Sample Preparation Techniques for the Analysis of Microplastics in Soil—A Review
Abstract
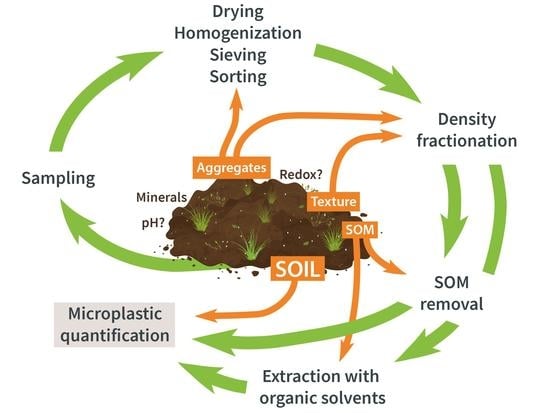
:1. Introduction
2. From the Field to the Lab
2.1. Sampling Strategies
2.2. Soil Characterization
3. Sample Preparation
3.1. (Freeze) Drying
3.2. Homogenization, Sieving, and Sorting
3.3. Dispersion of Soil Aggregates
3.4. Density Separation
3.4.1. Separation Principle
3.4.2. Density Solutions
3.4.3. Recycling of Salt Solutions
3.4.4. Instrumental Setups
3.4.5. Sample Collection
3.5. Removal of Soil Organic Matter
3.6. Extraction with Organic Solvents
3.7. Recent Developments
4. Options for Subsequent Microplastic Quantification
4.1. Microscopy
4.2. Spectroscopy
4.3. Thermoanalysis
4.4. Further Techniques
5. Suggestions for Best-Practice Sample Preparation
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| ABS | acrylonitrile butadiene styrene |
| ASE | accelerated solvent extraction |
| ATR | attenuated total reflection |
| DCM | dichloromethane |
| EVA | ethylene-vinyl acetate |
| FTIR | Fourier transformed infrared |
| FPA | focal plane array |
| GC | gas chromatography |
| GPC | gel permeation chromatography |
| HFIP | hexafluoroisopropanol |
| LC | liquid chromatographical |
| MS | mass spectrometry |
| NIR | near-infrared |
| NMR | nuclear magnetic resonance |
| PA | polyamide |
| PBAT | polybutylene adipate terephthalate |
| PBS | polybutylene sebacate |
| PC | polycarbonate |
| PE | polyethylene |
| PET | polyethylene terephthalate |
| PLA | polylactic acid |
| PMMA | poly(methyl methacrylate) |
| PP | polypropylene |
| PU | polyurethane |
| PS | polystyrene |
| PVC | polyvinyl chloride |
| PU | polyurethane |
| Py | pyrolysis |
| SEC | size-exclusion chromatography |
| SOM | soil organic matter |
| SPT | sodium polytungstate |
| TED | thermoextraction and desorption |
| TFA | trifluoroacetic acid |
| TGA | thermogravimetry |
| THF | tetrahydrofurane |
| TCB | trichlorobenzene |
| TOF | time-of-flight |
References
- Geyer, R.; Jambeck, J.R.; Law, K.L. Production, use, and fate of all plastics ever made. Sci. Adv. 2017, 3, e1700782. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kawecki, D.; Nowack, B. Polymer-Specific Modeling of the Environmental Emissions of Seven Commodity Plastics As Macro- and Microplastics. Environ. Sci. Technol. 2019, 53, 9664–9676. [Google Scholar] [CrossRef] [PubMed]
- PlasticsEurope. Plastics—The Facts 2019: An Analysis of European Plastics Production, Demand and Waste Data; PlasticsEurope: Brussels, Belgium, 2019. [Google Scholar]
- Burgstaller, M.; Potrykus, A.; Weißenbacher, J.; Kabasci, D.S.; Merrettig-Bruns, D.U.; Sayder, B. Study on the Treatment of Biodegradable Plastics; Technical Report; Umweltbundesamt: Dessau, Germany, 2018. [Google Scholar]
- Thompson, R.C. Plastic Debris in the Marine Environment: Consequences and Solutions; Technical Report; Marine Nature Conservation in Europe: Stralsund, Germany, 2006. [Google Scholar]
- Horton, A.A.; Svendsen, C.; Williams, R.J.; Spurgeon, D.J.; Lahive, E. Large microplastic particles in sediments of tributaries of the River Thames UK–Abundance, sources and methods for effective quantification. Mar. Pollut. Bull. 2017, 114, 218–226. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- De Souza Machado, A.A.; Lau, C.W.; Kloas, W.; Bergmann, J.; Bachelier, J.B.; Faltin, E.; Becker, R.; Görlich, A.S.; Rillig, M.C. Microplastics Can Change Soil Properties and Affect Plant Performance. Environ. Sci. Technol. 2019, 53, 6044–6052. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- De Souza Machado, A.A.; Horton, A.A.; Davis, T.; Maaß, S. Microplastics and Their Effects on Soil Function as a Life-Supporting System. In Microplastics in Terrestrial Environments: Emerging Contaminants and Major Challenges; He, D., Luo, Y., Eds.; The Handbook of Environmental Chemistry, Springer International Publishing: Cham, Switzerland, 2020; pp. 199–222. [Google Scholar] [CrossRef]
- Boots, B.; Russell, C.W.; Green, D.S. Effects of Microplastics in Soil Ecosystems: Above and Below Ground. Environ. Sci. Technol. 2019, 53, 11496–11506. [Google Scholar] [CrossRef] [PubMed]
- Lei, L.; Liu, M.; Song, Y.; Lu, S.; Hu, J.; Cao, C.; Xie, B.; Shi, H.; He, D. Polystyrene (nano)microplastics cause size-dependent neurotoxicity, oxidative damage and other adverse effects in Caenorhabditis elegans. Environ. Sci. Nano 2018, 5, 2009–2020. [Google Scholar] [CrossRef]
- Rillig, M.C.; Lehmann, A.; Machado, A.A.d.S.; Yang, G. Microplastic Effects on Plants. New Phytol. 2019, 223, 1066–1070. [Google Scholar] [CrossRef] [Green Version]
- Büks, F.; Loes van Schaik, N.; Kaupenjohann, M. What Do We Know about How the Terrestrial Multicellular Soil Fauna Reacts to Microplastic? SOIL 2020, 6, 245–267. [Google Scholar] [CrossRef]
- He, D.; Luo, Y.; Lu, S.; Liu, M.; Song, Y.; Lei, L. Microplastics in soils: Analytical methods pollution characteristics and ecological risks. TrAC Trends Anal. Chem. 2018, 109, 163–172. [Google Scholar] [CrossRef]
- Hurley, R.R.; Nizzetto, L. Fate and occurrence of micro(nano)plastics in soils: Knowledge gaps and possible risks. Curr. Opin. Environ. Sci. Health 2018, 1, 6–11. [Google Scholar] [CrossRef]
- Wang, J.; Liu, X.; Li, Y.; Powell, T.; Wang, X.; Wang, G.; Zhang, P. Microplastics as contaminants in the soil environment: A mini-review. Sci. Total Environ. 2019, 691, 848–857. [Google Scholar] [CrossRef]
- Steinmetz, Z.; Wollmann, C.; Schaefer, M.; Buchmann, C.; David, J.; Tröger, J.; Muñoz, K.; Frör, O.; Schaumann, G.E. Plastic mulching in agriculture. Trading short-term agronomic benefits for long-term soil degradation? Sci. Total Environ. 2016, 550, 690–705. [Google Scholar] [CrossRef]
- Bianco, A.; Passananti, M. Atmospheric Micro and Nanoplastics: An Enormous Microscopic Problem. Sustainability 2020, 12, 7327. [Google Scholar] [CrossRef]
- Bergmann, M.; Mützel, S.; Primpke, S.; Tekman, M.; Trachsel, J.; Gerdts, G. White and wonderful? Microplastics prevail in snow from the Alps to the Arctic. Sci. Adv. 2019, 5, eaax1157. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huerta Lwanga, E.; Gertsen, H.; Gooren, H.; Peters, P.; Salánki, T.; van der Ploeg, M.; Besseling, E.; Koelmans, A.A.; Geissen, V. Incorporation of microplastics from litter into burrows of Lumbricus terrestris. Environ. Pollut. 2017, 220, 523–531. [Google Scholar] [CrossRef] [PubMed]
- Rillig, M.C.; Ziersch, L.; Hempel, S. Microplastic transport in soil by earthworms. Sci. Rep. 2017, 7, 1362. [Google Scholar] [CrossRef]
- Van den Berg, P.; Huerta-Lwanga, E.; Corradini, F.; Geissen, V. Sewage sludge application as a vehicle for microplastics in eastern Spanish agricultural soils. Environ. Pollut. 2020, 261, 114198. [Google Scholar] [CrossRef]
- Briassoulis, D.; Babou, E.; Hiskakis, M.; Kyrikou, I. Analysis of long-term degradation behaviour of polyethylene mulching films with pro-oxidants under real cultivation and soil burial conditions. Environ. Sci. Pollut. Res. Int. 2015, 22, 2584–2598. [Google Scholar] [CrossRef]
- Cai, L.; Wang, J.; Peng, J.; Wu, Z.; Tan, X. Observation of the degradation of three types of plastic pellets exposed to UV irradiation in three different environments. Sci. Total Environ. 2018, 628-629, 740–747. [Google Scholar] [CrossRef]
- Braun, U.; Jekel, M.; Gerdts, G.; Ivleva, N.P.; Reiber, J. Microplastics Analytics—Sampling, Preparation and Detection Methods; Bundesministerium für Bildung und Forschung: Berlin, Germany, 2018. [Google Scholar]
- Hartmann, N.; Hüffer, T.; Thompson, R.; Hassellöv, M.; Verschoor, A.; Daugaard, A.; Rist, S.; Karlsson, T.; Brennholt, N.; Cole, M.; et al. Are We Speaking the Same Language? Recommendations for a Definition and Categorization Framework for Plastic Debris. Environ. Sci. Technol. 2019, 53, 1039–1047. [Google Scholar] [CrossRef] [Green Version]
- Bläsing, M.; Amelung, W. Plastics in soil: Analytical methods and possible sources. Sci. Total Environ. 2018, 612, 422–435. [Google Scholar] [CrossRef] [PubMed]
- Möller, J.N.; Löder, M.G.J.; Laforsch, C. Finding Microplastics in Soils: A Review of Analytical Methods. Environ. Sci. Technol. 2020, 54, 2078–2090. [Google Scholar] [CrossRef] [PubMed]
- Dioses-Salinas, D.C.; Pizarro-Ortega, C.I.; la Torre, G.E.D. A methodological approach of the current literature on microplastic contamination in terrestrial environments: Current knowledge and baseline considerations. Sci. Total Environ. 2020, 730, 139164. [Google Scholar] [CrossRef] [PubMed]
- Blume, H.P.; Brümmer, G.W.; Fleige, H.; Horn, R.; Kandeler, E.; Kögel-Knabner, I.; Kretzschmar, R.; Stahr, K.; Wilke, B.M. Scheffer/Schachtschabel Soil Science, 1st ed.; Springer: Berlin, Germany, 2016. [Google Scholar]
- Bronick, C.; Lal, R. Soil structure and management: A review. Geoderma 2005, 124, 3–22. [Google Scholar] [CrossRef]
- Löder, M.G.J.; Imhof, H.K.; Ladehoff, M.; Löschel, L.A.; Lorenz, C.; Mintenig, S.; Piehl, S.; Primpke, S.; Schrank, I.; Laforsch, C.; et al. Enzymatic Purification of Microplastics in Environmental Samples. Environ. Sci. Technol. 2017, 51, 14283–14292. [Google Scholar] [CrossRef]
- Fischer, M.; Scholz-Böttcher, B.M. Simultaneous Trace Identification and Quantification of Common Types of Microplastics in Environmental Samples by Pyrolysis-Gas Chromatography–Mass Spectrometry. Environ. Sci. Technol. 2017, 51, 5052–5060. [Google Scholar] [CrossRef]
- Steinmetz, Z.; Kintzi, A.; Muñoz, K.; Schaumann, G.E. A simple method for the selective quantification of polyethylene polypropylene, and polystyrene plastic debris in soil by pyrolysis-gas chromatography/mass spectrometry. J. Anal. Appl. Pyrolysis 2020, 147, 104803. [Google Scholar] [CrossRef]
- Fojt, J.; David, J.; Přikryl, R.; Řezáčová, V.; Kučerík, J. A critical review of the overlooked challenge of determining micro-bioplastics in soil. Sci. Total Environ. 2020, 745, 140975. [Google Scholar] [CrossRef]
- Wang, Z.; Taylor, S.E.; Sharma, P.; Flury, M. Poor extraction efficiencies of polystyrene nano- and microplastics from biosolids and soil. PLoS ONE 2018, 13, e0208009. [Google Scholar] [CrossRef]
- Wahl, A.; Le Juge, C.; Davranche, M.; El Hadri, H.; Grassl, B.; Reynaud, S.; Gigault, J. Nanoplastic Occurrence in a Soil Amended with Plastic Debris. Chemosphere 2020, 262, 127784. [Google Scholar] [CrossRef]
- Zhu, F.; Zhu, C.; Wang, C.; Gu, C. Occurrence and Ecological Impacts of Microplastics in Soil Systems: A Review. Bull. Environ. Contam. Toxicol. 2019, 102, 741–749. [Google Scholar] [CrossRef] [PubMed]
- Wu, M.; Yang, C.; Du, C.; Liu, H. Microplastics in waters and soils: Occurrence analytical methods and ecotoxicological effects. Ecotoxicol. Environ. Saf. 2020, 202, 110910. [Google Scholar] [CrossRef]
- Meixner, K.; Kubiczek, M.; Fritz, I. Microplastic in soil–current status in Europe with special focus on method tests with Austrian samples. AIMS Environ. Sci. 2020, 7, 174–191. [Google Scholar] [CrossRef]
- ISO 18400-102. Soil Quality—Sampling—Part 102: Selection and Application of Sampling Techniques; Technical Report; International Organization for Standardization: Geneva, Switzerland, 2017. [Google Scholar]
- Piehl, S.; Leibner, A.; Löder, M.G.J.; Dris, R.; Bogner, C.; Laforsch, C. Identification and quantification of macro- and microplastics on an agricultural farmland. Sci. Rep. 2018, 8, 1–9. [Google Scholar] [CrossRef] [Green Version]
- Corradini, F.; Meza, P.; Eguiluz, R.; Casado, F.; Huerta-Lwanga, E.; Geissen, V. Evidence of microplastic accumulation in agricultural soils from sewage sludge disposal. Sci. Total Environ. 2019, 671, 411–420. [Google Scholar] [CrossRef]
- Sponagel, H.; Grottenthaler, W.; Hartmann, K.; Hartwich, R.; Janetzko, P.; Joisten, H.; Kühn, D.; Sabel, K.; Traidl, R. Bodenkundliche Kartieranleitung, 5th ed.; Schweizerbart: Stuttgart, Germany, 2005. [Google Scholar]
- BBodSchV. Federal Soil Protection and Contaminated Sites Ordinance; Number On the Basis of §§ 6, 8 Paragraphs 1 and 2 and § 13 Paragraph 1 Sentence 2 Federal Soil Protection Law of 17 March 1998 (Federal Law Gazette I p. 502); Bundesgesetzblatt: Berlin, Germany, 1999. [Google Scholar]
- Liu, M.; Lu, S.; Song, Y.; Lei, L.; Hu, J.; Lv, W.; Zhou, W.; Cao, C.; Shi, H.; Yang, X.; et al. Microplastic and mesoplastic pollution in farmland soils in suburbs of Shanghai, China. Environ. Pollut. 2018, 242, 855–862. [Google Scholar] [CrossRef]
- Schoeneberger, P.; Wysocki, D.; Benham, E.; Staff, S.S. Field Book for Describing and Sampling Soils, Version 3.0; Natural Resources Conservation Service, National Soil Survey Center: Lincoln, NE, USA, 2012.
- EPA, U. LSASD Operating Procedure for Soil Sampling; Technical Report LSASDPROC-300-R4; Laboratory Services and Applied Science Division: Athens, GA, USA, 2020.
- Scheurer, M.; Bigalke, M. Microplastics in Swiss Floodplain Soils. Environ. Sci. Technol. 2018, 52, 3591–3598. [Google Scholar] [CrossRef]
- Witzig, C.S.; Földi, C.; Wörle, K.; Habermehl, P.; Pittroff, M.; Müller, Y.K.; Lauschke, T.; Fiener, P.; Dierkes, G.; Freier, K.; et al. When Good Intentions Go Bad—False Positive Microplastic Detection Caused by Disposable Gloves. Environ. Sci. Technol. 2020. [Google Scholar] [CrossRef]
- Ramos, L.; Berenstein, G.; Hughes, E.A.; Zalts, A.; Montserrat, J.M. Polyethylene film incorporation into the horticultural soil of small periurban production units in Argentina. Sci. Total Environ. 2015, 523, 74–81. [Google Scholar] [CrossRef]
- Huerta Lwanga, E.; Vega, J.M.; Quej, V.K.; Chi Angeles, J.D.; Cid, L.S.D.; Chi, C.; Segura, G.E.; Gertsen, H.; Salánki, T.; van der Ploeg, M.; et al. Field evidence for transfer of plastic debris along a terrestrial food chain. Sci. Rep. 2017, 7, 1–7. [Google Scholar] [CrossRef]
- ISO 17892-4. Geotechnical Investigation and Testing—Laboratory Testing of Soil—Part 4: Determination of Particle Size Distribution; Technical report; International Organization for Standardization: Geneva, Switzerland, 2016. [Google Scholar]
- Hammersley, J.M. Stochastic Models for the Distribution of Particles in Space. Adv. Appl. Probab. 1972, 4, 47. [Google Scholar] [CrossRef]
- ISO 11277. Soil Quality—Determination of Particle Size Distribution in Mineral Soil Material—Method by Sieving and Sedimentation; Technical Report; International Organization for Standardization: Geneva, Switzerland, 2020. [Google Scholar]
- ISO 11272. Soil Quality—Determination of Dry Bulk Density; Technical Report; International Organization for Standardization: Geneva, Switzerland, 2017. [Google Scholar]
- DIN EN 15935. Sludge, Treated Biowaste, Soil And Waste—Determination of Loss on Ignition; Technical Report; Beuth: Berlin, Germany, 2020. [Google Scholar] [CrossRef]
- Zhang, S.; Yang, X.; Gertsen, H.; Peters, P.; Salánki, T.; Geissen, V. A simple method for the extraction and identification of light density microplastics from soil. Sci. Total. Environ. 2018, 616–617, 1056–1065. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- ISO 11464. Soil Quality—Pretreatment of Samples for Physico-Chemical Analysis; Technical report; International Organization for Standardization: Geneva, Switzerland, 2006. [Google Scholar]
- Beyler, C.; Hirschler, M. Thermal Decomposition of Polymers. In SFPE Handbook of Fire Protection Engineering; DiNenno, P.J., Ed.; National Fire Protection Association: Quincy, MA, USA, 2002; Volume 2. [Google Scholar]
- Enders, K.; Lenz, R.; Ivar do Sul, J.A.; Tagg, A.S.; Labrenz, M. When every particle matters: A QuEChERS approach to extract microplastics from environmental samples. MethodsX 2020, 7, 100784. [Google Scholar] [CrossRef]
- Zubris, K.A.V.; Richards, B.K. Synthetic fibers as an indicator of land application of sludge. Environ. Pollut. 2005, 138, 201–211. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Liu, Q.; Jia, W.; Yan, C.; Wang, J. Agricultural plastic mulching as a source of microplastics in the terrestrial environment. Environ. Pollut. 2020, 260, 114096. [Google Scholar] [CrossRef]
- Zhou, B.; Wang, J.; Zhang, H.; Shi, H.; Fei, Y.; Huang, S.; Tong, Y.; Wen, D.; Luo, Y.; Barceló, D. Microplastics in agricultural soils on the coastal plain of Hangzhou Bay, east China: Multiple sources other than plastic mulching film. J. Hazard. Mater. 2020, 388, 121814. [Google Scholar] [CrossRef]
- Garcés-Ordóñez, O.; Castillo-Olaya, V.A.; Granados-Briceño, A.F.; García, L.M.B.; Díaz, L.F.E. Marine litter and microplastic pollution on mangrove soils of the Ciénaga Grande de Santa Marta, Colombian Caribbean. Mar. Pollut. Bull. 2019, 145, 455–462. [Google Scholar] [CrossRef]
- Vermaire, J.C.; Pomeroy, C.; Herczegh, S.M.; Haggart, O.; Murphy, M. Microplastic abundance and distribution in the open water and sediment of the Ottawa River, Canada, and its tributaries. FACETS 2017, 2, 301–314. [Google Scholar] [CrossRef] [Green Version]
- Zhang, G.S.; Liu, Y.F. The distribution of microplastics in soil aggregate fractions in southwestern China. Sci. Total Environ. 2018, 642, 12–20. [Google Scholar] [CrossRef]
- Cerli, C.; Celi, L.; Kalbitz, K.; Guggenberger, G.; Kaiser, K. Separation of light and heavy organic matter fractions in soil—Testing for proper density cut-off and dispersion level. Geoderma 2012, 170, 403–416. [Google Scholar] [CrossRef]
- Enders, K.; Tagg, A.S.; Labrenz, M. Evaluation of Electrostatic Separation of Microplastics From Mineral-Rich Environmental Samples. Front. Environ. Sci. 2020, 8. [Google Scholar] [CrossRef]
- Liu, M.; Lu, S.; Chen, Y.; Cao, C.; Bigalke, M.; He, D. Analytical Methods for Microplastics in Environments: Current Advances and Challenges. In The Handbook of Environmental Chemistry; Springer International Publishing: Cham, Switzerland, 2020; pp. 3–24. [Google Scholar] [CrossRef]
- Chen, Y.; Leng, Y.; Liu, X.; Wang, J. Microplastic pollution in vegetable farmlands of suburb Wuhan, central China. Environ. Pollut. 2020, 257, 113449. [Google Scholar] [CrossRef] [PubMed]
- Herrera, A.; Garrido-Amador, P.; Martínez, I.; Samper, M.D.; López-Martínez, J.; Gómez, M.; Packard, T.T. Novel methodology to isolate microplastics from vegetal-rich samples. Mar. Pollut. Bull. 2018, 129, 61–69. [Google Scholar] [CrossRef] [PubMed]
- Nuelle, M.T.; Dekiff, J.H.; Remy, D.; Fries, E. A new analytical approach for monitoring microplastics in marine sediments. Environ. Pollut. 2014, 184, 161–169. [Google Scholar] [CrossRef]
- Liu, M.; Song, Y.; Lu, S.; Qiu, R.; Hu, J.; Li, X.; Bigalke, M.; Shi, H.; He, D. A method for extracting soil microplastics through circulation of sodium bromide solutions. Sci. Total Environ. 2019, 691, 341–347. [Google Scholar] [CrossRef] [PubMed]
- Quinn, B.; Murphy, F.; Ewins, C. Validation of density separation for the rapid recovery of microplastics from sediment. Anal. Methods 2017, 9, 1491–1498. [Google Scholar] [CrossRef] [Green Version]
- Xiong, X.; Zhang, K.; Chen, X.; Shi, H.; Luo, Z.; Wu, C. Sources and distribution of microplastics in China’s largest inland lake—Qinghai Lake. Environ. Pollut 2018, 235, 899–906. [Google Scholar] [CrossRef]
- Claessens, M.; van Cauwenberghe, L.; Vandegehuchte, M.B.; Janssen, C.R. New techniques for the detection of microplastics in sediments and field collected organisms. Mar. Pollut. Bull. 2013, 70, 227–233. [Google Scholar] [CrossRef]
- Frère, L.; Paul-Pont, I.; Rinnert, E.; Petton, S.; Jaffré, J.; Bihannic, I.; Soudant, P.; Lambert, C.; Huvet, A. Influence of environmental and anthropogenic factors on the composition concentration and spatial distribution of microplastics: A case study of the Bay of Brest (Brittany, France). Environ. Pollut. 2017, 225, 211–222. [Google Scholar] [CrossRef] [Green Version]
- Frias, J.; Pagter, E.; Nash, R.; O’Connor, I.; Carretero, O.; Filgueiras, A.; Viñas, L.; Gago, J.; Antunes, J.; Bessa, F.; et al. Standardised Protocol for Monitoring Microplastics in Sediments; Technical Report; JPI-Oceans BASEMAN: Brussels, Belgium, 2018. [Google Scholar]
- Campanale, C.; Savino, I.; Pojar, I.; Massarelli, C.; Uricchio, V.F. A Practical Overview of Methodologies for Sampling and Analysis of Microplastics in Riverine Environments. Sustainability 2020, 12, 6755. [Google Scholar] [CrossRef]
- Masura, J.; Baker, J.; Foster, G.; Arthur, C. Laboratory Methods for the Analysis of Microplastics in the Marine Environment: Recommendations for Quantifying Snythetic Particles in Waters and Sediment. NOAA Technical Memorandum. NOS-OR&R-48; Technical report; National Oceanic and Atmospheric Administration: Washington, DC, USA, 2015.
- Renner, G.; Nellessen, A.; Schwiers, A.; Wenzel, M.; Schmidt, T.C.; Schram, J. Hydrophobicity–Water/Air–Based Enrichment Cell for Microplastics Analysis within Environmental Samples: A Proof of Concept. MethodsX 2019. [Google Scholar] [CrossRef]
- Van Cauwenberghe, L.; Devriese, L.; Galgani, F.; Robbens, J.; Janssen, C.R. Microplastics in Sediments: A Review of Techniques, Occurrence and Effects. Mar. Environ. Res. 2015, 111, 5–17. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stolte, A.; Forster, S.; Gerdts, G.; Schubert, H. Microplastic concentrations in beach sediments along the German Baltic coast. Mar. Pollut. Bull. 2015, 99, 216–229. [Google Scholar] [CrossRef]
- Stock, F.; Kochleus, C.; Bänsch-Baltruschat, B.; Brennholt, N.; Reifferscheid, G. Sampling techniques and preparation methods for microplastic analyses in the aquatic environment—A review. TrAC Trends Anal. Chem. 2019, 113, 84–92. [Google Scholar] [CrossRef]
- ECHA. Potassium Formate; Technical Report 209-677-9; European Chemicals Agency: Helsinki, Finnland, 2020. [Google Scholar]
- Mahon, A.M.; O’Connell, B.; Healy, M.G.; O’Connor, I.; Officer, R.; Nash, R.; Morrison, L. Microplastics in Sewage Sludge: Effects of Treatment. Environ. Sci. Technol. 2017, 51, 810–818. [Google Scholar] [CrossRef] [PubMed]
- ECHA. Zinc Chloride; Technical Report 231-592-0; European Chemicals Agency: Helsinki, Finnland, 2020. [Google Scholar]
- ECHA. Sodium Iodide; Technical Report 231-679-3; European Chemicals Agency: Helsinki, Finnland, 2020. [Google Scholar]
- Zobkov, M.B.; Esiukova, E.E. Evaluation of the Munich Plastic Sediment Separator efficiency in extraction of microplastics from natural marine bottom sediments. Limnol. Oceanogr. Methods 2017, 15, 967–978. [Google Scholar] [CrossRef]
- Prendergast-Miller, M.T.; Katsiamides, A.; Abbass, M.; Sturzenbaum, S.R.; Thorpe, K.L.; Hodson, M.E. Polyester-derived microfibre impacts on the soil-dwelling earthworm Lumbricus terrestris. Environ. Pollut. 2019, 251, 453–459. [Google Scholar] [CrossRef]
- Ballent, A.; Corcoran, P.L.; Madden, O.; Helm, P.A.; Longstaffe, F.J. Sources and sinks of microplastics in Canadian Lake Ontario nearshore tributary and beach sediments. Mar. Pollut. Bull. 2016, 110, 383–395. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Enders, K.; Käppler, A.; Biniasch, O.; Feldens, P.; Stollberg, N.; Lange, X.; Fischer, D.; Eichhorn, K.J.; Pollehne, F.; Oberbeckmann, S.; et al. Tracing microplastics in aquatic environments based on sediment analogies. Sci. Rep. 2019, 9. [Google Scholar] [CrossRef]
- Corcoran, P.L.; Biesinger, M.C.; Grifi, M. Plastics and beaches: A degrading relationship. Mar. Pollut. Bull. 2009, 58, 80–84. [Google Scholar] [CrossRef]
- Hurley, R.R.; Lusher, A.L.; Olsen, M.; Nizzetto, L. Validation of a Method for Extracting Microplastics from Complex, Organic-Rich, Environmental Matrices. Environ. Sci. Technol. 2018, 52, 7409–7417. [Google Scholar] [CrossRef] [Green Version]
- Dekiff, J.H.; Remy, D.; Klasmeier, J.; Fries, E. Occurrence and spatial distribution of microplastics in sediments from Norderney. Environ. Pollut. 2014, 186, 248–256. [Google Scholar] [CrossRef]
- Zhou, Q.; Zhang, H.; Fu, C.; Zhou, Y.; Dai, Z.; Li, Y.; Tu, C.; Luo, Y. The distribution and morphology of microplastics in coastal soils adjacent to the Bohai Sea and the Yellow Sea. Geoderma 2018, 322, 201–208. [Google Scholar] [CrossRef]
- Anastas, P.; Eghbali, N. Green Chemistry: Principles and Practice. Chem. Soc. Rev. 2010, 39, 301–312. [Google Scholar] [CrossRef] [PubMed]
- Kedzierski, M.; Le Tilly, V.; César, G.; Sire, O.; Bruzaud, S. Efficient microplastics extraction from sand. A cost effective methodology based on sodium iodide recycling. Mar. Pollut. Bull. 2017, 115, 120–129. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, M.; Gonçalves, A.; Gonçalves, F.; Abrantes, N. Improving cost-efficiency for MPs density separation by zinc chloride reuse. MethodsX 2020, 7, 100785. [Google Scholar] [CrossRef]
- Imhof, H.K.; Schmid, J.; Niessner, R.; Ivleva, N.P.; Laforsch, C. A novel, highly efficient method for the separation and quantification of plastic particles in sediments of aquatic environments. Limnol. Oceanogr. Methods 2012, 10, 524–537. [Google Scholar] [CrossRef]
- Klein, S.; Worch, E.; Knepper, T.P. Occurrence and Spatial Distribution of Microplastics in River Shore Sediments of the Rhine-Main Area in Germany. Environ. Sci. Technol. 2015, 49, 6070–6076. [Google Scholar] [CrossRef]
- Mahat, S. Separation and Quantification of Microplastics from Beach and Sediment Samples Using the Bauta Microplastic-Sediment Separator. Master’s Thesis, Norwegian University of Life Sciences, As, Norway, 2017. [Google Scholar]
- Han, X.; Lu, X.; Vogt, R.D. An optimized density-based approach for extracting microplastics from soil and sediment samples. Environ. Pollut. 2019, 254, 113009. [Google Scholar] [CrossRef]
- Besley, A.; Vijver, M.G.; Behrens, P.; Bosker, T. A standardized method for sampling and extraction methods for quantifying microplastics in beach sand. Mar. Pollut. Bull. 2017, 114, 77–83. [Google Scholar] [CrossRef]
- Nakajima, R.; Lindsay, D.J.; Tsuchiya, M.; Matsui, R.; Kitahashi, T.; Fujikura, K.; Fukushima, T. A small, stainless-steel sieve optimized for laboratory beaker-based extraction of microplastics from environmental samples. MethodsX 2019, 6, 1677–1682. [Google Scholar] [CrossRef] [PubMed]
- Vermeiren, P.; Muñoz, C.; Ikejima, K. Microplastic identification and quantification from organic rich sediments: A validated laboratory protocol. Environ. Pollut. 2020, 262, 114298. [Google Scholar] [CrossRef] [PubMed]
- Coppock, R.L.; Cole, M.; Lindeque, P.K.; Queirós, A.M.; Galloway, T.S. A small-scale, portable method for extracting microplastics from marine sediments. Environ. Pollut. 2017, 230, 829–837. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mintenig, S.M.; Int-Veen, I.; Löder, M.; Primpke, S.; Gerdts, G. Identification of microplastic in effluents of waste water treatment plants using focal plane array-based micro-Fourier-transform infrared imaging. Water Res. 2017, 108, 365–372. [Google Scholar] [CrossRef]
- Löder, M.G.J.; Kuczera, M.; Mintenig, S.; Lorenz, C.; Gerdts, G. Focal plane array detector-based micro-Fourier-transform infrared imaging for the analysis of microplastics in environmental samples. Environ. Chem. 2015, 12, 563. [Google Scholar] [CrossRef]
- Dehaut, A.; Cassone, A.L.; Frère, L.; Hermabessiere, L.; Himber, C.; Rinnert, E.; Rivière, G.; Lambert, C.; Soudant, P.; Huvet, A.; et al. Microplastics in seafood: Benchmark protocol for their extraction and characterization. Environ. Pollut. 2016, 215, 223–233. [Google Scholar] [CrossRef] [Green Version]
- Kühn, S.; van Werven, B.; van Oyen, A.; Meijboom, A.; Bravo Rebolledo, E.L.; van Franeker, J.A. The use of potassium hydroxide (KOH) solution as a suitable approach to isolate plastics ingested by marine organisms. Mar. Pollut. Bull. 2017, 115, 86–90. [Google Scholar] [CrossRef]
- Duan, J.; Han, J.; Zhou, H.; Lau, Y.L.; An, W.; Wei, P.; Cheung, S.G.; Yang, Y.; Tam, N.F.Y. Development of a digestion method for determining microplastic pollution in vegetal-rich clayey mangrove sediments. Sci. Total Environ. 2020, 707, 136030. [Google Scholar] [CrossRef]
- Petigara, B.R.; Blough, N.V.; Mignerey, A.C. Mechanisms of Hydrogen Peroxide Decomposition in Soils. Environ. Sci. Technol. 2002, 36, 639–645. [Google Scholar] [CrossRef]
- Mikutta, R.; Kleber, M.; Kaiser, K.; Jahn, R. Organic matter removal from soils using hydrogen peroxide, sodium hypochlorite, and disodium peroxodisulfate. Soil Sci. Soc. Am. J. 2005, 69, 120. [Google Scholar] [CrossRef]
- Cole, M.; Webb, H.; Lindeque, P.K.; Fileman, E.S.; Halsband, C.; Galloway, T.S. Isolation of microplastics in biota-rich seawater samples and marine organisms. Sci. Rep. 2014, 4, 4528. [Google Scholar] [CrossRef] [Green Version]
- Courtene-Jones, W.; Quinn, B.; Murphy, F.; Gary, S.F.; Narayanaswamy, B.E. Optimisation of enzymatic digestion and validation of specimen preservation methods for the analysis of ingested microplastics. Anal. Methods 2017, 9, 1437–1445. [Google Scholar] [CrossRef] [Green Version]
- Railo, S.; Talvitie, J.; Setälä, O.; Koistinen, A.; Lehtiniemi, M. Application of an enzyme digestion method reveals microlitter in Mytilus trossulus at a wastewater discharge area. Mar. Pollut. Bull. 2018, 130, 206–214. [Google Scholar] [CrossRef]
- Lavers, J.L.; Stivaktakis, G.; Hutton, I.; Bond, A.L. Detection of ultrafine plastics ingested by seabirds using tissue digestion. Mar. Pollut. Bull. 2019, 142, 470–474. [Google Scholar] [CrossRef]
- Ljung, E.; Olesen, K.B.; Andersson, P.G.; Fältström, E.; Vollertsen, J.; Wittgren, H.B.; Hagman, M. Microplastics in the Water and Nutrient-Cycle; Technical Report 2018–13; Sweden Water Research: Bromma, Sweden, 2018. [Google Scholar]
- Fabbri, D.; Tartari, D.; Trombini, C. Analysis of poly(vinyl chloride) and other polymers in sediments and suspended matter of a coastal lagoon by pyrolysis-gas chromatography-mass spectrometry. Anal. Chim. Acta 2000, 413, 3–11. [Google Scholar] [CrossRef]
- Peez, N.; Becker, J.; Ehlers, S.M.; Fritz, M.; Fischer, C.B.; Koop, J.H.E.; Winkelmann, C.; Imhof, W. Quantitative analysis of PET microplastics in environmental model samples using quantitative 1H-NMR spectroscopy: Validation of an optimized and consistent sample clean-up method. Anal. Bioanal. Chem. 2019, 411, 7409–7418. [Google Scholar] [CrossRef]
- Dierkes, G.; Lauschke, T.; Becher, S.; Schumacher, H.; Földi, C.; Ternes, T. Quantification of microplastics in environmental samples via pressurized liquid extraction and pyrolysis-gas chromatography. Anal. Bioanal. Chem. 2019, 411, 6959–6968. [Google Scholar] [CrossRef]
- Fuller, S.; Gautam, A. A Procedure for Measuring Microplastics using Pressurized Fluid Extraction. Environ. Sci. Technol. 2016, 50, 5774–5780. [Google Scholar] [CrossRef] [Green Version]
- Okoffo, E.D.; Ribeiro, F.; O’Brien, J.W.; O’Brien, S.; Tscharke, B.J.; Gallen, M.; Samanipour, S.; Mueller, J.F.; Thomas, K.V. Identification and quantification of selected plastics in biosolids by pressurized liquid extraction combined with double-shot pyrolysis gas chromatography–mass spectrometry. Sci. Total Environ. 2020, 715, 136924. [Google Scholar] [CrossRef]
- Siotto, M.; Zoia, L.; Tosin, M.; Innocenti, F.D.; Orlandi, M.; Mezzanotte, V. Monitoring biodegradation of poly(butylene sebacate) by Gel Permeation Chromatography 1H-NMR and 31P-NMR techniques. J. Environ. Manag. 2013, 116, 27–35. [Google Scholar] [CrossRef] [PubMed]
- Nelson, T.F.; Remke, S.C.; Kohler, H.P.E.; McNeill, K.; Sander, M. Quantification of Synthetic Polyesters from Biodegradable Mulch Films in Soils. Environ. Sci. Technol. 2019. [Google Scholar] [CrossRef] [PubMed]
- Elert, A.M.; Becker, R.; Duemichen, E.; Eisentraut, P.; Falkenhagen, J.; Sturm, H.; Braun, U. Comparison of different methods for MP detection: What can we learn from them and why asking the right question before measurements matters? Environ. Pollut. 2017, 231, 1256–1264. [Google Scholar] [CrossRef] [PubMed]
- La Nasa, J.; Biale, G.; Mattonai, M.; Modugno, F. Microwave-assisted solvent extraction and double-shot analytical pyrolysis for the quali-quantitation of plasticizers and microplastics in beach sand samples. J. Hazard. Mater. 2020, 401, 123287. [Google Scholar] [CrossRef]
- Ceccarini, A.; Corti, A.; Erba, F.; Modugno, F.; Nasa, J.L.; Bianchi, S.; Castelvetro, V. The Hidden Microplastics: New Insights and Figures from the Thorough Separation and Characterization of Microplastics and of Their Degradation Byproducts in Coastal Sediments. Environ. Sci. Technol. 2018, 52, 5634–5643. [Google Scholar] [CrossRef] [PubMed]
- Felsing, S.; Kochleus, C.; Buchinger, S.; Brennholt, N.; Stock, F.; Reifferscheid, G. A new approach in separating microplastics from environmental samples based on their electrostatic behavior. Environ. Pollut. 2018, 234, 20–28. [Google Scholar] [CrossRef]
- Grbic, J.; Nguyen, B.; Guo, E.; You, J.B.; Sinton, D.; Rochman, C.M. Magnetic Extraction of Microplastics from Environmental Samples. Environ. Sci. Technol. Lett. 2019, 6, 68–72. [Google Scholar] [CrossRef]
- Crichton, E.M.; Noël, M.; Gies, E.A.; Ross, P.S. A novel density-independent and FTIR-compatible approach for the rapid extraction of microplastics from aquatic sediments. Anal. Methods 2017, 9, 1419–1428. [Google Scholar] [CrossRef]
- Mani, T.; Frehland, S.; Kalberer, A.; Burkhardt-Holm, P. Using castor oil to separate microplastics from four different environmental matrices. Anal. Methods 2019, 11, 1788–1794. [Google Scholar] [CrossRef] [Green Version]
- Scopetani, C.; Chelazzi, D.; Mikola, J.; Leiniö, V.; Heikkinen, R.; Cincinelli, A.; Pellinen, J. Olive oil-based method for the extraction quantification and identification of microplastics in soil and compost samples. Sci. Total Environ. 2020, 733, 139338. [Google Scholar] [CrossRef]
- Lusher, A.L.; Bråte, I.L.N.; Munno, K.; Hurley, R.R.; Welden, N.A. Is It or Isn’t It: The Importance of Visual Classification in Microplastic Characterization. Appl. Spectrosc. 2020. [Google Scholar] [CrossRef]
- Filella, M. Questions of size and numbers in environmental research on microplastics: Methodological and conceptual aspects. Environ. Chem. 2015, 12, 527. [Google Scholar] [CrossRef] [Green Version]
- Zhang, S.; Liu, X.; Hao, X.; Wang, J.; Zhang, Y. Distribution of low-density microplastics in the mollisol farmlands of northeast China. Sci. Total Environ. 2020, 708, 135091. [Google Scholar] [CrossRef]
- Corradini, F.; Bartholomeus, H.; Huerta Lwanga, E.; Gertsen, H.; Geissen, V. Predicting soil microplastic concentration using vis-NIR spectroscopy. Sci. Total Environ. 2019, 650, 922–932. [Google Scholar] [CrossRef] [PubMed]
- Helmberger, M.S.; Frame, M.K.; Grieshop, M. Counterstaining to Separate Nile Red-Stained Microplastic Particles from Terrestrial Invertebrate Biomass. Environ. Sci. Technol. 2020, 54, 5580–5588. [Google Scholar] [CrossRef]
- Nel, H.A.; Chetwynd, A.J.; Kelleher, L.; Lynch, I.; Mansfield, I.; Margenat, H.; Onoja, S.; Goldberg Oppenheimer, P.; Sambrook Smith, G.H. Detection Limits Are Central to Improve Reporting Standards When Using Nile Red for Microplastic Quantification. Chemosphere 2021, 263, 127953. [Google Scholar] [CrossRef]
- Anger, P.M.; von der Esch, E.; Baumann, T.; Elsner, M.; Niessner, R.; Ivleva, N.P. Raman microspectroscopy as a tool for microplastic particle analysis. TrAC Trends Anal. Chem. 2018, 109, 214–226. [Google Scholar] [CrossRef]
- Xu, J.L.; Thomas, K.V.; Luo, Z.; Gowen, A.A. FTIR and Raman imaging for microplastics analysis: State of the art challenges and prospects. TrAC Trends Anal. Chem. 2019, 119, 115629. [Google Scholar] [CrossRef]
- Lares, M.; Ncibi, M.C.; Sillanpää, M.; Sillanpää, M. Intercomparison study on commonly used methods to determine microplastics in wastewater and sludge samples. Environ. Sci. Pollut. Res. 2019, 26, 12109–12122. [Google Scholar] [CrossRef] [Green Version]
- Simon, M.; van Alst, N.; Vollertsen, J. Quantification of microplastic mass and removal rates at wastewater treatment plants applying Focal Plane Array (FPA)-based Fourier Transform Infrared (FT-IR) imaging. Water Res. 2018, 142, 1–9. [Google Scholar] [CrossRef]
- David, J.; Steinmetz, Z.; Kučerík, J.; Schaumann, G.E. Quantitative Analysis of Poly(ethylene terephthalate) Microplastics in Soil via Thermogravimetry-Mass Spectrometry. Anal. Chem. 2018, 90, 8793–8799. [Google Scholar] [CrossRef]
- Boyron, O.; Marre, T.; Delauzun, A.; Cozic, R.; Boisson, C. An Advanced Technique for Linear Low-Density Polyethylene Composition Determination: TGA–IST16–GC–MS Coupling. Macromol. Chem. Phys. 2019, 220, 1900162. [Google Scholar] [CrossRef]
- Dümichen, E.; Eisentraut, P.; Bannick, C.G.; Barthel, A.K.; Senz, R.; Braun, U. Fast identification of microplastics in complex environmental samples by a thermal degradation method. Chemosphere 2017, 174, 572–584. [Google Scholar] [CrossRef] [PubMed]
- Fischer, M.; Scholz-Böttcher, B.M. Microplastics analysis in environmental samples—Recent pyrolysis-gas chromatography-mass spectrometry method improvements to increase the reliability of mass-related data. Anal. Methods 2019, 11, 2489–2497. [Google Scholar] [CrossRef]
- La Nasa, J.; Biale, G.; Fabbri, D.; Modugno, F. A review on challenges and developments of analytical pyrolysis and other thermoanalytical techniques for the quali-quantitative determination of microplastics. J. Anal. Appl. Pyrolysis 2020, 149, 104841. [Google Scholar] [CrossRef]
- Nguyen, B.; Claveau-Mallet, D.; Hernandez, L.M.; Xu, E.G.; Farner, J.M.; Tufenkji, N. Separation and Analysis of Microplastics and Nanoplastics in Complex Environmental Samples. Accounts Chem. Res. 2019, 52, 858–866. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sullivan, G.; Gallardo, J.D.; Jones, E.; Hollliman, P.; Watson, T.; Sarp, S. Detection of trace sub-micron (nano) plastics in water samples using pyrolysis-gas chromatography time of flight mass spectrometry (PY-GCToF). Chemosphere 2020, 249, 126179. [Google Scholar] [CrossRef] [PubMed]
- Becker, R.; Altmann, K.; Sommerfeld, T.; Braun, U. Quantification of microplastics in a freshwater suspended organic matter using different thermoanalytical methods—Outcome of an interlaboratory comparison. J. Anal. Appl. Pyrolysis 2020, 148, 104829. [Google Scholar] [CrossRef]
- Schirinzi, G.F.; Llorca, M.; Seró, R.; Moyano, E.; Barceló, D.; Abad, E.; Farré, M. Trace analysis of polystyrene microplastics in natural waters. Chemosphere 2019, 236, 124321. [Google Scholar] [CrossRef]
- Drzeżdżon, J.; Jacewicz, D.; Sielicka, A.; Chmurzyński, L. A review of new approaches to analytical methods to determine the structure and morphology of polymers. TrAC Trends Anal. Chem. 2019, 118, 470–476. [Google Scholar] [CrossRef]
- Peez, N.; Janiska, M.C.; Imhof, W. The first application of quantitative 1H NMR spectroscopy as a simple and fast method of identification and quantification of microplastic particles (PE PET and PS). Anal. Bioanal. Chem. 2018, 411, 823–833. [Google Scholar] [CrossRef]
- Peez, N.; Imhof, W. Quantitative 1H-NMR spectroscopy as an efficient method for identification and quantification of PVC ABS and PA microparticles. Analyst 2020, 145, 5363–5371. [Google Scholar] [CrossRef] [PubMed]
- Watteau, F.; Dignac, M.F.; Bouchard, A.; Revallier, A.; Houot, S. Microplastic Detection in Soil Amended With Municipal Solid Waste Composts as Revealed by Transmission Electronic Microscopy and Pyrolysis/GC/MS. Front. Sustain. Food Syst. 2018, 2. [Google Scholar] [CrossRef] [Green Version]
- Du, C.; Wu, J.; Gong, J.; Liang, H.; Li, Z. ToF-SIMS characterization of microplastics in soils. Surf. Interface Anal. 2020, 52, 293–300. [Google Scholar] [CrossRef]
- Paul, A.; Wander, L.; Becker, R.; Goedecke, C.; Braun, U. High-throughput NIR spectroscopic (NIRS) detection of microplastics in soil. Environ. Sci. Pollut. Res. 2019, 26, 7364–7374. [Google Scholar] [CrossRef]

| Density Solution | Density [g cm—3] | Evaluated Polymer Type(s) | Sample Type | Remarks | Ref. |
|---|---|---|---|---|---|
| Ethanol (96%) | 0.8 | Light-density SOM | Plant material | Flotation of light-density SOM; no microplastic separation | [71] |
| Deionized water | 1.0 | PE, PP | Clay soil, loess, and sandy soil | Not suitable for high-density polymers | [57] |
| NaCl | 1.2 | PE, PP, PS, PA, PC, PMMA, ABS | Farmland soil, marine sediment | Not suitable for high-density polymers | [45,72] |
| NaBr | 1.4–1.6 | PE, PP, PS, PET, PVC, PA, PMMA | Agricultural and floodplain soil, sediment | [73,74] | |
| CaCl2 | 1.3–1.5 | PE, PP, PS, PET, PVC, PC, PA, PU, ABS | Organic-rich topsoil | Ca2+ may cause flocculation of SOM | [48] |
| Potassium formate | 1.5–1.6 | PE, PP, PS, PET | Lakeshore sediments | No validation performed | [75] |
| ZnCl2 | 1.5–1.7 | PS | Biosolids, soil | Expensive†, corrosive, and harmful to the environment, may alter microplastics and cause foaming | [35] |
| ZnBr2 | 1.7 | PE, PP, PS, PET, PVC, PA | Sediment | Expensive†, corrosive, and harmful to the environment | [74] |
| NaI | 1.6–1.8 | PE, PP, PS, PET, PVC, PA, PU | Agricultural soil, sediment | Expensive†, harmful to the environment | [62,72,74,76] |
| SPT | 1.4–1.8 | PE, PET, PVC, PA | (Beach) sediment | Expensive† | [68,77] |
| Sodium tungstate dihydrate | 1.4 | PE, PP, PS, PET, PVC, PC, PA, PU, PMMA, EVA | Sediment | Expensive† | [78] |
| Reagent | Sample Type | Evaluated Polymers | Extraction Time [d] | Temp. [°C] | SOM Removal Efficiency [%] | Deteriorated Polymers | Ref. |
|---|---|---|---|---|---|---|---|
| KOH | Loamy sand | PE, PP, PS, PET, PA, PC, PMMA | 1 | 60 | 30 ± 20 | PC | [94] |
| NaOH | Loamy sand | PE, PP, PS, PET, PA, PC, PMMA | 1 | 60 | 70 ± 20 (1 M), 60 ± 40 (10 M) | PET, PC | [94] |
| HNO3 | Floodplain soil | PE, PP, PS, PET, PVC, PA, PC, PU, ABS | 2 | 90 | Higher than NaOH, H2SO4, H2O2 | ABS, PA, PET | [48] |
| H2SO4 | Floodplain soil | PE, PP, PS, PET, PVC, PA, PC, PU, ABS | 1, 4, 7 | 90 | Lower than with HNO3 | Not tested | [48] |
| H2O2 | Loamy sand | PE, PP, PS, PET, PA, PC, PMMA | 1 | 60 | 100 ± 10 | PS | [94] |
| H2O2 | Agricultural soil | PE, PP, PS, PET, PVC, PA, PC, PMMA, ABS | 1 | 60 | Not reported | Not reported | [73] |
| H2O2 | Sediment | PE, PP, PS, PET, PVC, PA, PC, PU, ABS | 7 | RT | Not reported | PET, PVC, PC, PA, PUR, PP, LDPE | [72] |
| H2O2 | Loamy sand | PS, PP, PE, PET, PA, PC, PMMA | 1 | 70 | 110 ± 10 | PA, PS | [94] |
| Fenton reagent | Loamy sand | PE, PP, PS, PET, PA, PC, PMMA | 1 | 40 | 110 ± 10 | None | [94] |
| Method | Sample | Polymer | Extraction | Recovery | Ref. | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| Type | Mass | Type | Spiked conc. | Solvent(s) | Volume | Temperature | Time | |||
| [g] | [mg g—1] | [mL] | [°C] | [h] | [%] | |||||
| ASE | Municipal waste, soil | 2 | PE, PP, PS, PET, PVC | 5–25 | DCM | 80 | 180 | 0.25 | 85–94 | [123] |
| ASE | Roadside and potting soil | 1 | PE, PP, PS | 0.05–0.75 | THF | 35 | 185 | 1 | 77–123 | [122] |
| ASE | Agricultural soil | 2.5–5 | PBAT | 1 | Chloroform/methanol (9:1) | 40–50 | 120 | 0.5 | 100 | [126] |
| ASE | Biosolids | 1 | PE, PP, PS, PET, PVC, PC, PMMA | 0.02–0.1 | DCM | 80 | 180 | 0.25 | 85–128 | [124] |
| Batch extraction | Agricultural soil | 0.5 | PS | 5 | THF | 2 | 45 | 24 | 100 | [127] |
| Batch extraction | Agricultural soil | 0.5 | PET | 20 | HFIP | 2 | 45 | 24 | 80 | [127] |
| Batch extraction | Sediment | 2.5 | PET | 0.8 | Chloroform/TFA (4:1) | 1 | RT | 2–4 | 91–108 | [121] |
| Batch extraction | Agricultural soil | 4 | PE, PP, PS | 0.05–0.25 | 1,2,4-TCB | 8 | 120 | 1 | 70–128 | [33] |
| Microwave | Beach sand | 1 | PS | 0.05 | DCM | 1 | 80 | 1 | 97 | [128] |
| Kumagawa apparatus | Beach sand | 160 | PE, PS | 0.36–0.82 | (1) DCM, (2) xylenes † | 90 | (1) 37, (2) 135–140 † | 3–6 | 95–97 | [129] |
| Soxhlet | Sediment, suspended matter | 10 | PVC | 1 | DCM | 300 | >40 | 16 | 85 | [120] |
| Soxhlet | Agricultural soil | 150 | PBS | 4 | Chloroform | NA | >61 | 8 | 83 | [125] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Thomas, D.; Schütze, B.; Heinze, W.M.; Steinmetz, Z. Sample Preparation Techniques for the Analysis of Microplastics in Soil—A Review. Sustainability 2020, 12, 9074. https://doi.org/10.3390/su12219074
Thomas D, Schütze B, Heinze WM, Steinmetz Z. Sample Preparation Techniques for the Analysis of Microplastics in Soil—A Review. Sustainability. 2020; 12(21):9074. https://doi.org/10.3390/su12219074
Chicago/Turabian StyleThomas, Daniela, Berit Schütze, Wiebke Mareile Heinze, and Zacharias Steinmetz. 2020. "Sample Preparation Techniques for the Analysis of Microplastics in Soil—A Review" Sustainability 12, no. 21: 9074. https://doi.org/10.3390/su12219074
APA StyleThomas, D., Schütze, B., Heinze, W. M., & Steinmetz, Z. (2020). Sample Preparation Techniques for the Analysis of Microplastics in Soil—A Review. Sustainability, 12(21), 9074. https://doi.org/10.3390/su12219074