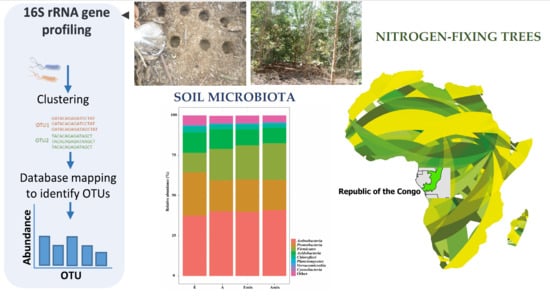
Influence of Acacia mangium on Soil Fertility and Bacterial Community in Eucalyptus Plantations in the Congolese Coastal Plains
Abstract
:1. Introduction
2. Materials and Methods
2.1. Site Description, Experimental Design, and Sampling
2.1.1. Location, Soil Classification, Climate, and Previous Vegetation Cover
2.1.2. Experimental Design and History
2.1.3. Soil Sampling
2.2. Soil Carbon, Nitrogen, Sulfur Concentration, and Available Phosphorus Analyses
2.3. DNA Extraction and Quality Assessment
2.4. Illumina 16S Library Construction and Sequencing
2.5. Bioinformatic and Statistical Analysis
3. Results
3.1. Soil Nitrogen, Carbon, Sulfur Concentrations, and Available Phosphorus
3.2. Sequencing Data and Overall Composition of Bacterial Community along with the Field Sites
3.3. Bacterial Alpha and Beta Diversity
3.4. Relationship between Soil Microbiota and Soil Characteristics
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Bernhard-Reversat, F. Dynamics of Litter and Organic Matter at the Soil-Litter Interface in Fast-Growing Tree Plantations on Sandy Ferrallitic Soils (Congo). Acta Ecol. 1993, 14, 179–195. [Google Scholar]
- Laclau, J.P.; Bouillet, J.P.; Gonçalves, J.L.M.; Silva, E.V.; Jourdan, C.; Cunha, M.C.S.; Moreira, M.R.; Saint-Andr, L.; Maquère, V.; Nouvellon, Y.; et al. Mixed-Species Plantations of Acacia Mangium and Eucalyptus Grandis in Brazil: 1. Growth Dynamics and Aboveground Net Primary Production. For. Ecol. Manag. 2008, 255, 3905–3917. [Google Scholar] [CrossRef]
- Bouillet, J.P.; Laclau, J.P.; Gonçalves, J.L.M.; Voigtlaender, M.; Gava, J.L.; Leite, F.P.; Hakamada, R.; Mareschal, L.; Mabiala, A.; Tardy, F.; et al. Eucalyptus and Acacia Tree Growth over Entire Rotation in Single- and Mixed-Species Plantations across Five Sites in Bra References 24 and 64 are the same. Please check whether it should be replaced with another publication or not. If not, please delete one of them and renumber the references, and revise citation in main text.zil and Congo. For. Ecol. Manag. 2013, 301, 89–101. [Google Scholar] [CrossRef]
- Epron, D.; Nouvellon, Y.; Mareschal, L.; Moreira, R.M.E.; Koutika, L.-S.; Geneste, B.; Delgado-Rojas, J.S.; Laclau, J.-P.; Sola, G.; Gonçalves, J.L.M.; et al. Partitioning of Net Primary Production in Eucalyptus and Acacia Stands and in Mixed-Species Plantations: Two Case-Studies in Contrasting Tropical Environments. For. Ecol. Manag. 2013, 301, 102–111. [Google Scholar] [CrossRef]
- Koutika, L.S.; Epron, D.; Bouillet, J.P.; Mareschal, L. Changes in N and C Concentrations, Soil Acidity and P Availability in Tropical Mixed Acacia and Eucalypt Plantations on a Nutrient-Poor Sandy Soil. Plant Soil 2014, 379, 205–216. [Google Scholar] [CrossRef]
- Koutika, L.S.; Mareschal, L. Acacia and Eucalypt Change P, N and C Concentrations in POM of Arenosols in the Congolese Coastal Plains. Geoderma Reg. 2017, 11, 37–43. [Google Scholar] [CrossRef]
- Tchichelle, S.V.; Epron, D.; Mialoundama, F.; Koutika, L.S.; Harmand, J.M.; Bouillet, J.P.; Mareschal, L. Differences in Nitrogen Cycling and Soil Mineralisation between a Eucalypt Plantation and a Mixed Eucalypt and Acacia Mangium Plantation on a Sandy Tropical Soil. South. For. 2017, 79, 1–8. [Google Scholar] [CrossRef] [Green Version]
- Koutika, L.S. Afforesting Savannas with Acacia Mangium and Eucalyptus Improves P Availability in Arenosols of the Congolese Coastal Plains. Geoderma Reg. 2019, e00207. [Google Scholar] [CrossRef]
- Binkley, D. Mixtures Nitrogen-Fixing and Non-Nitrogen-Fixing Tree Species. In The Ecology of Mixed-Species Stands of Trees; Cannell, M.G.R., Malcolm, D.C., Robertson, P.A., Eds.; Blackwell Scientific Publications: Oxford, UK, 1992; pp. 99–124. [Google Scholar]
- Inagaki, M.; Kamo, K.; Miyamoto, K.; Titin, J.; Jamalung, L.; Lapongan, J.; Miura, S. Nitrogen and Phosphorus Retranslocation and N:P Ratios of Litterfall in Three Tropical Plantations: Luxurious N and Efficient P Use by Acacia Mangium. Plant Soil 2011, 341, 295–307. [Google Scholar] [CrossRef]
- Koutika, L.S.; Mareschal, L.; Epron, D. Soil P Availability under Eucalypt and Acacia on Ferralic Arenosols, Republic of the Congo. Geoderma Reg. 2016, 7, 153–158. [Google Scholar] [CrossRef]
- Waithaisong, K.; Robin, A.; Mareschal, L.; Bouillet, J.P.; Laclau, J.P.; Deleporte, P.; Gonçalves, J.L.M.; Harmand, J.M.; Plassard, C. Introducing N2-Fixing Trees (Acacia Mangium) in Eucalypt Plantations Rapidly Modifies the Pools of Organic P and Low Molecular Weight Organic Acids in Tropical Soils. Sci. Total Environ. 2020, 742, 140535. [Google Scholar] [CrossRef] [PubMed]
- Koutika, L.S.; Cafiero, L.; Bevivino, A.; Merino, A. Organic Matter Quality of Forest Floor as a Driver of C and P Dynamics in Acacia and Eucalypt Plantations Established on a Ferralic Arenosols, Congo. For. Ecosyst. 2020, 7, 40. [Google Scholar] [CrossRef]
- Paquette, A.; Messier, C. The Role of Plantations in Managing the World’s Forests in the Anthropocene. Front. Ecol. Environ. 2010, 8, 27–34. [Google Scholar] [CrossRef] [Green Version]
- Kaiser, K.; Wemheuer, B.; Korolkow, V.; Wemheuer, F.; Nacke, H.; Schöning, I.; Schrumpf, M.; Daniel, R. Driving Forces of Soil Bacterial Community Structure, Diversity, and Function in Temperate Grasslands and Forests. Sci. Rep. 2016, 6, 33696. [Google Scholar] [CrossRef] [Green Version]
- Lladó, S.; López-Mondéjar, R.; Baldrian, P. Forest Soil Bacteria: Diversity, Involvement in Ecosystem Processes, and Response to Global Change. Microbiol. Mol. Biol. Rev. 2017, 81, e00063. [Google Scholar] [CrossRef] [Green Version]
- Rumpel, C.; Amiraslani, F.; Chenu, C.; Garcia Cardenas, M.; Kaonga, M.; Koutika, L.S.; Ladha, J.; Madari, B.; Shirato, Y.; Smith, P.; et al. The 4p1000 Initiative: Opportunities, Limitations and Challenges for Implementing Soil Organic Carbon Sequestration as a Sustainable Development Strategy. Ambio 2020, 49, 350–360. [Google Scholar] [CrossRef] [Green Version]
- Mercado-Blanco, J.; Abrantes, I.; Barra Caracciolo, A.; Bevivino, A.; Ciancio, A.; Grenni, P.; Hrynkiewicz, K.; Kredics, L.; Proença, D.N. Belowground Microbiota and the Health of Tree Crops. Front. Microbiol. 2018, 9, 1006. [Google Scholar] [CrossRef] [Green Version]
- Fonseca, E.D.S.; Peixoto, R.S.; Rosado, A.S.; Balieiro, F.D.C.; Tiedje, J.M.; Rachid, C.T.C.D.C. The Microbiome of Eucalyptus Roots under Different Management Conditions and Its Potential for Biological Nitrogen Fixation. Microb. Ecol. 2018, 75, 183–191. [Google Scholar] [CrossRef]
- Bini, D.; Dos Santos, C.A.; Bouillet, J.-P.; Gonçalves, J.L.M.; Cardoso, E.J.B.N. Eucalyptus Grandis and Acacia Mangium in Monoculture and Intercropped Plantations: Evolution of Soil and Litter Microbial and Chemical Attributes during Early Stages of Plant Development. Appl. Soil Ecol. 2013, 63, 57–66. [Google Scholar] [CrossRef]
- Bini, D.; Figueiredo, A.F.; Silva, M.C.P.; Vasconcellos, R.L.F.; Cardoso, E.J.B.N. Microbial Biomass and Activity in Litter during the Initial Development of Pure and Mixed Plantations of Eucalyptus Grandis and Acacia Mangium. Rev. Bras. Ciência Solo 2013, 37, 76–85. [Google Scholar] [CrossRef] [Green Version]
- De Araujo Pereira, A.P.; De Andrade, P.A.M.; Bini, D.; Durrer, A.; Robin, A.; Bouillet, J.P.; Andreote, F.D.; Cardoso, E.J.B.N. Shifts in the Bacterial Community Composition along Deep Soil Profiles in Monospecific and Mixed Stands of Eucalyptus Grandis and Acacia Mangium. PLoS ONE 2017, 12, e018037. [Google Scholar] [CrossRef] [Green Version]
- Pereira, A.P.A.; Zagatto, M.R.G.; Brandani, C.B.; Mescolotti, D.D.L.; Cotta, S.R.; Gonçalves, J.L.M.; Cardoso, E.J.B.N. Acacia Changes Microbial Indicators and Increases C and N in Soil Organic Fractions in Intercropped Eucalyptus Plantations. Front. Microbiol. 2018, 9, 655. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hartmann, M.; Niklaus, P.A.; Zimmermann, S.; Schmutz, S.; Kremer, J.; Abarenkov, K.; Lüscher, P.; Widmer, F.; Frey, B. Resistance and Resilience of the Forest Soil Microbiome to Logging-Associated Compaction. ISME J. 2014, 8, 226–244. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.; Liu, S.; You, Y.; Wen, Y.; Wang, H.; Wang, J. Microbial Community and Associated Enzymes Activity Influence Soil Carbon Chemical Composition in Eucalyptus Urophylla Plantation with Mixing N2-Fixing Species in Subtropical China. Plant Soil 2017, 414, 199–212. [Google Scholar] [CrossRef]
- Santos, F.M.; Chaer, G.M.; Diniz, A.R.; Balieiro, F.C. Nutrient Cycling over Five Years of Mixed-Species Plantations of Eucalyptus and Acacia on a Sandy Tropical Soil. For. Ecol. Manag. 2017, 384, 110–121. [Google Scholar] [CrossRef]
- Chernov, T.I.; Zhelezova, A.D.; Tkhakakhova, A.K.; Bgazhba, N.A.; Zverev, A.O. Microbiomes of Virgin Soils of Southern Vietnam Tropical Forests. Microbiology 2019, 88, 489–498. [Google Scholar] [CrossRef]
- Li, Y.; Tian, D.; Wang, J.; Niu, S.; Tian, J.; Ha, D.; Qu, Y.; Jing, G.; Kang, X.; Song, B. Differential Mechanisms Underlying Responses of Soil Bacterial and Fungal Communities to Nitrogen and Phosphorus Inputs in a Subtropical Forest. PeerJ 2019, 7, e7631. [Google Scholar] [CrossRef]
- Xu, Y.; Fan, J.; Ding, W.; Bol, R.; Chen, Z.; Luo, J.; Bolan, N. Stage-Specific Response of Litter Decomposition to N and S Amendments in a Subtropical Forest Soil. Biol. Fertil. Soils 2016, 52, 711–724. [Google Scholar] [CrossRef]
- Plassard, C.; Louche, J.; Ali, M.A.; Duchemin, M.; Legname, E.; Cloutier-Hurteau, B. Diversity in Phosphorus Mobilisation and Uptake in Ectomycorrhizal Fungi. Ann. For. Sci. 2011, 68, 33–43. [Google Scholar] [CrossRef] [Green Version]
- Dukunde, A.; Schneider, D.; Schmidt, M.; Veldkamp, E.; Daniel, R. Tree Species Shape Soil Bacterial Community Structure and Function in Temperate Deciduous Forests. Front. Microbiol. 2019, 10, 1519. [Google Scholar] [CrossRef]
- Bernhard-Reversat, F.; Schwartz, D. Change in Lignin Content during Litter Decomposition in Tropical Forest Soils (Congo): Comparison of Exotic Plantations and Native Stands. Comptes Rendus l’Académie Sci. Ser. IIA Earth Planet. Sci. 1997, 325, 427–432. [Google Scholar] [CrossRef]
- Spaargaren, O.C.; Deckers, J. The World Reference Base for Soil Resources. In Soils of Tropical Forest Ecosystems; Schulte, A., Ruhiyat, D., Eds.; Springer: Berlin/Heidelberg, Germany, 1998; pp. 21–28. [Google Scholar] [CrossRef]
- Mareschal, L.; Nzila, J.D.D.; Turpault, M.P.; Thongo M’Bou, A.; Mazoumbou, J.C.; Bouillet, J.P.; Ranger, J.; Laclau, J.P. Mineralogical and Physico-Chemical Properties of Ferralic Arenosols Derived from Unconsolidated Plio-Pleistocenic Deposits in the Coastal Plains of Congo. Geoderma 2011, 162, 159–170. [Google Scholar] [CrossRef]
- Epron, D.; Laclau, J.P.; Almeida, J.C.R.; Gonalves, J.L.M.; Ponton, S.; Sette, C.R.; Delgado-Rojas, J.S.; Bouillet, J.P.; Nouvellon, Y. Do Changes in Carbon Allocation Account for the Growth Response to Potassium and Sodium Applications in Tropical Eucalyptus Plantations? Tree Physiol. 2012, 32, 667–679. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- d’Annunzio, R.; Conche, S.; Landais, D.; Saint-André, L.; Joffre, R.; Barthès, B.G. Pairwise Comparison of Soil Organic Particle-Size Distributions in Native Savannas and Eucalyptus Plantations in Congo. For. Ecol. Manag. 2008. [Google Scholar] [CrossRef]
- Zagatto, M.R.G.; Pereira, A.P.A.; Souza, A.J.; Pereira, R.F.; Baldesin, L.F.; Pereira, C.M.; Lopes, R.V.; Cardoso, E.J.B.N. Interactions between Mesofauna, Microbiological and Chemical Soil Attributes in Pure and Intercropped Eucalyptus Grandis and Acacia Mangium Plantations. For. Ecol. Manag. 2008, 255, 1050–1056. [Google Scholar] [CrossRef]
- Moir, J.; Tiessen, H. Characterization of Available P by Sequential Extraction. In Soil Sampling and Methods of Analysis, Second Edition; Carter, M.R., Gregorich, E.G., Eds.; Lewis Publishers: Boca Raton, FL, USA, 2007; pp. 75–86. [Google Scholar] [CrossRef]
- Takahashi, S.; Tomita, J.; Nishioka, K.; Hisada, T.; Nishijima, M. Development of a Prokaryotic Universal Primer for Simultaneous Analysis of Bacteria and Archaea Using Next-Generation Sequencing. PLoS ONE 2014, 9, e105592. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Caporaso, J.G.; Kuczynski, J.; Stombaugh, J.; Bittinger, K.; Bushman, F.D.; Costello, E.K.; Fierer, N.; Peña, A.G.; Goodrich, J.K.; Gordon, J.I.; et al. Correspondence QIIME Allows Analysis of High- Throughput Community Sequencing Data Intensity Normalization Improves Color Calling in SOLiD Sequencing. Nat. Publ. Gr. 2010, 7, 335–336. [Google Scholar] [CrossRef]
- Kopylova, E.; Navas-Molina, J.A.; Mercier, C.; Xu, Z.Z.; Mahé, F.; He, Y.; Zhou, H.-W.; Rognes, T.; Caporaso, J.G.; Knight, R. Open-Source Sequence Clustering Methods Improve the State of the Art. mSystems 2016, 1, e00003-15. [Google Scholar] [CrossRef] [Green Version]
- Quast, C.; Pruesse, E.; Yilmaz, P.; Gerken, J.; Schweer, T.; Yarza, P.; Peplies, J.; Glöckner, F.O. The SILVA Ribosomal RNA Gene Database Project: Improved Data Processing and Web-Based Tools. Nucleic Acids Res. 2013, 41, D590–D596. [Google Scholar] [CrossRef] [PubMed]
- Edgar, R.C. Search and Clustering Orders of Magnitude Faster than BLAST. Bioinformatics 2010, 26, 2460–2461. [Google Scholar] [CrossRef] [Green Version]
- Ramette, A. Multivariate Analyses in Microbial Ecology. FEMS Microbiol. Ecol. 2007, 62, 142–160. [Google Scholar] [CrossRef] [Green Version]
- Vázquez-Baeza, Y.; Pirrung, M.; Gonzalez, A.; Knight, R. EMPeror: A Tool for Visualizing High-Throughput Microbial Community Data. Gigascience 2013, 2. [Google Scholar] [CrossRef] [Green Version]
- Legendre, P.; Andersson, M.J. Distance-Based Redundancy Analysis: Testing Multispecies Responses in Multifactorial Ecological Experiments. Ecol. Monogr. 1999, 69, 1–24. [Google Scholar] [CrossRef]
- Faith, D.P. Conservation Evaluation and Phylogenetic Diversity. Biol. Conserv. 1992, 61, 1–10. [Google Scholar] [CrossRef]
- Fierer, N. Embracing the Unknown: Disentangling the Complexities of the Soil Microbiome. Nat. Rev. Microbiol. 2017, 15, 579–590. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Yang, Z.; Dang, P.; Zhu, H.; Gao, Y.; Ha, V.N.; Zhao, Z. Response of Soil Microbial Community Dynamics to Robinia Pseudoacacia, L. Afforestation in the Loess Plateau: A Chronosequence Approach. Plant Soil 2018, 423, 327–338. [Google Scholar] [CrossRef]
- Li, J.G.; Shen, M.C.; Hou, J.F.; Li, L.; Wu, J.X.; Dong, Y.H. Effect of Different Levels of Nitrogen on Rhizosphere Bacterial Community Structure in Intensive Monoculture of Greenhouse Lettuce. Sci. Rep. 2016, 6, 25305. [Google Scholar] [CrossRef] [PubMed]
- Stevenson, A.; Hallsworth, J.E. Water and Temperature Relations of Soil Actinobacteria. Environ. Microbiol. Rep. 2014, 6, 744–755. [Google Scholar] [CrossRef] [PubMed]
- Gao, P.; Zheng, X.; Wang, L.; Liu, B.; Zhang, S. Changes in the Soil Bacterial Community in a Chronosequence of Temperate Walnut-Based Intercropping Systems. Forests 2019, 10, 299. [Google Scholar] [CrossRef] [Green Version]
- Wang, Q.; Wang, C.; Yu, W.W.; Turak, A.; Chen, D.; Huang, Y.; Ao, J.; Jiang, Y.; Huang, Z. Effects of Nitrogen and Phosphorus Inputs on Soil Bacterial Abundance, Diversity, and Community Composition in Chinese Fir Plantations. Front. Microbiol. 2018, 9, 1543. [Google Scholar] [CrossRef] [PubMed]
- Yan, G.; Xing, Y.; Xu, L.; Wang, J.; Dong, X.; Shan, W.; Guo, L.; Wang, Q. Effects of Different Nitrogen Additions on Soil Microbial Communities in Different Seasons in a Boreal Forest. Ecosphere 2017, 8, e01879. [Google Scholar] [CrossRef] [Green Version]
- Treseder, K.K. Nitrogen Additions and Microbial Biomass: A Meta-Analysis of Ecosystem Studies. Ecol. Lett. 2008, 11, 1111–1120. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Peerawat, M.; Blaud, A.; Trap, J.; Chevallier, T.; Alonso, P.; Gay, F.; Thaler, P.; Spor, A.; Sebag, D.; Choosai, C.; et al. Rubber Plantation Ageing Controls Soil Biodiversity after Land Conversion from Cassava. Agric. Ecosyst. Environ. 2018, 257, 92–102. [Google Scholar] [CrossRef] [Green Version]
- Koutika, L.S.; Mareschal, L.; Rudowsky, S. Fate of Acacia Mangium in Eucalypt Mixed-Species Plantations during Drought Conditions in the Congolese Coastal Plains. Bosque 2018, 39, 131–136. [Google Scholar] [CrossRef] [Green Version]
- Acosta-Martínez, V.; Dowd, S.E.; Sun, Y.; Wester, D.; Allen, V. Pyrosequencing Analysis for Characterization of Soil Bacterial Populations as Affected by an Integrated Livestock-Cotton Production System. Appl. Soil Ecol. 2010, 45, 13–25. [Google Scholar] [CrossRef]
- Battistuzzi, F.U.; Hedges, S.B. A Major Clade of Prokaryotes with Ancient Adaptations to Life on Land. Mol. Biol. Evol. 2009, 26, 335–343. [Google Scholar] [CrossRef]
- Paula, R.R.; Bouillet, J.P.; Ocheuze Trivelin, P.C.; Zeller, B.; Leonardo de Moraes Gonçalves, J.; Nouvellon, Y.; Bouvet, J.M.; Plassard, C.; Laclau, J.P. Evidence of Short-Term Belowground Transfer of Nitrogen from Acacia Mangium to Eucalyptus Grandis Trees in a Tropical Planted Forest. Soil Biol. Biochem. 2015, 91, 99–108. [Google Scholar] [CrossRef] [Green Version]
- López-Mondéjar, R.; Voříšková, J.; Větrovský, T.; Baldrian, P. The Bacterial Community Inhabiting Temperate Deciduous Forests Is Vertically Stratified and Undergoes Seasonal Dynamics. Soil Biol. Biochem. 2015, 87, 43–50. [Google Scholar] [CrossRef]
- Lauber, C.L.; Hamady, M.; Knight, R.; Fierer, N. Pyrosequencing-Based Assessment of Soil PH as a Predictor of Soil Bacterial Community Structure at the Continental Scale. Appl. Environ. Microbiol. 2009, 75, 5111–5120. [Google Scholar] [CrossRef] [Green Version]
- Peralta, R.M.; Ahn, C.; Gillevet, P.M. Characterization of Soil Bacterial Community Structure and Physicochemical Properties in Created and Natural Wetlands. Sci. Total Environ. 2013, 443, 725–732. [Google Scholar] [CrossRef]
- Voigtlaender, M.; Laclau, J.P.; de Gonçalves, J.L.M.; de Piccolo, M.C.; Moreira, M.Z.; Nouvellon, Y.; Ranger, J.; Bouillet, J.P. Introducing Acacia Mangium Trees in Eucalyptus Grandis Plantations: Consequences for Soil Organic Matter Stocks and Nitrogen Mineralization. Plant Soil 2012, 352, 99–111. [Google Scholar] [CrossRef]
- Rachid, C.T.C.C.; Balieiro, F.C.; Peixoto, R.S.; Pinheiro, Y.A.S.; Piccolo, M.C.; Chaer, G.M.; Rosado, A.S. Mixed Plantations Can Promote Microbial Integration and Soil Nitrate Increases with Changes in the N Cycling Genes. Soil Biol. Biochem. 2013, 66, 146–153. [Google Scholar] [CrossRef]
- Nielsen, U.N.; Osler, G.H.R.; Campbell, C.D.; Burslem, D.F.R.P.; van der Wal, R. The Influence of Vegetation Type, Soil Properties and Precipitation on the Composition of Soil Mite and Microbial Communities at the Landscape Scale. J. Biogeogr. 2010, 37, 1317–1328. [Google Scholar] [CrossRef]
- Lejon, D.P.H.; Chaussod, R.; Ranger, J.; Ranjard, L. Microbial Community Structure and Density under Different Tree Species in an Acid Forest Soil (Morvan, France). Microb. Ecol. 2005, 50, 614–625. [Google Scholar] [CrossRef] [PubMed]
- De Bellis, T.; Kernaghan, G.; Widden, P. Plant Community Influences on Soil Microfungal Assemblages in Boreal Mixed-Wood Forests. Mycologia 2007, 99, 356–367. [Google Scholar] [CrossRef]
- Mendes, R.; Garbeva, P.; Raaijmakers, J.M. The Rhizosphere Microbiome: Significance of Plant Beneficial, Plant Pathogenic, and Human Pathogenic Microorganisms. FEMS Microbiol. Rev. 2013, 37, 634–663. [Google Scholar] [CrossRef]
- Bevivino, A.; Paganin, P.; Bacci, G.; Florio, A.; Pellicer, M.S.; Papaleo, M.C.; Mengoni, A.; Ledda, L.; Fani, R.; Benedetti, A.; et al. Soil Bacterial Community Response to Differences in Agricultural Management along with Seasonal Changes in a Mediterranean Region. PLoS ONE 2014, 9, e105515. [Google Scholar] [CrossRef] [Green Version]
- Schmidt, M.; Veldkamp, E.; Corre, M.D. Tree Species Diversity Effects on Productivity, Soil Nutrient Availability and Nutrient Response Efficiency in a Temperate Deciduous Forest. For. Ecol. Manag. 2015, 338, 114–123. [Google Scholar] [CrossRef]
- Nannipieri, P.; Ascher, J.; Ceccherini, M.T.; Landi, L.; Pietramellara, G.; Renella, G. Microbial Diversity and Soil Functions. Eur. J. Soil Sci. 2003, 68, 12–26. [Google Scholar] [CrossRef]
- Lauber, C.L.; Strickland, M.S.; Bradford, M.A.; Fierer, N. The Influence of Soil Properties on the Structure of Bacterial and Fungal Communities across Land-Use Types. Soil Biol. Biochem. 2008, 40, 2407–2415. [Google Scholar] [CrossRef]
- Zhang, Y.; Cong, J.; Lu, H.; Yang, C.; Yang, Y.; Zhou, J.; Li, D. An Integrated Study to Analyze Soil Microbial Community Structure and Metabolic Potential in Two Forest Types. PLoS ONE 2014, 9, e93773. [Google Scholar] [CrossRef] [PubMed]
- Dong, Q.; Shi, H.; Liu, Y. Microbial Character Related Sulfur Cycle under Dynamic Environmental Factors Based on the Microbial Population Analysis in Sewerage System. Front. Microbiol. 2017, 8, 64. [Google Scholar] [CrossRef] [Green Version]
- Scholz, R.W.; Ulrich, A.E.; Eilittä, M.; Roy, A. Sustainable Use of Phosphorus: A Finite Resource. Sci. Total Environ. 2013, 461–462, 799–803. [Google Scholar] [CrossRef]
- Bini, D.; dos Santos, C.A.; da Silva, M.C.P.; Bonfim, J.A.; Cardoso, E.J.B.N. Intercropping Acacia Mangium Stimulates AMF Colonization and Soil Phosphatase Activity in Eucalyptus Grandis. Sci. Agric. 2018, 75, 102–110. [Google Scholar] [CrossRef]
- Wu, H.; Xiang, W.; Ouyang, S.; Forrester, D.I.; Zhou, B.; Chen, L.; Ge, T.; Lei, P.; Chen, L.; Zeng, Y.; et al. Linkage between Tree Species Richness and Soil Microbial Diversity Improves Phosphorus Bioavailability. Funct. Ecol. 2019, 33, 1549–1560. [Google Scholar] [CrossRef]





| R1Y7 | R2Y2 | R2Y5 | |||||||
|---|---|---|---|---|---|---|---|---|---|
| 100 A | 50 A 50 E | 100 E | 100 A | 50 A 50 E | 100 E | 100 A | 50 A 50 E | 100 E | |
| pH-H2O | 4.2 ± 0.03 c | 4.4 ± 0.02 b | 4.5 ± 0.04 a | 4.4 ± 0.02 a | 4.3 ± 0.03 b | 4.4 ± 0.03 a | 3.9 ± 0.05 b | 4.0 ± 0.03 b | 4.2 ± 0.04 a |
| pH-KCl | 3.5 ± 0.02 a | 3.5 ± 0.02 a | 3.5 ± 0.03 a | 3.3 ± 0.02 a | 3.2 ± 0.02 b | 3.3 ± 0.03 a | 3.5 ± 0.02 a | 3.5 ± 0.02 a | 3.5 ± 0.04 a |
| ΔpH | 0.8 ± 0.03 c | 0.90 ± 0.01 b | 1.0 ± 0.02 a | 1.1 ± 0.02 a | 1.1 ± 0.02 b | 1.1 ± 0.03 a | 0.4 ± 0.06 ab | 0.5 ± 0.03 b | 0.6 ± 0.05 a |
| N (%) | 0.058 ± 0.003 ab | 0.064 ± 0.003 b | 0.050 ± 0.004 a | 0.050 ± 0.002 a | 0.065 ± 0.011 b | 0.061 ± 0.016 ab | 0.150 ± 0.015 a | 0.168 ± 0.011a | 0.164 ± 0.016a |
| C (%) | 0.99 ± 0.074 ab | 1.18 ± 0.078 b | 0.87 ± 0.091 a | 1.01 ± 0.055 a | 1.50 ± 0.088 b | 1.41 ± 0.149 ab | 1.42 ± 0.091 a | 1.49 ± 0.086 a | 1.36 ± 0.140 a |
| Available P (mg kg−1) | 8.07 ± 0.63 a | 6.94 ± 0.45 b | 8.46 ± 0.79 a | 8.46 ± 0.42 c | 9.34 ± 0.44 b | 10.65 ± 1.05 a | 1.47 ± 0.01 a | 1.46 ± 0.01 a | 1.46 ± 0.01 a |
| Stands | N (%) | C (%) | C/N (%) | S (%) |
|---|---|---|---|---|
| 100 A | 0.11 ± 0.03 a | 1.5 ± 0.25 a | 14.2 ± 2.39 a | 0.15 ± 0.10 a |
| 50 A 50 E (Ac) | 0.18 ± 0.03 ab | 1.57 ± 0.18 a | 9.8 ± 2.31 a | 0.07 ± 0.10 a |
| 50 A 50 E (Eu) | 0.14 ± 0.03 ab | 1.7 ± 0.31 a | 13.1 ± 2.39 a | 0.06 ± 0.10 a |
| 100 E | 0.14 ± 0.03 ab | 1.6 ± 0.32 a | 12.1 ± 2.74 a | 0.08 ± 0.10 a |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Koutika, L.-S.; Fiore, A.; Tabacchioni, S.; Aprea, G.; Pereira, A.P.d.A.; Bevivino, A. Influence of Acacia mangium on Soil Fertility and Bacterial Community in Eucalyptus Plantations in the Congolese Coastal Plains. Sustainability 2020, 12, 8763. https://doi.org/10.3390/su12218763
Koutika L-S, Fiore A, Tabacchioni S, Aprea G, Pereira APdA, Bevivino A. Influence of Acacia mangium on Soil Fertility and Bacterial Community in Eucalyptus Plantations in the Congolese Coastal Plains. Sustainability. 2020; 12(21):8763. https://doi.org/10.3390/su12218763
Chicago/Turabian StyleKoutika, Lydie-Stella, Alessia Fiore, Silvia Tabacchioni, Giuseppe Aprea, Arthur Prudêncio de Araujo Pereira, and Annamaria Bevivino. 2020. "Influence of Acacia mangium on Soil Fertility and Bacterial Community in Eucalyptus Plantations in the Congolese Coastal Plains" Sustainability 12, no. 21: 8763. https://doi.org/10.3390/su12218763
APA StyleKoutika, L. -S., Fiore, A., Tabacchioni, S., Aprea, G., Pereira, A. P. d. A., & Bevivino, A. (2020). Influence of Acacia mangium on Soil Fertility and Bacterial Community in Eucalyptus Plantations in the Congolese Coastal Plains. Sustainability, 12(21), 8763. https://doi.org/10.3390/su12218763