Abiotic Soil Health Indicators that Respond to Sustainable Management Practices in Sugarcane Cultivation
Abstract
:1. Introduction
2. Material and Methods
2.1. Description and History of the Area
2.2. Experimental Design
2.3. Soil Sampling and Laboratory Analysis
2.4. Soil Quality Index
2.5. Categorization of the Soil Quality Index
2.6. Decision Tree Induction and Generated Model Validation
2.7. Statistical Analysis
3. Results
3.1. Selection of Indicators
3.2. Soil Quality Index
4. Discussion
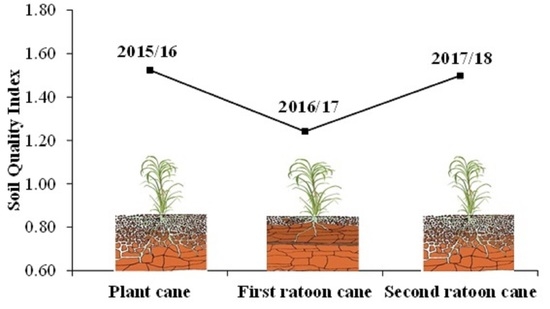
4.1. Impact of Production Cycles on Soil Quality
4.2. Impact of Cover Crops and Tillage Systems
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Conab—Companhia Nacional de Abastecimento. Acompanhamento de Safra Brasileira: Cana-de-Açúcar, Primeiro Levantamento, Safra 2019/20; CONAB: Brasília, Brasil, 2020; 62p. [Google Scholar]
- Esteban, D.A.A.; De Souza, Z.M.; Tormena, C.A.; Lovera, L.H.; Lima, E.D.S.; De Oliveira, I.N.; Ribeiro, N.D.P. Soil compaction, root system and productivity of sugarcane under different row spacing and controlled traffic at harvest. Soil Tillage Res. 2019, 187, 60–71. [Google Scholar] [CrossRef]
- Bordonal, R.O.; Carvalho, J.L.N.; Lal, R.; De Figueiredo, E.B.; De Oliveira, B.G.; Júnior, N.L.S. Sustainability of sugarcane production in Brazil. A review. Agron. Sustain. Dev. 2018, 38, 1–23. [Google Scholar] [CrossRef] [Green Version]
- Barbosa, L.C.; Magalhães, P.S.G.; Bordonal, R.O.; Cherubin, M.R.; Castioni, G.A.; Tenelli, S.; Franco, H.C.J.; Carvalho, J.L.N. Soil physical quality associated with tillage practices during sugarcane planting in south-central Brazil. Soil Tillage Res. 2019, 195, 104383. [Google Scholar] [CrossRef]
- Busari, M.A.; Kukal, S.S.; Kaur, A.; Bhatt, R.; Dulazi, A.A. Conservation tillage impacts on soil, crop and the environment. Int. Soil Water Conserv. Res. 2015, 3, 119–129. [Google Scholar] [CrossRef] [Green Version]
- Farhate, C.V.V.; Souza, Z.M.; Júnior, N.L.S.; Sousa, A.C.M.; Santos, A.P.G.; Carvalho, J.L.N.; Júnior, N.L.S. Soil tillage and cover crop on soil CO 2 emissions from sugarcane fields. Soil Use Manag. 2019, 35, 273–282. [Google Scholar] [CrossRef]
- D’Hose, T.; Cougnon, M.; De Vliegher, A.; Vandecasteele, B.; Viaene, N.; Cornelis, W.; Van Bockstaele, E.; Reheul, D. The positive relationship between soil quality and crop production: A case study on the effect of farm compost application. Appl. Soil Ecol. 2014, 75, 189–198. [Google Scholar] [CrossRef]
- Obade, V.D.P.; Lal, R. A standardized soil quality index for diverse field conditions. Sci. Total. Environ. 2016, 541, 424–434. [Google Scholar] [CrossRef]
- Karlen, D.L.; Mausbach, M.J.; Doran, J.W.; Cline, R.G.; Harris, R.F.; Schuman, G.E. Soil Quality: A Concept, Definition, and Framework for Evaluation (A Guest Editorial). Soil Sci. Soc. Am. J. 1997, 61, 4–10. [Google Scholar] [CrossRef] [Green Version]
- Bünemann, E.K.; Bongiorno, G.; Bai, Z.; Creamer, R.E.; Deyn, G.D.; Goede, R.; Fleskens, L.; Geissen, V.; Kuyper, T.W.; Mäder, P.; et al. Soil quality—A critical review. Soil Biol. Biochem. 2018, 120, 105–125. [Google Scholar] [CrossRef]
- Hussain, I.; Olson, K.R.; Wander, M.; Karlen, D. Adaptation of soil quality indices and application to three tillage systems in southern Illinois. Soil Tillage Res. 1999, 50, 237–249. [Google Scholar] [CrossRef]
- Cherubin, M.R.; Franco, A.L.; Cerri, C.E.P.; Oliveira, D.M.D.S.; Davies, C.A.; Cerri, C.C. Sugarcane expansion in Brazilian tropical soils—Effects of land use change on soil chemical attributes. Agric. Ecosyst. Environ. 2015, 211, 173–184. [Google Scholar] [CrossRef]
- Cherubin, M.R.; Karlen, D.L.; Franco, A.L.; Tormena, C.A.; Cerri, C.E.; Davies, C.A.; Cerri, C.E.P. Soil physical quality response to sugarcane expansion in Brazil. Geoderma 2016, 267, 156–168. [Google Scholar] [CrossRef]
- Cherubin, M.R.; Karlen, D.L.; Franco, A.L.; Cerri, C.E.P.; Tormena, C.A. A Soil Management Assessment Framework (SMAF) Evaluation of Brazilian Sugarcane Expansion on Soil Quality. Soil Sci. Soc. Am. J. 2016, 80, 215–226. [Google Scholar] [CrossRef]
- Cherubin, M.R.; Karlen, D.L.; Cerri, C.E.P.; Franco, A.L.; Tormena, C.A.; Davies, C.A.; Cerri, C.C. Soil Quality Indexing Strategies for Evaluating Sugarcane Expansion in Brazil. PLoS ONE 2016, 11, e0150860. [Google Scholar] [CrossRef]
- Cherubin, M.R.; Franco, A.L.; Guimarães, R.M.L.; Tormena, C.A.; Cerri, C.E.P.; Karlen, D.L.; Cerri, C.E.P. Assessing soil structural quality under Brazilian sugarcane expansion areas using Visual Evaluation of Soil Structure (VESS). Soil Tillage Res. 2017, 173, 64–74. [Google Scholar] [CrossRef] [Green Version]
- Wauters, E.; Bielders, C.; Poesen, J.; Govers, G.; Mathijs, E. Adoption of soil conservation practices in Belgium: An examination of the theory of planned behaviour in the agri-environmental domain. Land Use Policy 2010, 27, 86–94. [Google Scholar] [CrossRef]
- Awe, G.; Reichert, J.; Fontanela, E. Sugarcane production in the subtropics: Seasonal changes in soil properties and crop yield in no-tillage, inverting and minimum tillage. Soil Tillage Res. 2020, 196, 104447. [Google Scholar] [CrossRef]
- Poeplau, C.; Kätterer, T.; Bolinder, M.A.; Börjesson, G.; Berti, A.; Lugato, E. Low stabilization of aboveground crop residue carbon in sandy soils of Swedish long-term experiments. Geoderma 2015, 237–238, 246–255. [Google Scholar] [CrossRef]
- Voyant, C.; Notton, G.; Kalogirou, S.; Nivet, M.-L.; Paoli, C.; Motte, F.; Fouilloy, A. Machine learning methods for solar radiation forecasting: A review. Renew. Energy 2017, 105, 569–582. [Google Scholar] [CrossRef]
- Dongming, L.; Yan, L.; Chao, Y.; Chaoran, L.; Huan, L.; Lijuan, Z. The application of decision tree C4.5 algorithm to soil quality grade forecasting model. In Proceedings of the 2016 First IEEE International Conference on Computer Communication and the Internet (ICCCI), Wuhan, China, 13–15 October 2016; pp. 552–555. [Google Scholar]
- Saghebian, S.M.; Sattari, M.T.; Mirabbasi, R.; Pal, M. Ground water quality classification by decision tree method in Ardebil region, Iran. Arab. J. Geosci. 2013, 7, 4767–4777. [Google Scholar] [CrossRef]
- Alvares, C.A.; Stape, J.L.; Sentelhas, P.C.; Gonçalves, J.L.M.; Sparovek, K. Köppen’s climate classification map for Brazil. Meteorol. Z. 2013, 22, 711–728. [Google Scholar] [CrossRef]
- Santos, H.G.; Jacomine, P.K.T.; Anjos, L.H.C.; Oliveira, V.A.; Lumbreras, J.F.; Coelho, M.R.; Almeida, J.A.; Araujo Filho, J.C.; Oliveira, J.B.; Cunha, T.J.F. Sistema Brasileiro de Classificação de Solos, 5th ed.; Embrapa: Brasília, Brasil, 2018; p. 353. [Google Scholar]
- Soil Survey Staff. Keys to Soil Taxonomy, 12th ed.; Department of Agriculture, Natural Resources Conservation Service: Washington, DC, USA, 2014; p. 372.
- USDA—United States Department of Agriculture. Soil Science Division Staff. Soil Survey Manual; Ditzler, C., Scheffe, K., Monger, H.C., Eds.; USDA Handbook 18; Government Printing Office: Washington, DC, USA, 2017.
- Teixeira, P.C.; Donagemma, G.K.; Fontana, A.; Teixeira, W.G. Manual de Métodos de Análise de Solos, 3rd ed.; Embrapa: Brasília, Brasil, 2017; p. 573. [Google Scholar]
- Dexter, A.; Kroesbergen, B. Methodology for determination of tensile strength of soil aggregates. J. Agric. Eng. Res. 1985, 31, 139–147. [Google Scholar] [CrossRef]
- Kemper, W.D.; Chepil, W.S. Size distribution of aggregates. In Methods of Soil Analysis: Physical and Mineralogical Properties, Including Statistics of Measurement and Sampling. Part 1.; Black, C.A., Ed.; American Society of Agronomy: Madison, WI, USA, 1965; pp. 499–510. [Google Scholar]
- Van Raij, B.; Andrade, J.C.; Cantarella, H.; Quaggio, J.A. Análise Química Para Avaliação da Fertilidade de Solos Tropicais; Instituto Agronômico: Campinas, Brasil, 2001; p. 285. [Google Scholar]
- Nelson, D.W.; Sommers, L.E. Total carbon, organic carbon and organic matter. In Methods of Soil Analysis. Part 3: Chemical Methods; Soil Science Society of America and American Society of Agronom: Madison, WI, USA, 1996; pp. 963–1010. [Google Scholar]
- Andrews, S.S.; Karlen, D.L.; Cambardella, C.A. The Soil Management Assessment Framework. Soil Sci. Soc. Am. J. 2004, 68, 1945–1962. [Google Scholar] [CrossRef]
- Kaiser, H.F. The varimax criterion for analytic rotation in factor analysis. Psychometrika 1958, 23, 187–200. [Google Scholar] [CrossRef]
- Chaves, H.M.L.; Lozada, C.M.C.; Gaspar, R.O. Soil quality index of an Oxisol under different land uses in the Brazilian savannah. Geoderma Reg. 2017, 10, 183–190. [Google Scholar] [CrossRef]
- Quinlan, J.R. C4.5: Programs for Empirical Learning; Morgan Kaufmann: San Francisco, CA, USA, 1993; p. 302. [Google Scholar]
- Koch, J.R.L.G. The Measurement of Observer Agreement for Categorical Data. Biometrics 1977, 33, 159. [Google Scholar] [CrossRef] [Green Version]
- Souza, G.S.; Souza, Z.M.; Silva, R.B.; Barbosa, R.S.; Araújo, F.S. Controle de tráfego e seu efeito na qualidade física do solo e no cultivo da cana-de-açúcar. Rev. Bras. Ciênc. Solo. 2014, 38, 135–146. [Google Scholar] [CrossRef] [Green Version]
- Satiro, L.S.; Cherubin, M.R.; Safanelli, J.L.; Lisboa, I.P.; Junior, P.R.D.R.; Cerri, C.E.P.; Cerri, C.C. Sugarcane straw removal effects on Ultisols and Oxisols in south-central Brazil. Geoderma Reg. 2017, 11, 86–95. [Google Scholar] [CrossRef]
- Carvalho, J.L.N.; Hudiburg, T.W.; Franco, H.C.J.; DeLucia, E.H. Contribution of above- and belowground bioenergy crop residues to soil carbon. GCB Bioenergy 2017, 9, 1333–1343. [Google Scholar] [CrossRef]
- Esteban, D.A.A.; De Souza, Z.M.; Da Silva, R.B.; Lima, E.D.S.; Lovera, L.H.; De Oliveira, I.N. Impact of permanent traffic lanes on the soil physical and mechanical properties in mechanized sugarcane fields with the use of automatic steering. Geoderma 2020, 362, 114097. [Google Scholar] [CrossRef]
- Leal, M.R.L.V.; Galdos, M.; Scarpare, F.V.; Seabra, J.E.A.; Walter, A.C.S.; Oliveira, C.O. Sugarcane straw availability, quality, recovery and energy use: A literature review. Biomass Bioenergy 2013, 53, 11–19. [Google Scholar] [CrossRef]
- Castioni, G.A.; Cherubin, M.R.; Menandro, L.; Sanches, G.M.; Bordonal, R.O.; Barbosa, L.C.; Franco, H.C.J.; Carvalho, J.L.N. Soil physical quality response to sugarcane straw removal in Brazil: A multi-approach assessment. Soil Tillage Res. 2018, 184, 301–309. [Google Scholar] [CrossRef]
- Da Silva, R.B.; Iori, P.; De Souza, Z.M.; Pereira, D.D.M.G.; Filho, O.J.V.; Silva, F.A.D.M. Contact pressures and the impact of farm equipment on Latosol with the presence and absence of sugarcane straw. Ciênc. Agrotec. 2016, 40, 265–278. [Google Scholar] [CrossRef] [Green Version]
- Castioni, G.A.F.; Cherubin, M.R.; Bordonal, R.D.O.; Barbosa, L.C.; Menandro, L.M.S.; Carvalho, J.L.N. Straw Removal Affects Soil Physical Quality and Sugarcane Yield in Brazil. BioEnergy Res. 2019, 12, 789–800. [Google Scholar] [CrossRef]
- Filho, O.J.V.; De Souza, Z.M.; Da Silva, R.B.; De Lima, C.C.; Pereira, D.D.M.G.; De Lima, M.E.; De Sousa, A.C.M.; De Souza, G.S. Capacidade de suporte de carga de Latossolo Vermelho cultivado com cana-de-açúcar e efeitos da mecanização no solo. Pesqui. Agropecu. Bras. 2015, 50, 322–332. [Google Scholar] [CrossRef] [Green Version]
- Pimentel, L.G.; Cherubin, M.R.; Oliveira, D.M.; Cerri, C.E.; Cerri, C.C. Decomposition of sugarcane straw: Basis for management decisions for bioenergy production. Biomass Bioenergy 2019, 122, 133–144. [Google Scholar] [CrossRef]
- Han, E.; Kautz, T.; Perkons, U.; Lüsebrink, M.; Pude, R.; Köpke, U. Quantification of soil biopore density after perennial fodder cropping. Plant Soil 2015, 394, 73–85. [Google Scholar] [CrossRef]
- Hinsinger, P.; Bengough, A.G.; Vetterlein, D.; Young, I.M. Rhizosphere: Biophysics, biogeochemistry and ecological relevance. Plant Soil 2009, 321, 117–152. [Google Scholar] [CrossRef]
- Kautz, T. Research on subsoil biopores and their functions in organically managed soils: A review. Renew. Agric. Food Syst. 2014, 30, 318–327. [Google Scholar] [CrossRef]
- Silva, A.P.M.; Bono, J.A.M.; Pereira, F.A.R. Fertigation with vinasse in sugarcane crop: Effect on the soil and on productivity. Rev. Bras. Eng. Agríc. 2014, 18, 38–43. [Google Scholar] [CrossRef] [Green Version]
- Almeida Júnior, A.B.; Nascimento, C.W.A.; Sobral, M.F.; Silva, F.B.V.; Gomes, W.A. Soil fertility and uptake of nutrients by sugarcane fertilized with filter cake. Rev. Bras. Eng. Agríc. Ambient. 2011, 15, 1004–1013. [Google Scholar] [CrossRef] [Green Version]
- Weiler, D.A.; Moro, V.J.; Awe, G.O.; Oliveira, D.M.S.; Cerri, C.P.P.; Reichert, J.M.; Giacomini, S.J. Carbon Balance in Sugarcane Areas Under Different Tillage Systems. Bioenergy Res. 2019, 12, 778–788. [Google Scholar] [CrossRef]
- De Oliveira, I.N.; De Souza, Z.M.; Lovera, L.H.; Farhate, C.V.V.; Lima, E.D.S.; Esteban, D.A.A.; Fracarolli, J. Least limiting water range as influenced by tillage and cover crop. Agric. Water Manag. 2019, 225, 105777. [Google Scholar] [CrossRef]
- Sarkar, S.K.; Hazra, S.K.; Sen, H.S.; Karmakar, P.G.; Tripathi, M.K. Sunn Hemp in India; ICAR-Central Research Institute for Jute and Allied Fibres (ICAR): Barrackpore, India, 2015; p. 14. [Google Scholar]
- Shekinah, D.E.; Stute, J.K. Sunn Hemp: A Legume Cover Crop with Potential for the Midwest? Sustain. Agric. Res. 2018, 7, 63–69. [Google Scholar]
- Foloni, J.S.S.; Lima, S.L.L.; Büll, L.T. Shoot and root growth of soybean and cover crops as affected by soil compaction. Rev. Bras. Ciênc. Solo. 2006, 30, 49–57. [Google Scholar] [CrossRef] [Green Version]
- Dantas, R.D.A.; Carmona, R.; De Carvalho, A.M.; Rein, T.A.; Malaquias, J.V.; Junior, J.D.D.G.D.S. Produção de matéria seca e controle de plantas daninhas por leguminosas consorciadas com cana-de-açúcar em cultivo orgânico. Pesqui. Agropecu. Bras. 2015, 50, 681–689. [Google Scholar] [CrossRef]
- Chagas, M.F.; Bordonal, R.O.; Cavalett, O.; Carvalho, J.L.N.; Bonomi, A.; Júnior, N.L.S. Environmental and economic impacts of different sugarcane production systems in the ethanol biorefinery. Biofuels Bioprod. Biorefining 2015, 10, 89–106. [Google Scholar] [CrossRef]
- Perin, A.; Santos, R.H.S.; Urquiaga, S.; Guerra, J.G.M.; Cecon, P.R. Produção de fitomassa, acúmulo de nutrientes e fixação biológica de nitrogênio por adubos verdes em cultivo isolado e consorciado. Pesqui. Agropecu. Bras. 2004, 39, 35–40. [Google Scholar] [CrossRef] [Green Version]
- Teodoro, R.B.; Oliveira, F.; Da Silva, D.M.N.; Fávero, C.; Quaresma, M.A.L. Aspectos agronômicos de leguminosas para adubação verde no Cerrado do Alto Vale do Jequitinhonha. Rev. Bras. Ciênc. Solo 2011, 35, 635–640. [Google Scholar] [CrossRef]
- Perin, A.; Santos, R.H.S.; Urquiaga, S.S.; Cecon, P.R.; Guerra, J.G.M.; Freitas, G.B. Sunn hemp and millet as green manure for tropical maize production. Sci. Agric. 2006, 63, 453–459. [Google Scholar] [CrossRef]
- Ambrosano, E.J.; Cantarella, H.; Dias, F.L.F.; Rossi, F.; Trivelin, P.C.O.; Muraoka, T. The role of green manure nitrogen use by corn and sugarcane crops in Brazil. Agric. Sci. 2013, 4, 89–108. [Google Scholar] [CrossRef] [Green Version]
- Júnior, J.B.D.; Coelho, F.C. Adubos verdes e seus efeitos no rendimento da cana-de-açúcar em sistema de plantio direto. Bragantia 2008, 67, 723–732. [Google Scholar] [CrossRef] [Green Version]
- Stallings, A.M.; Balkcom, K.S.; Wood, C.W.; Guertal, E.A.; Weaver, D.B. Nitrogen Mineralization from ‘Au Golden’ Sunn Hemp Residue. J. Plant Nutr. 2016, 40, 50–62. [Google Scholar] [CrossRef]
- Pereira-Filho, I.A.; Rodrigues, J.A.S.; Karam, D.; Coelho, A.M.; Alvarenga, R.C.; Cruz, J.C.; Cabezas, W.L. Manejo da cultura do milheto. In Milheto: Tecnologias de Produção e Agronegócio; Netto, D.A.M., Durões, F.O.M., Eds.; Embrapa-Informações Tecnológicas: Brasília, Brasil, 2005; pp. 59–87. [Google Scholar]
- Padovan, M.P.; Motta, I.S.; Carneiro, L.F.; Moitinho, M.R.; Salomão, G.B. Dynamics of mass accumulation and nutrient by millet for green manure production systems under agroecological agroecosystem. Rev. Bras. Agroecol. 2012, 7, 95–103. [Google Scholar]
- Calvo, C.L.; Foloni, J.S.S.; Brancalião, S. Produtividade de fitomassa e relação C/N de monocultivos e consórcios de guandu-anão, milheto e sorgo em três épocas de corte. Bragantia 2010, 69, 77–86. [Google Scholar] [CrossRef]
- Moreira, F.M.S.; Siqueira, J.O. Microbiologia e Bioquímica do Solo; Editora UFLA: Lavras-MG, Brasil, 2002; p. 626. [Google Scholar]
- Lynch, M.J.; Mulvaney, M.J.; Hodges, S.C.; Thompson, T.L.; Thomason, W.E. Decomposition, nitrogen and carbon mineralization from food and cover crop residues in the central plateau of Haiti. SpringerPlus 2016, 5, 973. [Google Scholar] [CrossRef] [Green Version]
- Ribeiro, R.H.; Besen, M.R.; Figueroa, L.V.; Bogo, T.; Brancaleoni, E.; Ronsani, S.C.; Guginski-Piva, C.A.; Piva, J.T. Efeito da adubação nitrogenada na cobertura do solo e produção de fitomassa de espécies de inverno. Varia Sci. Agrár. 2017, 4, 41–53. [Google Scholar]
- Menezes, L.A.S.; Leandro, W.M.; Oliveira Junior, J.P.; Ferreira, A.C.B.; Santana, J.G.; Barros, R.G. Produção de fitomassa de diferentes espécies, isoladas e consorciadas, com potencial de utilização para cobertura do solo. Biosci. J. 2009, 25, 7–12. [Google Scholar]
- Soratto, R.P.; Crusciol, C.A.C.; Costa, C.H.M.; Ferrari Neto, J.; Castro, G.S.A. Production, decomposition and nutrient cycling in residues of sunnhemp and pearl millet in monocropped and intercropped systems. Pesqui. Agropecu. Bras. 2012, 47, 1462–1470. [Google Scholar] [CrossRef] [Green Version]




| Layers | Particle-Size Fraction | Texture * | Soil Horizon | |||
|---|---|---|---|---|---|---|
| Sand | Silt | Clay | ||||
| 0.00–0.10 | 736 ± 17 | 97 ± 2 | 169 ± 14 | Sandy loam | A | |
| 0.10–0.20 | 694 ± 28 | 111 ± 2 | 195 ± 18 | Sandy loam | A | |
| 0.20–0.30 | 631 ± 28 | 102 ± 6 | 267 ± 17 | Sandy clay loam | AB | |
| 0.30–0.70 | 571 ± 59 | 107 ± 9 | 322 ± 58 | Sandy clay loam | Bt | |
| Physical Attributes | ||||||
| BD | Pd | MaP | MiP | MWD | SRP | |
| 0.00–0.10 | 1.55 ± 0.05 | 2.67 ± 0.04 | 0.11 ± 0.07 | 0.30 ± 0.05 | 1.94 ± 0.19 | 1.01 ± 0.26 |
| 0.10–0.20 | 1.61 ± 0.02 | 2.69 ± 0.03 | 0.14 ± 0.05 | 0.27 ± 0.02 | 1.83 ± 0.28 | 1.59 ± 0.59 |
| 0.20–0.30 | 1.66 ± 0.08 | 2.71 ± 0.07 | 0.11 ± 0.04 | 0.27 ± 0.05 | 1.28 ± 0.23 | 1.60 ± 0.36 |
| 0.30–0.70 | 1.51 ± 0.01 | 2.70 ± 0.01 | 0.10 ± 0.01 | 0.33 ± 0.01 | 0.67 ± 0.06 | 1.79 ± 0.63 |
| Chemical Attributes | ||||||
| pH | P | Ca | Mg | K | TC | |
| 0.00–0.10 | 4.67 ± 0.06 | 3.67 ± 0.58 | 0.97 ± 0.15 | 0.53 ± 0.12 | 0.25 ± 0.20 | 8.83 ± 0.12 |
| 0.10–0.20 | 4.80 ± 0.01 | 2.00 ± 0.01 | 1.00 ± 0.10 | 0.47 ± 0.06 | 0.08 ± 0.04 | 6.38 ± 0.36 |
| 0.20–0.30 | 4.93 ± 0.31 | 2.33 ± 0.58 | 1.03 ± 0.21 | 0.50 ± 0.10 | 0.04 ± 0.01 | 5.42 ± 0.71 |
| 0.30–0.70 | 5.13 ± 0.06 | 2.33 ± 1.15 | 1.33 ± 0.15 | 0.60 ± 0.10 | 0.03 ± 0.02 | 4.66 ± 0.29 |
| Cover Crops | Soil Tillage Systems |
|---|---|
| Peanut | No-tillage (NT) |
| Subsoiling at 0.40 m (MT) | |
| Deep subsoiling at 0.70 m (MT/DS) | |
| Sunn hemp | No-tillage (NT) |
| Subsoiling at 0.40 m (MT) | |
| Deep subsoiling at 0.70 m (MT/DS) | |
| Millet | No-tillage (NT) |
| Subsoiling at 0.40 m (MT) | |
| Deep subsoiling at 0.70 m (MT/DS) | |
| Sorghum | No-tillage (NT) |
| Subsoiling at 0.40 m (MT) | |
| Deep subsoiling at 0.70 m (MT/DS) | |
| Control—Sugarcane planted under conventional tillage * | |
| Indicator | Description | Abbreviation | Unit | Method |
|---|---|---|---|---|
| Physical | Bulk density | BD | Mg·m−3 | [27] |
| Macroporosity | MaP | m3·m−3 | [27] | |
| Microporosity | MiP | m3·m−3 | [27] | |
| Soil resistance to penetration | SRP | MPa | Electronic penetrometer * | |
| Tensile strength | TS | kPa | [28] | |
| Mean weight diameter | MWD | mm | [29] | |
| Chemical | Active acidity (CaCl2) | pH | - | [30] |
| Phosphorus available (Resin) | P | mg·dm−3 | [30] | |
| Exchangeable Potassium | K | mmolc·dm−3 | [30] | |
| Exchangeable Calcium | Ca | mmolc·dm−3 | [30] | |
| Exchangeable Magnesium | Mg | mmolc·dm−3 | [30] | |
| Potential acidity | H+Al | mmolc·dm−3 | [30] | |
| Total carbon | TC | g·kg−1 | [31] |
| Class | Limits |
|---|---|
| High | ≥ 1.52 |
| Medium | 1.34 ≤ SQI < 1.51 |
| Low | <1.33 |
| Principal Components | |||||
|---|---|---|---|---|---|
| PC1 | PC2 | PC3 | PC4 | PC5 | |
| Eigenvalues | 2.58 | 2.25 | 1.96 | 1.49 | 1.03 |
| Variance (%) | 19.84 | 17.27 | 15.09 | 11.49 | 7.91 |
| Cumulative (%) | 19.84 | 37.12 | 52.21 | 63.70 | 71.61 |
| Indicator | Eigenvectors | ||||
| BD | −0.42 | 0.35 | −0.17 | 0.67 | −0.20 |
| MaP | 0.82 | 0.01 | −0.19 | −0.28 | 0.24 |
| MiP | −0.61 | −0.32 | 0.40 | −0.27 | −0.16 |
| SRP | −0.53 | 0.29 | −0.07 | 0.36 | 0.20 |
| TS | −0.49 | 0.18 | 0.22 | 0.12 | 0.34 |
| MWD | −0.21 | 0.25 | 0.11 | −0.19 | 0.81 |
| P | 0.09 | 0.70 | −0.31 | −0.37 | −0.18 |
| pH | 0.49 | 0.33 | 0.61 | 0.30 | 0.00 |
| K | 0.17 | 0.78 | −0.26 | 0.01 | −0.09 |
| Ca | 0.07 | 0.29 | 0.75 | −0.10 | −0.06 |
| Mg | −0.08 | 0.19 | 0.69 | −0.30 | −0.19 |
| H+Al | −0.60 | −0.20 | −0.31 | −0.49 | −0.07 |
| TC | −0.36 | 0.69 | −0.09 | −0.38 | −0.10 |
| BD | MaP | MiP | SRP | TS | MWD | P | pH | K | Ca | Mg | H+Al | TC | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| BD | 1.00 | ||||||||||||
| MaP | −0.54 * | 1.00 | |||||||||||
| MiP | −0.17 * | −0.63 * | 1.00 | ||||||||||
| SRP | 0.39 * | −0.37 * | 0.08 | 1.00 | |||||||||
| TS | 0.20 * | −0.30 * | 0.18 * | 0.25 * | 1.00 | ||||||||
| MWD | −0.04 | −0.06 | 0.06 | 0.17 * | 0.19 * | 1.00 | |||||||
| P | 0.03 | 0.17 * | −0.21 * | 0.05 | −0.08 | 0.06 | 1.00 | ||||||
| pH | −0.03 | 0.17 * | −0.17 * | −0.09 | −0.03 | 0.00 | 0.03 | 1.00 | |||||
| K | 0.21 * | 0.12 * | −0.33 * | 0.05 | 0.01 | 0.07 | 0.54 * | 0.17 * | 1.00 | ||||
| Ca | −0.07 | −0.03 | 0.14 * | 0.01 | 0.07 | 0.10 | 0.00 | 0.46 * | 0.03 | 1.00 | |||
| Mg | −0.09 | −0.09 | 0.22 * | −0.06 | 0.16 * | 0.04 | 0.03 | 0.28 * | −0.07 | 0.45 * | 1.00 | ||
| H+Al | 0.00 | −0.24 * | 0.32 * | 0.11 * | 0.13 * | 0.05 | 0.05 | −0.62 * | −0.19 * | −0.17 * | −0.01 | 1.00 | |
| TC | 0.15 * | −0.18 * | 0.06 | 0.23 * | 0.18 * | 0.20 * | 0.51 * | −0.10 | 0.41 * | 0.13 * | 0.19 * | 0.22 * | 1.00 |
| Model Parameters | Values | ||||
|---|---|---|---|---|---|
| Accuracy rate | 73% | ||||
| Error rate | 27% | ||||
| Kappa coefficient | 0.59 | ||||
| Observed vs. Predicted | High | Medium | Low | Total | Accuracy by class |
| High | 20 | 12 | 0 | 32 | 71% |
| Medium | 7 | 27 | 5 | 39 | 63% |
| Low | 1 | 4 | 32 | 37 | 86% |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Farhate, C.V.V.; Souza, Z.M.d.; Cherubin, M.R.; Lovera, L.H.; Oliveira, I.N.d.; Carneiro, M.P.; La Scala Jr., N. Abiotic Soil Health Indicators that Respond to Sustainable Management Practices in Sugarcane Cultivation. Sustainability 2020, 12, 9407. https://doi.org/10.3390/su12229407
Farhate CVV, Souza ZMd, Cherubin MR, Lovera LH, Oliveira INd, Carneiro MP, La Scala Jr. N. Abiotic Soil Health Indicators that Respond to Sustainable Management Practices in Sugarcane Cultivation. Sustainability. 2020; 12(22):9407. https://doi.org/10.3390/su12229407
Chicago/Turabian StyleFarhate, Camila Viana Vieira, Zigomar Menezes de Souza, Maurício Roberto Cherubin, Lenon Herique Lovera, Ingrid Nehmi de Oliveira, Marina Pedroso Carneiro, and Newton La Scala Jr. 2020. "Abiotic Soil Health Indicators that Respond to Sustainable Management Practices in Sugarcane Cultivation" Sustainability 12, no. 22: 9407. https://doi.org/10.3390/su12229407
APA StyleFarhate, C. V. V., Souza, Z. M. d., Cherubin, M. R., Lovera, L. H., Oliveira, I. N. d., Carneiro, M. P., & La Scala Jr., N. (2020). Abiotic Soil Health Indicators that Respond to Sustainable Management Practices in Sugarcane Cultivation. Sustainability, 12(22), 9407. https://doi.org/10.3390/su12229407