and diazotrophic community responses to artificial warming in permafrost and tallgrass prairie soils. Front. Microbiol. 2015, 6, 746. [Google Scholar] [CrossRef] [Green Version]
- Marx, M.C.; Wood, M.; Jarvis, S.C. A microplate fluorimetric assay for the study of enzyme diversity in soils. Soil Biol. Biochem. 2001, 33, 1633–1640. [Google Scholar] [CrossRef]
- Sinsabaugh, R.L.; Hill, B.H.; Shah, J.J.F. Ecoenzymatic stoichiometry of microbial organic nutrient acquisition in soil and sediment. Nature 2009, 462, 795–798. [Google Scholar] [CrossRef] [PubMed]
- Allison, S.D.; Treseder, K.K. Warming and drying suppress microbial activity and carbon cycling in boreal forest soils. Glob. Chang. Biol. 2008, 14, 2898–2909. [Google Scholar] [CrossRef] [Green Version]
- Gorham, E. Northern peatlands: Role in the carbon cycle and probable responses to climatic warming. Ecol. Appl. 1991, 1, 182–195. [Google Scholar] [CrossRef] [PubMed]
- Turunen, J.; Tomppo, E.; Tolonen, K.; Reinikainen, A. Estimating carbon accumulation rates of undrained mires in Finland—Application to boreal and subarctic regions. Holocene 2002, 12, 69–80. [Google Scholar] [CrossRef]
- Schlesinger, W.H. Biogeochemistry: An Analysis of Global Change; Academic Press: San Diego, CA, USA, 1997. [Google Scholar]
- Weedon, J.T.; Kowalchuk, G.A.; Aerts, R.; van Hal, J.; van Logtestijn, R.; Tas, N.; Roling, W.F.M.; van Bodegom, P.M. Summer warming accelerates sub-arctic peatland nitrogen cycling without changing enzyme pools or microbial community structure. Glob. Chang. Biol. 2012, 18, 138–150. [Google Scholar] [CrossRef]
- Lin, X.J.; Tfaily, M.M.; Green, S.J.; Steinweg, J.M.; Chanton, P.; Imvittaya, A.; Chanton, J.P.; Cooper, W.; Schadt, C.; Kostka, J.E. Microbial metabolic potential for carbon degradation and nutrient (nitrogen and phosphorus) acquisition in an ombrotrophic peatland. Appl. Environ. Microb. 2014, 80, 3531–3540. [Google Scholar] [CrossRef]
- Guo, J.T.; Hu, Y.M.; Xiong, Z.P.; Yan, X.L.; Li, C.L.; Bu, R.C. Variations in growing-season NDVI and its response to permafrost degradation in Northeast China. Sustainability 2017, 9, 551. [Google Scholar] [CrossRef]
- Cui, Q.; Song, C.C.; Wang, X.W.; Shi, F.X.; Yu, X.Y.; Tan, W.W. Effects of warming on N2O fluxes in a boreal peatland of Permafrost region, Northeast China. Sci. Total Environ. 2018, 616, 427–434. [Google Scholar] [CrossRef] [PubMed]
- Gong, S.W.; Zhang, T.; Guo, R.; Cao, H.B.; Shi, L.X.; Guo, J.X.; Sun, W. Response of soil enzyme activity to warming and nitrogen addition in a meadow steppe. Soil Res. 2015, 53, 242–252. [Google Scholar] [CrossRef]
- Ma, X.M.; Razavi, B.S.; Holz, M.; Blagodatskaya, E.; Kuzyakov, Y. Warming increases hotspot areas of enzyme activity and shortens the duration of hot moments in the root-detritusphere. Soil Biol. Biochem. 2017, 107, 226–233. [Google Scholar] [CrossRef]
- Shi, F.S.; Wu, Y.; Wu, N.; Luo, P. Different growth and physiological responses to experimental warming of two dominant plant species Elymus nutans and Potentilla anserina in an alpine meadow of the eastern Tibetan Plateau. Photosynthetica 2010, 48, 437–445. [Google Scholar] [CrossRef]
- Wu, J.; Joergensen, R.G.; Pommerening, B.; Chaussod, R.; Brookes, P.C. Measurement of soil microbial biomass C by fumigation–extraction–an automated procedure. Soil Biol. Biochem. 1990, 22, 1167–1169. [Google Scholar] [CrossRef]
- Ghani, A.; Dexter, M.; Perrott, K.W. Hot-water extractable carbon in soils: A sensitive measurement for determining impacts of fertilisation, grazing and cultivation. Soil Biol. Biochem. 2003, 35, 1231–1243. [Google Scholar] [CrossRef]
- Zhang, Z.; Schwartz, S.; Wagner, L.; Miller, W. A greedy algorithm for aligning DNA sequences. J. Comput. Biol. 2000, 7, 203–214. [Google Scholar] [CrossRef] [PubMed]
- Tabatabai, M.A. Methods of Soil Analysis: Microbiological and Biochemical Properties; Soil Science Society of America: Baltimore, MD, USA, 1994; pp. 236–268. [Google Scholar]
- Guan, S.Y. Soil Enzymology and Research Method; Agricultural Press: Beijing, China, 1986. [Google Scholar]
- Zhao, L.P.; Jiang, Y. Measure method of soil phosphatase. Chin. J. Soil Sci. 1986, 17, 138–141. [Google Scholar]
- Zhang, H.; Tang, J.; Liang, S.; Li, Z.Y.; Yang, P.; Wang, J.J.; Wang, S.N. The emissions of carbon dioxide, methane, and nitrous oxide during winter without cultivation in local saline-alkali rice and maize fields in northeast China. Sustainability 2017, 9, 1916. [Google Scholar] [CrossRef]
- Yang, G.; Wang, M.; Chen, H.; Liu, L.F.; Wu, N.; Zhu, D.; Tian, J.Q.; Peng, C.H.; Zhu, Q.; He, Y.X. Responses of CO2 emission and pore water DOC concentration to soil warming and water table drawdown in Zoige Peatlands. Atmos. Environ. 2017, 152, 323–329. [Google Scholar] [CrossRef]
- Song, Y.Y.; Song, C.C.; Hou, A.X.; Ren, J.S.; Wang, X.W.; Cui, Q.; Wang, M.Q. Effects of temperature and root additions on soil carbon and nitrogen mineralization in a predominantly permafrost peatland. Catena 2018, 165, 381–389. [Google Scholar] [CrossRef]
- Rinnan, R.; Michelsen, A.; Bååth, E.; Jonasson, S. Fifteen years of climate change manipulations alter soil microbial communities in a subarctic heath ecosystem. Glob. Chang. Biol. 2007, 13, 28–39. [Google Scholar] [CrossRef]
- Hou, R.X.; Ouyang, Z.; Maxim, D.; Wilson, G.; Kuzyakov, Y. Lasting effect of soil warming on organic matter decomposition depends on tillage practices. Soil Biol. Biochem. 2016, 95, 243–249. [Google Scholar] [CrossRef] [Green Version]
- Chen, D.D.; Zhao, L.; Li, Q.; Cai, H.; Li, J.M.; Xu, S.X.; Zhao, X.Q. Response of soil carbon and nitrogen to 15-year experimental warming in two alpine habitats (kobresia meadow and potentilla shrubland) on the Qinghai-Tibetan Plateau. Pol. J. Environ. Stud. 2016, 25, 2305–2313. [Google Scholar] [CrossRef]
- Yue, H.W.; Wang, M.M.; Wang, S.P.; Gilbert, J.A.; Sun, X.; Wu, L.W.; Lin, Q.Y.; Hu, Y.G.; Li, X.Z.; He, Z.L.; et al. The microbe-mediated mechanisms affecting topsoil carbon stock in Tibetan grasslands. ISME J. 2015, 9, 2012–2020. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rustad, L.E.; Campbell, J.L.; Marion, G.M.; Norby, R.J.; Mitchell, M.J.; Hartley, A.E.; Cornelissen, J.H.C.; Gurevitch, J.; Gcte, N. A meta-analysis of the response of soil respiration, net nitrogen mineralization, and aboveground plant growth to experimental ecosystem warming. Oecologia 2001, 126, 543–562. [Google Scholar] [CrossRef]
- Xu, Z.F.; Hu, R.; Xiong, P.; Wan, C.; Cao, G.; Liu, Q. Initial soil responses to experimental warming in two contrasting forest ecosystems, Eastern Tibetan Plateau, China: Nutrient availabilities, microbial properties and enzyme activities. Appl. Soil Ecol. 2010, 46, 291–299. [Google Scholar] [CrossRef] [Green Version]
- Sardans, J.; Peñuelas, J.; Estiarte, M. Changes in soil enzymes related to C and N cycle and in soil C and N content under prolonged warming and drought in a Mediterranean shrubland. Appl. Soil Ecol. 2008, 39, 223–235. [Google Scholar] [CrossRef]
- Alatalo, J.M.; Jägerbrand, A.K.; Juhanson, J.; Michelsen, A.; Ľuptáčik, P. Impacts of twenty years of experimental warming on soil carbon, nitrogen, moisture and soil mites across alpine/subarctic tundra communities. Sci. Rep. 2017, 7, 44489. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chang, R.Y.; Wang, G.X.; Yang, Y.H.; Chen, X.P. Experimental warming increased soil nitrogen sink in the Tibetan permafrost. J. Geophys. Res. Biogeosci. 2017, 122, 1870–1879. [Google Scholar] [CrossRef]
- Wallenstein, M.D.; Hall, E.K. A trait-based framework for predicting when and where microbial adaptation to climate change will affect ecosystem functioning. Biogeochemistry 2012, 109, 35–47. [Google Scholar] [CrossRef]
- Stark, S.; Männistö, M.K.; Ganzert, L.; Tiirola, M.; Häggblom, M.M. Grazing intensity in subarctic tundra affects the temperature adaptation of soil microbial communities. Soil Biol. Biochem. 2015, 84, 147–157. [Google Scholar] [CrossRef]
- Wang, H.; Yang, J.P.; Yang, S.H.; Yang, Z.C.; Lv, Y.M. Effect of a 10 degrees C-elevated temperature under different water contents on the microbial community in a tea orchard soil. Eur. J. Soil Biol. 2014, 62, 113–120. [Google Scholar] [CrossRef]
- Bárcenas Moreno, G.; Gómez Brandón, M.; Rousk, J.; Bååth, E. Adaptation of soil microbial communities to temperature: Comparison of fungi and bacteria in a laboratory experiment. Glob. Chang. Biol. 2009, 15, 2950–2957. [Google Scholar] [CrossRef]
- Hayden, H.L.; Mele, P.M.; Bougoure, D.S.; Allan, C.Y.; Norng, S.; Piceno, Y.M.; Brodie, E.L.; DeSantis, T.Z.; Andersen, G.L.; Williams, A.L.; et al. Changes in the microbial community structure of bacteria, archaea and fungi in response to elevated CO2 and warming in an Australian native grassland soil. Environ. Microb. 2012, 14, 3081–3096. [Google Scholar] [CrossRef] [PubMed]
- Rui, J.P.; Li, J.B.; Wang, S.P.; An, J.X.; Liu, W.T.; Lin, Q.Y.; Yang, Y.F.; He, Z.L.; Li, X.Z. Responses of bacterial communities to simulated climate changes in alpine meadow soil of the Qinghai-Tibet Plateau. Appl. Environ. Microb. 2015, 81, 6070–6077. [Google Scholar] [CrossRef] [PubMed]
- Zumsteg, A.; Bääth, E.; Stierli, B.; Zeyer, J.; Frey, B. Bacterial and fungal community responses to reciprocal soil transfer along a temperature and soil moisture gradient in a glacier forefield. Soil Biol. Biochem. 2013, 61, 121–132. [Google Scholar] [CrossRef]
- Bastida, F.; Torres, I.F.; Moreno, J.L.; Baldrian, P.; Ondono, S.; Ruiz Navarro, A.; Hernandez, T.; Richnow, H.H.; Starke, R.; Garcia, C.; et al. The active microbial diversity drives ecosystem multifunctionality and is physiologically related to carbon availability in Mediterranean semi-arid soils. Mol. Ecol. 2016, 25, 4660–4673. [Google Scholar] [CrossRef]
- Wang, H.; He, Z.L.; Lu, Z.M.; Zhou, J.Z.; Van Nostrand, J.D.; Xu, X.H.; Zhang, Z.J. Genetic linkage of soil carbon pools and microbial functions in subtropical freshwater wetlands in response to experimental warming. Appl. Environ. Microb. 2012, 78, 7652–7661. [Google Scholar] [CrossRef]
- Liu, D.; Keiblinger, K.M.; Schindlbacher, A.; Wegner, U.; Sun, H.; Fuchs, S.; Lassek, C.; Riedel, K.; Zechmeister-Boltenstern, S. Microbial functionality as affected by experimental warming of a temperate mountain forest soil-A metaproteomics survey. Appl. Soil Ecol. 2017, 117, 196–202. [Google Scholar] [CrossRef]
- Rinnan, R.; Rousk, J.; Yergeau, E.; Kowalchuk, G.A.; Bååth, E. Temperature adaptation of soil bacterial communities along an Antarctic climate gradient: Predicting responses to climate warming. Glob. Chang. Biol. 2009, 15, 2615–2625. [Google Scholar] [CrossRef]
- Liu, Y.; Li, M.; Zheng, J.W.; Li, L.Q.; Zhang, X.H.; Zheng, J.F.; Pan, G.X.; Yu, X.Y.; Wang, J.F. Short-term responses of microbial community and functioning to experimental CO2 enrichment and warming in a Chinese paddy field. Soil Biol. Biochem. 2014, 77, 58–68. [Google Scholar] [CrossRef]
- Bomberg, M.; Münster, U.; Pumpanen, J.; Ilvesniemi, H.; Heinonsalo, J. Archaeal communities in boreal forest tree rhizospheres respond to changing soil temperatures. Microb. Ecol. 2011, 62, 205–217. [Google Scholar] [CrossRef] [PubMed]
- Rasche, F.; Knapp, D.; Kaiser, C.; Koranda, M.; Kitzler, B.; Zechmeister-Boltenstern, S.; Richter, A.; Sessitsch, A. Seasonality and resource availability control bacterial and archaeal communities in soils of a temperate beech forest. ISME J. 2011, 5, 389–402. [Google Scholar] [CrossRef] [PubMed]
- Martins, C.S.C.; Nazaries, L.; Delgado-Baquerizo, M.; Macdonald, C.A.; Anderson, I.C.; Hobbie, S.E.; Venterea, R.T.; Reich, P.B.; Singh, B.K. Identifying environmental drivers of greenhouse gas emissions under warming and reduced rainfall in boreal-temperate forests. Funct. Ecol. 2017, 31, 2356–2368. [Google Scholar] [CrossRef]
- Han, J.; Jung, J.; Park, M.; Hyun, S.; Park, W. Short-term effect of elevated temperature on the abundance and diversity of bacterial and archaeal amoA genes in Antarctic soils. J. Microbiol. Biotechnol. 2013, 23, 1187–1196. [Google Scholar] [CrossRef]
- Fierer, N.; Carney, K.M.; Horner-Devine, M.C.; Megonigal, J.P. The biogeography of ammonia-oxidizing bacterial communities in Soil. Microb. Ecol. 2009, 58, 435–445. [Google Scholar] [CrossRef]
- Fuchs, A.; Lyautey, E.; Montuelle, B.; Casper, P. Effects of increasing temperatures on methane concentrations and methanogenesis during experimental incubation of sediments from oligotrophic and mesotrophic lakes. J. Geophys. Res. Biogeosci. 2016, 121, 1394–1406. [Google Scholar] [CrossRef]
- Conrad, R.; Klose, M.; Noll, M. Functional and structural response of the methanogenic microbial community in rice field soil to temperature change. Environ. Microbiol. 2009, 11, 1844–1853. [Google Scholar] [CrossRef]
- Wilson, R.M.; Hopple, A.M.; Tfaily, M.M.; Sebestyen, S.D.; Schadt, C.W.; Pfeifer-Meister, L.; Medvedeff, C.; McFarlane, K.J.; Kostka, J.E.; Kolton, M.; et al. Stability of peatland carbon to rising temperatures. Nat. Commun. 2016, 7, 13723. [Google Scholar] [CrossRef] [Green Version]
- Liu, D.Y.; Tago, K.; Hayatsu, M.; Tokida, T.; Sakai, H.; Nakamura, H.; Usui, Y.; Hasegawa, T.; Asakawa, S. Effect of elevated CO2 concentration, elevated temperature and no nitrogen fertilization on methanogenic archaeal and methane-oxidizing bacterial community structures in paddy soil. Microbes Environ. 2016, 31, 349–356. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Yang, S.H.; Yang, J.P.; Lv, Y.M.; Zhao, X.; Pang, J.L. Temporal changes in soil bacterial and archaeal communities with different fertilizers in tea orchards. J. Zhejiang Univ. Sci. B. 2014, 15, 953–965. [Google Scholar] [CrossRef] [PubMed]
- Pajares, S.; Merino Ibarra, M.; Macek, M.; Alcocer, J. Vertical and seasonal distribution of picoplankton and functional nitrogen genes in a high-altitude warm-monomictic tropical lake. Freshw. Biol. 2017, 62, 1180–1193. [Google Scholar] [CrossRef]
- Szukics, U.; Abell, G.C.J.; Hoedl, V.; Mitter, B.; Sessitsch, A.; Hackl, E.; Zechmeister-Boltenstern, S. Nitrifiers and denitrifiers respond rapidly to changed moisture and increasing temperature in a pristine forest soil. FEMS Microbiol. Ecol. 2010, 72, 395–406. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Di, H.J.; Cameron, K.C.; Podolyan, A.; Robinson, A. Effect of soil moisture status and a nitrification inhibitor, dicyandiamide, on ammonia oxidizer and denitrifier growth and nitrous oxide emissions in a grassland soil. Soil Biol. Biochem. 2014, 73, 59–68. [Google Scholar] [CrossRef]
- Hallin, S.; Jones, C.M.; Schloter, M.; Philippot, L. Relationship between N-cycling communities and ecosystem functioning in a 50-year-old fertilization experiment. ISME J. 2009, 3, 597–605. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Graham, D.W.; Trippett, C.; Dodds, W.K.; O’Brien, J.M.; Banner, E.B.K.; Head, I.M.; Smith, M.S.; Yang, R.K.; Knapp, C.W. Correlations between in situ denitrification activity and nir-gene abundances in pristine and impacted prairie streams. Environ. Pollut. 2010, 158, 3225–3229. [Google Scholar] [CrossRef]
- Zhang, X.M.; Liu, W.; Schloter, M.; Zhang, G.M.; Chen, Q.S.; Huang, J.H.; Li, L.H.; Elser, J.J.; Han, X.G. Response of the abundance of key soil microbial nitrogen-cycling genes to multi-factorial global changes. PLoS ONE 2013, 8, e76500. [Google Scholar] [CrossRef]
- Cantarel, A.A.M.; Bloor, J.M.G.; Pommier, T.; Guillaumaud, N.; Moirot, C.; Soussana, J.F.; Poly, F. Four years of experimental climate change modifies the microbial drivers of N2O fluxes in an upland grassland ecosystem. Glob. Chang. Biol. 2012, 18, 2520–2531. [Google Scholar] [CrossRef]
- Jung, J.; Yeom, J.; Kim, J.; Han, J.; Lim, H.S.; Park, H.; Hyun, S.; Park, W. Change in gene abundance in the nitrogen biogeochemical cycle with temperature and nitrogen addition in Antarctic soils. Res. Microbiol. 2011, 162, 1018–1026. [Google Scholar] [CrossRef]
- Schindlbacher, A.; Schnecker, J.; Takriti, M.; Borken, W.; Wanek, W. Microbial physiology and soil CO2 efflux after 9 years of soil warming in a temperate forest—No indications for thermal adaptations. Glob. Chang. Biol. 2015, 21, 4265–4277. [Google Scholar] [CrossRef] [PubMed]
- Eliasson, P.E.; McMurtrie, R.E.; Pepper, D.A.; Stromgren, M.; Linder, S.; Agren, G.I. The response of heterotrophic CO2 flux to soil warming. Glob. Chang. Biol. 2005, 11, 167–181. [Google Scholar] [CrossRef]
- Wallenstein, M.; Allison, S.D.; Ernakovich, J.; Steinweg, J.M.; Sinsabaugh, R. Controls on the temperature sensitivity of soil enzymes: A key driver of situ enzyme activity rates. In Soil Enzymology; Springer: Berlin/Heidelberg, Germany, 2010; pp. 245–258. [Google Scholar]
- German, D.P.; Chacon, S.S.; Allison, S.D. Substrate concentration and enzyme allocation can affect rates of microbial decomposition. Ecology 2011, 92, 1471–1480. [Google Scholar] [CrossRef] [PubMed]
- Zantua, M.I.; Bremner, J.M. Stability of urease in soils. Soil Biol. Biochem. 1977, 9, 135–140. [Google Scholar] [CrossRef]
- Amador, J.A.; Glucksman, A.M.; Lyons, J.B.; Gorres, J.H. Spatial distribution of soil phosphatase activity within a riparian forest. Soil Sci. 1997, 162, 808–825. [Google Scholar] [CrossRef]
- Sardans, J.; Peñuelas, J.; Estiarte, M. Warming and drought alter soil phosphatase activity and soil P availability in a Mediterranean shrubland. Plant Soil. 2006, 289, 227–238. [Google Scholar] [CrossRef]
- Delarue, F.; Buttler, A.; Bragazza, L.; Grasset, L.; Jassey, V.E.J.; Gogo, S.; Laggoun-Défarge, F. Experimental warming differentially affects microbial structure and activity in two contrasted moisture sites in a Sphagnum-dominated peatland. Sci. Total Environ. 2015, 511, 576–583. [Google Scholar] [CrossRef] [PubMed] [Green Version]







| Bacteria 16S rRNA | Archaea 16S rRNA | mcrA | pmoA | amoA | nirS | nirK | |
|---|---|---|---|---|---|---|---|
| MBC | 0.359 | –0.427 | 0.288 | 0.653 ** | −0.529 * | 0.146 | 0.608 * |
| DOC | 0.084 | –0.262 | 0.395 | 0.229 | 0.134 | 0.558 * | 0.477 |
| NH4+–N | 0.417 | −0.046 | 0.422 | 0.726 ** | −0.609 * | 0.321 | 0.607 * |
| NO3−N | 0.150 | 0.321 | 0.146 | −0.043 | 0.201 | −0.009 | −0.095 |
| TC | 0.121 | −0.135 | -0.051 | −0.026 | 0.022 | −0.265 | −0.344 |
| TN | 0.164 | 0.141 | 0.299 | 0.512 * | −0.402 | 0.008 | 0.297 |
| β-Glucosidase | Invertase | Urease | Acid Phosphotase | |
|---|---|---|---|---|
| MBC | 0.791 ** | 0.633 ** | 0.204 | –0.370 |
| DOC | 0.059 | 0.848 ** | 0.690 ** | –0.238 |
| NH4+–N | 0.735 ** | 0.597 * | 0.164 | –0.470 |
| NO3−–N | −0.016 | –0.199 | −0.155 | 0.424 |
| TC | 0.153 | –0.426 | −0.377 | 0.366 |
| TN | 0.595 * | –0.077 | −0.273 | 0.005 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Song, Y.; Song, C.; Ren, J.; Ma, X.; Tan, W.; Wang, X.; Gao, J.; Hou, A. Short-Term Response of the Soil Microbial Abundances and Enzyme Activities to Experimental Warming in a Boreal Peatland in Northeast China. Sustainability 2019, 11, 590. https://doi.org/10.3390/su11030590
Song Y, Song C, Ren J, Ma X, Tan W, Wang X, Gao J, Hou A. Short-Term Response of the Soil Microbial Abundances and Enzyme Activities to Experimental Warming in a Boreal Peatland in Northeast China. Sustainability. 2019; 11(3):590. https://doi.org/10.3390/su11030590
Chicago/Turabian StyleSong, Yanyu, Changchun Song, Jiusheng Ren, Xiuyan Ma, Wenwen Tan, Xianwei Wang, Jinli Gao, and Aixin Hou. 2019. "Short-Term Response of the Soil Microbial Abundances and Enzyme Activities to Experimental Warming in a Boreal Peatland in Northeast China" Sustainability 11, no. 3: 590. https://doi.org/10.3390/su11030590
APA StyleSong, Y., Song, C., Ren, J., Ma, X., Tan, W., Wang, X., Gao, J., & Hou, A. (2019). Short-Term Response of the Soil Microbial Abundances and Enzyme Activities to Experimental Warming in a Boreal Peatland in Northeast China. Sustainability, 11(3), 590. https://doi.org/10.3390/su11030590