1. Introduction
The activated sludge process, which produces waste activated sludge (WAS), is employed worldwide in wastewater treatment plants (WWTP). WAS management accounts for up to 60% of total operating expenses in WWTP [
1]. With the increasing demand for WAS management, the rising growth of WAS has become a challenge worldwide. To reduce expenses, the dewatering of WAS is necessary for subsequent transportation, treatment and, ultimately, disposal.
As a result of highly hydrophilic extracellular polymeric substances (EPS) and the widespread negative surface charge of colloid particles, advanced dewatering of WAS is always an issue. Accounting for over 80% of the total mass of WAS, EPS mainly consists of protein (PN), polysaccharide (PS) and humic substances [
2,
3]. PN and PS make up 70~80% of the extracellular organic carbon in WAS [
4]. EPS can be stratified into three fractions including slime EPS (S-EPS), loosely bound EPS (LB-EPS) and tightly bound EPS (TB-EPS) [
5]. A high correlation between EPS and dewaterability has been widely reported [
6,
7]. On the other hand, there were abundant negative surface charges of colloid particles in WAS, which enhanced the stability of colloidal particles and further deteriorated dewaterability [
8].
In previous research, various methods have been employed to enhance WAS dewaterability, including thermal treatment [
9], freezing treatment [
10], acid treatment [
11], alkaline treatment [
12], ultrasonic treatment [
13], Fenton treatment [
14], electrochemistry treatment [
15] and, in addition, skeleton builders [
16]. Recently, advanced oxidation processes (AOPs), based on sulfate radicals (•SO
42−), have been one of the main focuses of research, thanks to its security and efficiency. Sulfate radicals can be generated from peroxymonosulfate (PMS) and peroxydisulfate (PDS). PDS is more stable than PMS [
17], hence, it is more likely to be chosen as the source of the sulfate radicals. PDS can be thermally decomposed to produce two equivalents of the sulfate radicals by the symmetrical fission of the peroxide bond (Reaction 1), during which the energy required for the thermal rupture of O-O bond is 140.2 kJ/mol [
18]. Sulfate radicals (E
0 (•SO
4−/SO
42−) = 2.43 V
NHE) [
19] can be generated from PDS by various activation methods, such as ultraviolet (UV) photolysis [
20], thermal activation [
17], alkaline activation [
21] and transition metal catalysis [
2]. PDS activated by UV in WAS is impractical because of the turbidity and opacity of the WAS. Alkali enhances negative surface charges of colloid particle in WAS and therefore works against dewaterability. Although transition metal catalysis (especially Fe) was proven to be effective in PDS activation [
2], it causes a series of subsequent questions, such as, the excessive consumption of transition metal, the high residue of the transition metal and the difficulty of phosphorus recovery. Thermal activated PDS (TAP) avoids the above problems. Therefore, it can be used to enhance WAS dewaterability better.
Sulfate radical generation:
Phosphorus is a nonrenewable and irreplaceable limited resource [
22]. It is a plant nutrient essential to all life and, therefore, cannot be substituted in food production [
23]. Nowadays, phosphorus is mostly obtained from mined rock phosphate and is often combined in mineral fertilizers with sulphuric acid, nitrogen and potassium [
24]. Approximately 90% of phosphate rock is used for food production: 82% for fertilizers, 5% for animal feed and 2–3% for food additives [
25]. By 2050, the world’s population is estimated to reach 9.1 billion; in order to feed the growing population, agricultural production would need to increase by 70% overall and nearly 100% in developing countries [
26]. Currently explored and economically feasible global reserves may be depleted within only a few generations [
27]. On the other hand, the distribution of phosphorus is severely uneven. Morocco, for instance, controls 75% of the remaining world’s phosphorus reserves [
28]. Around the world, over 90% of phosphorus is dependent on imports [
29]. All in all, phosphorus resources are inextricably linked to the question of global food security.
Over 90% of phosphorus in influent transfers into sludge in WWTP [
1]. It is necessary to recover phosphorus from WAS. In Europe, phosphorus is mostly recovered by precipitation or crystallization processes in the form of HAP (hydroxyapatite, Ca
5 (PO
4)
3OH) or struvite (MgNH
4PO
4·6H
2O) [
30]. Orthophosphate and ammonia nitrogen can be simultaneously recovered in the molar ratio of 1:1 through the magnesium ammonium phosphate (MAP) method (Reaction 2). Struvite (MgNH
4PO
4 •6 H
2O), the main product of the MAP method, is an effective slow release fertilizer in agriculture [
31]. Disrupting metals (Ca, Al and Fe) disrupts the struvite precipitation (Reaction 2, 3, 4 and 5). Unfortunately, H
+, a by-product of TAP, enhances the release of Ca, Al and Fe to supernatant. It is necessary to remove disrupting metals to prepare high purity struvite and cation exchange is optional.
Main reaction (struvite precipitation):
Several recent studies have demonstrated that sludge dewaterability is enhanced by TAP and clarified its internal mechanisms [
17,
32,
33]. In these studies, TAP was proven to solubilize WAS, which manifested the release of total organic carbon, dissolved organics and NH
4+. However, the release of phosphorus was not examined. Moreover, there is still a lack of subsequent treatment for TAP treated WAS to recover the resource.
In this study, an integrated WAS resource recovery scheme (TAP treatment/struvite precipitation) was present. In the TAP treatment, the effects of activation temperature, PDS dose and initial pH on WAS dewaterability were explored and optimal conditions were attained. Further, based on sludge morphology, particle size distribution, Zeta potential and EPS concentration, the mechanism of dewaterability enhanced by TAP treatment was clarified. On the other hand, the effect of PDS dose on WAS solubilization was explored. Further, the contrastive effects of TAP treatment, thermal-alkaline hydrolysis, thermal-acidic hydrolysis and thermal hydrolysis on sludge solubilization were explored. Disrupting metals in TAP treated supernatant were removed through cation exchange. In struvite precipitation, the effects of Mg/P and initial pH were explored and optimal conditions were attained. Further, the environmental, economic and technical sustainability of combined TAP treatment and struvite precipitation were discussed.
2. Materials and Methods
2.1. Waste Activated Sludge
The WAS used in our experiments was drawn from the secondary settling tank of a wastewater treatment plant (113.36° E, 23.12° N) located in Guangzhou, China. Anoxic/anaerobic/oxic processes are employed to treat 200,000 m
3 of domestic sewage per day in this WWTP. Al
2 (SO
4)
3 was dosed in the secondary settling tank to enhance settleability. After sampling, the WAS was transported to the laboratory in 1 h and stored at 4 °C. To thicken the WAS, supernatant was removed after 12 h of gravity settling. The WAS was thickened to 96~97% moisture content and stored at 4 °C. The basic characteristics of the WAS were listed in
Table 1. All experiments were conducted in 7 days.
2.2. Conditioning Experiments
In single-factor experiments, the main influence factors, including activation temperature, PDS dose and initial pH, were investigated. PDS dose and initial pH were specified at 2.0 mmol/g TSS and 6.8 respectively in the activation temperature experiment. The activation temperature and initial pH were specified at 80 °C and 6.8 respectively in the PDS dose experiment. The activation temperature and PDS dose were specified at 80 °C and 2.0 mmol/g TSS, respectively, in the activation temperature experiment. In airtight 2 l three-necked flask, 1000 mL of the WAS was heated by thermostatic water bath with stirring at 300 rpm. The WAS was adjusted to the pH initially specified prior to experiment, heated to specified temperature in 10 min and sustained within ± 1 °C. Specified doses of sodium peroxydisulfate (Na2S2O8, > 98%, Sinopharm Chemical Reagent Co., China) were dosed into the reactor to initiate reaction. At predetermined time intervals, samples were withdrawn and immediately immersed into an ice bath to quench further reaction. Subsequently, the dewaterability of samples was evaluated. To clarify the mechanism of dewaterability enhanced by TAP treatment, sludge morphology, particle size distribution, Zeta potential and EPS were analyzed.
2.3. Solubilization Experiments
On the basis of conditioning experiments, solubilization experiments were conducted to investigate the effect of PDS dose on the release of PO43−-P, NH4+-N and SCOD in the supernatant. The activation temperature and reaction time were specified at 80 °C and 40 min respectively. Samples were withdrawn and immediately immersed into an ice bath. The treated WAS was centrifuged (4000× g, 10 min) and the concentrations of PO43−, NH4+ and SCOD in supernatants were determined.
Further, contrastive experiments of TAP treatment (80 °C, 1.5 mmol/g TSS), thermal-alkaline hydrolysis (80 °C, initial pH 11), thermal-acidic hydrolysis (80 °C, initial pH 3) and thermal hydrolysis (80 °C) were conducted.
2.4. Cation Exchange Experiments
A variety of commercial cation exchange resin (732, Boxu Environmental Technology Ltd., China) was used to remove disrupting metals such as Al, Ca and Fe. The cation exchange resin was immersed in 1% HCl solution and 1% NaOH solution for 12 h, respectively, and filled into a cation exchange column (Φ 3 cm × 15 cm). The concentrations of PO43−-P, NH4+-N, SCOD, Al, Ca and Fe were determined before and after cation exchange.
2.5. Struvite Experiments
In single-factor experiments, the main influencing factors, including initial pH and n (Mg2+)/n (PO43−) (Mg/P), were investigated. The reaction time was specified at 15 min. The initial pH was specified at 10.0 in Mg/P experiment and Mg/P was specified at 1.4 in the PDS dose experiment. In a 500 mL beaker, 250 mL of supernatant after cation exchange was stirred by magnetic stirrers. The supernatant was adjusted to the specified initial pH with stirring at 200 rpm. According to specified Mg/P, magnesium sulfate (MgSO4, 99%, Guangzhou Chemical Reagent Factory, GCRF, China) was dosed to initiate reaction. Mixed liquors were centrifuged (4000× g, 10 min) to separate sediments from solutions. The concentrations of PO43−-P and NH4+-N in solutions were determined, and the purity of sediments was determined. Further, scanning electron microscope (SEM), energy dispersive X-ray spectroscopy (EDX) and X-ray diffraction (XRD) were used to identify sediments.
2.6. Analytical Methods
Sulfuric acid (H2SO4, 98%, GCRF, China) and sodium hydroxide (NaOH, 98%, GCRF, China) were used to prepare 1 mol/L and 0.1 mol/L solution which adjusted pH. pH was determined with a pH meter (PHSJ 6L, INESA Ltd., China). The dewaterability of WAS was evaluated by capillary suction time (CST), which was determined with a standard apparatus (304 M, Triton Ltd., UK) equipped with standard CST filter papers.
Sludge morphology was analyzed with a SEM (Merlin, Carl Zeiss AG, Germany). Particle size distribution was analyzed with a Mastersizer (Mastersizer 3000E, Malvern Ltd., UK). Zeta potential was analyzed with a Zetasizer (Nano ZSE, Malvern Ltd., UK). XRD (Empyrean, Malvern Ltd., UK) was used to identify sediments.
EPS was extracted with a modified thermal method. WAS samples were centrifuged (4000× g, 10 min) and the supernatants were withdrawn as S-EPS. The deposits were fully resuspended to the original volume using NaCl solution (70 °C, 0.05%) and centrifuged (4000× g, 10 min), and the supernatants were withdrawn as LB-EPS. The deposits were fully resuspended to the original volume using NaCl solution (70 °C, 0.05%), heated by water bath (60 °C, 30 min), centrifuged (4000× g, 10 min) and the supernatants were withdrawn as TB-EPS.
The PS was analyzed with anthrone-sulfuric acid method using glucose as the standard. PN was analyzed with the Coomassie Brilliant Blue G-250 method using bovine serum albumin as the standard [
34]. The concentrations of Al, Ca and Fe were determined by inductively coupled plasma optical emission spectrometer (ICP-OES, iCAP 7200, Thermo Fisher, USA). The concentrations of PO
43−-P, NH
4+-N and COD were determined according to APHA standard methods. Sediment was dissolved in 0.1 mol/L HCl, and the concentration of NH
4+-N in solution was determined to analyze the purity of struvite. Based on SCOD and TCOD, the disintegration degree (DD
SCOD) was calculated as follows [
14]:
3. Results and Discussion
3.1. Effect of TAP Treatment on WAS Dewaterability
As exhibited in
Figure 1a, the time-dependent variation of the WAS dewaterability with different PDS doses was monitored. In the absence of PDS, elevated temperature deteriorated WAS dewaterability with CST increased from 94.2 s to 3717.9 s in 60 min, which coincided with previous research [
35]. As previous research has reported [
36], this phenomenon was attributed to EPS destruction and the intensified negative surface charges of WAS (exhibited in
Figure 2g). A slight dose of PDS (≤0.5mmol/g TSS) was unable to eliminate the negative effects of thermal hydrolysis completely, though CST only increased to 176.8 s in 60 min with 0.5mmol/g TSS of PDS. In the PDS dose range of 1.0~2.0 mmol/g TSS, WAS dewaterability increased significantly with the PDS dose. In contrast, a PDS dose of over 2.0 mmol/g TSS did not enhance dewaterability further. The optimal PDS dose, which decreased WAS CST from 94.2 s to 28.9 s in 40 min, was 2.0 mmol/g TSS. Previous studies [
17,
33] have suggested that the optimal PDS dose was 2.0 mmol/g VSS at most, which can possibly be attributed to the different characteristics of WAS.
As shown in
Figure 1b, the time-dependent variation of the WAS dewaterability with different initial pH was monitored. In the initial 5 min, WAS dewaterability deteriorated significantly at an initial pH of 10.0 with CST increased to 3843.7 s. In the subsequent 55 min, WAS CST ranged from 1816.6s to 2011.6 s, which remained an indication of seriously deteriorated dewaterability. As for an initial pH of 8.5, CST increased to 1138.9 s in the initial 5 min but gradually decreased to 55.0 s in the subsequent 55 min. TAP treatment effectively enhanced WAS dewaterability in mildly alkaline conditions (pH ≤ 8.5). Nevertheless, the effect of TAP was limited in alkaline conditions, which coincides with previous research [
33]. As previous research [
37] has reported, •SO
42− transformed to •OH in alkaline conditions, which limits oxidation. In neutral and acidic conditions, the effect of TAP on dewaterability, which finally decreased CST to approximately 28.5 s, was similar. The only difference was that CST decreased rapidly within the initial 20 min in acidic conditions, which was attributed to the fact that the activation energy in acidic conditions (100–116 kJ/mol) was less than in neutral conditions (119–129 kJ/mol) [
38]. As for conventional WAS in neutral conditions, it is unnecessary to adjust pH prior to TAP treatment. As exhibited in
Figure 1c, the time-dependent variation of the WAS dewaterability with different activation temperatures was monitored. The application of PDS without an elevated temperature deteriorated WAS dewaterability, with CST increased to 603.1 s in 60 min. This phenomenon has not been reported in recent research. Previous research [
39] suggested that cations are an integral part of the floc structure and an excess of monovalent cations relative to divalent cations in the influent to an activated sludge treatment plant can lead to poorly dewatering sludge. As mentioned above, the PDS dose in this research (2.0 mmol/g TSS), which possibly deteriorated WAS dewaterability by cation exchange, was more than in recent research (2.0 mmol/g VSS at most) [
17,
33]. Lower temperatures (≤70 °C) ineffectively activate PDS. The optimal activation temperature was 80 °C, which coincided with recent studies [
17,
32,
33].
3.2. Variations of Sludge Morphology, Particle SIZE distribution, Zeta Potential and EPS
As exhibited in
Figure 2a,b, no significant changes in microstructure were observed before and after TAP treatment. TAP treatment (80 °C, 40 min) has no severe destructive effect on the WAS microstructure.
The particle size distribution of WAS was exhibited in
Figure 2c. The peak patterns were remarkably similar. The major peaks of raw WAS, WAS treated for 20 min and WAS treated for 40 min were centered at 76 μm, 59 μm and 52 μm, respectively. According to cumulative particle size distribution, D10 (i.e., 10% of particles are smaller than this size), D50 (i.e., 50% of particles are smaller than this size) and D90 (i.e., 10% of particles are smaller than this size) are exhibited in
Figure 2d. When treated with TAP for 40 min, D10, D50 and D90 decreased 11.99%, 14.43% and 12.97%, respectively, which demonstrated the disintegration of WAS particles and the release of bound water. On the other hand, WAS particles were not fragmented to massive tiny particles. Massive tiny particles were difficult to gather. It has been reported [
40] that solids smaller than 10 μm have the most critical effect on the WAS dewaterability. This pattern of WAS disintegration facilitated the gathering of colloid particles. Similar observations have also been reported in previous research [
33]. In addition, thermal-acidic hydrolysis (80 °C, initial pH 3, 40 min) similarly disintegrates WAS particles, though it was not as effective as TAP treatment.
The PN and PS in EPS were exhibited in
Figure 2e,f respectively. With TAP treatment, PN and PS in TB-EPS decreased 93.74% and 87.27% in 40 min, respectively. TAP treatment solubilized PS and PN to a great extent, and further disintegrated EPS. In 20 min, PN and PS in TB-EPS decreased 72.47% and 49.71%, respectively, which demonstrated that PN was more vulnerable than PS. Treated with TAP for 40 min, increments of PN and PS in S-EPS were more than decrements of PN and PS in B-EPS, respectively, which demonstrated the solubilization of intracellular polymers. Single thermal hydrolysis (80 °C, 40 min) played a positive role in EPS disintegration though it was negative for general dewaterability. In the presence of H
+ (initial pH 3), the effect of thermal hydrolysis on EPS was enhanced. EPS disintegration resulted from the synergistic effect between radical oxidation, thermal hydrolysis and acidification. The discussion above coincided with recent studies [
32,
33].
As exhibited in
Figure 2g, Zeta potential increased from −12.50 mV to −2.91 mV after 40 min of TAP treatment, which indicated that the negative surface charge was neutralized to a great extent. According to DLVO theory [
41], the reduction of negative surface charge weakens the electrostatic repulsion and increases interaction energy, which further enhances WAS dewaterability by facilitating the sediment and flocculation of sludge particles. Based on the conservation of charge, along with the decomposition of S
2O
82−, two equivalents of H
+ were ultimately generated as by-products. WAS acidification caused negative surface charge neutralization. An identical process was occurring in thermal-acidic hydrolysis (80 °C, initial pH 3, 40 min). In addition, single thermal hydrolysis (80 °C, 40 min) enhanced the negative surface charge of WAS, which corresponded to the deteriorated dewaterability exhibited in
Figure 1a.
3.3. Mechanism of Dewaterability Enhanced by TAP Treatment
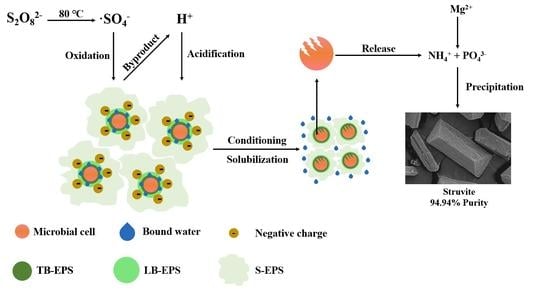
TAP treatment of WAS is a combined process of thermal hydrolysis, radical oxidation reaction and acidification.
Before PDS addition, thermal hydrolysis, which enhanced the negative surface charge of colloid particle, was proceeding in WAS. Thermal hydrolysis deteriorated WAS dewaterability.
After the addition of PDS, the oxidation reaction was initiated to disintegrate organic components. PDS was thermally decomposed to produce two equivalents of •SO
4− by the symmetrical fission of the peroxide bond (Reaction 1) [
17]. SO
4− oxidatively disintegrated LB-EPS and TB-EPS to a great extent, and further released bound water. On the other hand, WAS particles were not fragmented into massive tiny particle. This pattern of WAS disintegration facilitated the gathering of colloid particles. The radical oxidation reaction enhanced WAS dewaterability to a great extent.
Along with the decomposition of S2O82−, two equivalents of H+ were ultimately generated as by-product. H+ transferred rapidly at elevated temperatures and neutralized the negative surface charge of colloid particles to a great extent. Acidification enhanced the radical oxidation reaction by improving radical oxidizing ability. In addition, thermal-acidic hydrolysis of PN and PS disintegrated EPS to some extent. Acidification enhanced WAS dewaterability to some extent.
3.4. Effect of TAP Treatment on Sludge Solubilization
Based on conditioning experiments, the effects of the PDS dose on WAS solubilization (TP, PO
43−-P, TN, NH
4+-N and SCOD) were investigated. The effects on TP, PO
43−-P, TN and NH
4+-N in supernatant are exhibited in
Figure 3a. TP, PO
43−-P, TN and NH
4+-N in supernatant increased with the PDS dose. The release of TN and NH
4+-N (883.71 mg/L and 333.02 mg/L at 2.0 mmol/TSS, respectively) were more than the release of TP and PO
43−-P (184.94 mg/L and 181.48 mg/L at 2.0 mmol/TSS, respectively). However, the growth rates of TP and PO
43−-P with PDS dose were significantly higher. The TP curve was almost identical to the PO
43−-P curve, which indicated that the phosphorus was all in the form of PO
43−. In term of phosphorus release, a decelerated growth was observed at PDS dose of 1.5~2.5 mmol/g TSS. Effects on SCOD and DD
SCOD were exhibited in
Figure 3b. The SCOD curve was similar to the TP curve. At a PDS dose of 2.0 mmol/g TSS, SCOD and DD
SCOD were 13,013 mg/L and 26.64%, respectively. Considering the enhanced dewaterability in
Figure 1a, the optimal PDS dose was 2.0 mmol/g TSS.
3.5. Contrastive Effects of TAP Treatment, Thermal-Alkaline Hydrolysis, Thermal-Acidic Hydrolysis and Thermal Hydrolysis on Sludge Solubilization
The effect of different treatments on NH
4+-N release, PO
43−-P release and DD
SCOD were exhibited in
Figure 4a–c, respectively.
With NH4+-N release increased to 287.22 mg/L and 266.50 mg/L in 40 min, respectively, the effects of TAP and thermal-acidic hydrolysis on NH4+-N release were similar. With NH4+-N release increased to 158.67 mg/L in 40 min, thermal hydrolysis enhanced NH4+-N release to some extent. As a result of ammonia volatilization in alkaline conditions in the supernatant of thermal-alkaline hydrolysis treated WAS, the NH4+-N concentration was only 16.08 mg/L. To rank the effects on NH4+-N release: TAP = thermal-acidic hydrolysis > thermal hydrolysis > thermal-alkaline hydrolysis.
As for PO
43−-P release, with an increase to 370.23 mg/L in 40 min, thermal-alkaline hydrolysis was significantly effective. With a performance of 179.33 mg/L PO
43−-P release in 40 min, TAP treatment was also effective. Thermal-acidic hydrolysis and thermal hydrolysis, which released PO
43−-P 65.8 mg/L 1 and 27.57 mg/L, respectively, was inefficient. Phosphorus was the most precious in the released substances, and the recovery of phosphorus was always a concern of previous research. PO
43− release in thermal-alkaline hydrolysis mainly originated from the disrupted phospholipids bilayer of cell membrane [
31]. Al
3+ (especially Al
2 (SO
4)
3) was dosed to enhance sludge settleability and remove phosphorus in the secondary settling tank. Compared with thermal-alkaline hydrolysis, TAP was less effective in disrupting the phospholipids bilayer. However, acidification caused the solubilization of AlPO
4, which compensated for the low phosphorus release in radical oxidation. To rank the effects on PO
43−-P release: thermal-alkaline hydrolysis > TAP > thermal-acidic hydrolysis > thermal hydrolysis.
Similar to PO43−-P release, thermal-alkaline hydrolysis effectively disintegrated WAS with the DDSCOD of 38.14%. With the DDSCOD of 21.95% and 20.73%, respectively, TAP and thermal-acidic hydrolysis performed similarly. In 40 min, the DDSCOD of thermal hydrolysis was only 9.19%. To rank the effects on DDSCOD: thermal-alkaline hydrolysis > TAP = thermal-acidic hydrolysis > thermal hydrolysis.
In sum, TAP performed effective in releasing PO
43−, NH
4+ and SCOD. Combined with the discussion in
Section 3.1, TAP simultaneously enhances dewaterability and solubilization of WAS.
3.6. Effect of Cation Exchange
The basic characteristics in TAP treated supernatant before and after cation exchange were exhibited in
Table 2. Precipitations of Ca
3 (PO
4)
2 (K
sp = 2.07 × 10
−33), AlPO
4 (K
sp = 9.83 × 10
−21) and FePO
4 (K
sp = 9.92 × 10
−29) were prior to struvite (K
sp = 2.5 × 10
−13) precipitation; hence, it was necessary to remove disrupting metals in the TAP treated supernatant. Ca and Al, whose concentrations were 331.735 mg/L and 589.873 mg/L, respectively, were the main disrupting metals in the TAP treated supernatant. With an apparent nonselective effect of cation exchange, the removal rates of Ca, Al and Fe were all more than 98%. At less than 10 mg/L, the effect of disrupting metal on struvite precipitation was negligible. In cation exchange, the loss rates of PO
43−-P, NH
4+-N and SCOD were 5.82%, 20.64% and 23.98% respectively. The loss of PO
43−-P was not severe, and the molar ratio of NH
4+ to PO
43− was 3.53, which was beneficial to recover phosphorus adequately. Overall, cation exchange was an appropriate pretreatment before struvite precipitation.
3.7. Effect of Struvite Precipitation
The effects of Mg/P on PO
43−-P concentration and PO
43−-P recovery were exhibited in
Figure 5a. In the Mg/P range of 1.0~1.4, PO
43−-P recovery rate increased with Mg/P. With Mg/P of 1.4, PO
43−-P recovery was 95.06%. Mg/P of over 1.4 did not improve PO
43−-P recovery further. As exhibited in
Figure 5c, the effect of Mg/P on struvite purity was similar to effect on PO
43−-P recovery. In the Mg/P range of 1.0~2.0, high struvite purity was observed. With Mg/P of 1.4, struvite purity was 94.34%.
The effects of the initial pH on PO
43−-P concentration and PO
43−-P recovery rate were exhibited in
Figure 6a. PO
43−-P recovery rate increased with the initial pH. With a pH of 10.0, PO
43−-P recovery was 95.03%. A pH of over 10.0 did not improve PO
43−-P recovery further. More phosphate was transformed to PO
43− at an elevated initial pH, which improve PO
43−-P recovery. The effect of the initial pH on struvite purity was exhibited in
Figure 6c. Struvite purity increased with the initial pH in the range of 8.0~10.0 and decreased with he initial pH in the range of 10.0~11.0. With a pH of 10.0, struvite purity was 94.94%. Optimal conditions of struvite precipitation were Mg/P 1.4 and an initial pH of 10.0.
As exhibited in
Figure 5b and
Figure 6b, similar to PO
43−-P recovery, NH
4+-N recovery also increased with Mg/P and initial pH, respectively. The only difference was that NH
4+-N recovery did not further increase at the Mg/P range of 1.6~2.0, while NH
4+-N recovery increased slightly at the initial pH range of 10~11. Because strong alkalinity accelerated ammonia volatilization. There remained 173.66 mg/L of NH
4+-N in supernatant after struvite precipitation. A total of 9893 mg/L of SCOD and 173.66 mg/L of NH
4+-N means that the mass ratio of C to N was 100:1.75 (over 100:5, general C/N of aerobic microbial treatment). In the case of avoiding shock load, the supernatant after struvite precipitation can be returned to a biochemical treatment process as a carbon source. Considering that the supernatant after struvite precipitation was at a pH range of 9.3~9.5, it is feasible that the supernatant was heated slightly (<50 °C) by the waste heat of TAP treatment to strip ammonia and prepare a purer carbon source.
A Corresponding product was exhibited in
Figure 7a–c, respectively. The product was mainly in the form of a columnar crystal. Product XRD was highly consistent with powder diffraction file (PDF, 15-0762).
As exhibited in
Table 3, the elemental composition of the product was calculated from EDX. EDX is a semi-quantitative analysis, in which there can be large errors. Only the N measured value was close to the theoretical value. The phenomenon that the P measured value differed greatly from theoretical value may be due to the fact that P and Pt interfered with each other during the EDX measurement. The EDX indicated that the product consisted of N, O, Mg and P. Organic matter and heavy metals were not detected. Considering the XRD result and struvite purity, struvite (approximately 95%) was major and Mg
3 (PO
4)
2 (approximately 5%) was minor in product. Compared with the products in previous research [
42,
43,
44], phosphorus recovery (95.06%) and struvite purity (94.94%) were considerable. Commercial enriched struvite purity is mostly over 98% and, unfortunately, the product of this research was not pure enough to be a laboratory reagent (LR). Supernatant used to precipitate struvite was disinfected by TAP treatment (80 °C, 40 min, strong oxidability from sulfate radicals and strong acidity from H
+) and, hence, the product was harmless enough to be used as a slow release fertilizer.
3.8. Environmental, Technical and Economic Sustainability of TAP Treatment Combined with Struvite Precipitation
TAP treatment combined with struvite precipitation is environmentally sustainable. TAP treatment simultaneously enhanced the dewaterability and solubilization of WAS. Enhanced WAS dewaterability facilitates subsequent sludge treatment and disposal. WAS was disinfected by TAP treatment (80 °C, 40 min, strong oxidability from sulfate radical and strong acidity from H+) and, hence, harmful microorganisms were restrained. PO43−, NH4+ and SCOD solubilization of WAS made nutrient recovery possible. Cation exchange can be used to remove most Ca and Al from the TAP treated supernatant, which guaranteed subsequent struvite purity. Struvite precipitation recovered PO43− and NH4+ as slow-release fertilizer and supernatant after struvite precipitation can be returned to biochemical treatment process as a carbon source.
It is also technically reliable. Even if peroxydisulfate is strong oxidant, it steadily and safely produces sulfate radicals at the activation temperature. The activation temperature of PDS was 80 °C and, hence, TAP treatment can be carried out in normal atmospheric pressure, which reduces the need for equipment. Struvite precipitation was rapid at room temperature.
The cost of TAP treatment, combined with struvite precipitation, was listed in
Table 4. TAP treatment accounts for an impressive 98.9% of all costs. On the one hand, the high specific heat capacity of water leads to large amounts of electricity consumption. On the other, the high molecular weight of sodium peroxydisulfate (238.104 g/mol) leads to much higher agent cost. According to the results of this research, 1.13 kg of struvite (95% purity) was prepared per ton of WAS. Considering that the selling price of struvite is 420
$/ton [
45], the income obtained by selling struvite is 0.45
$/ton of WAS, which adequately covers the cost of struvite precipitation (0.14
$/ton). When it comes to the biochemical treatment with design of MLSS (mixed liquor suspended solids) of 3000 mg/L, 13.33 tons of wastewater produces 1 ton of WAS (moisture content 96%) and the cost of WAS treatment is 0.96
$/ton wastewater. In the condition of cheaper electricity and PDS, the cost is further reduced.