Fluidization Roasting Technology of Jingtieshan Iron Ore in the Absence of Carbon Additives
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods and Equipment
2.2.1. Experimental Apparatus and Procedure
2.2.2. Characterization Methods
3. Results and Discussion
3.1. Thermodynamic Analysis
3.2. Magnetization Roasting and Magnetic Separation
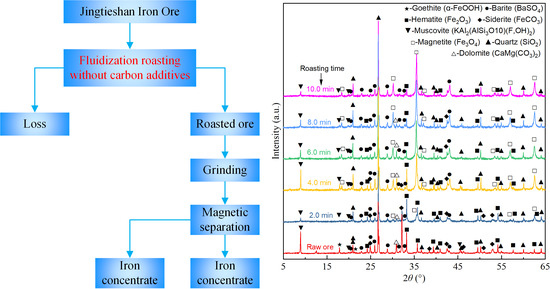
3.3. XRD Analysis
3.4. OM and BSE-EDS Analysis
3.5. VSM Analysis
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- National Minerals Information Center. Mineral Commodity Summaries; US Geological Survey: Reston, VA, USA, 2021.
- Han, H.; Lu, L. Chapter 13-Thermal Beneficiation of Refractory Iron Ore. In Iron Ore, 2nd ed.; Lu, L., Ed.; Woodhead Publishing: Sawston, UK, 2022; pp. 421–456. [Google Scholar]
- Yu, J.; Han, Y.; Li, Y.; Gao, P. Recent advances in magnetization roasting of refractory iron ores: A technological review in the past decade. Miner. Process. Extr. Metall. Rev. 2020, 41, 349–359. [Google Scholar] [CrossRef]
- Zhou, W.; Han, Y.; Sun, Y.; Li, Y. Strengthening iron enrichment and dephosphorization of high-phosphorus oolitic hematite using high-temperature pretreatment. Int. J. Miner. Metall. Mater. 2020, 27, 443–453. [Google Scholar] [CrossRef]
- Wan, J.; Chen, T.; Zhou, X.; Luo, Y.; Liu, W.; Lu, Q. Efficient improvement for the direct reduction of high-iron red mud by co-reduction with high-manganese iron ore. Miner. Eng. 2021, 174, 107024. [Google Scholar] [CrossRef]
- Huang, J.; Liu, J.; Zhang, H.; Guo, Y. Sustainable risk analysis of China’s overseas investment in iron ore. Resour. Policy 2020, 68, 101771. [Google Scholar] [CrossRef]
- Zhang, X.; Han, Y.; Sun, Y.; Li, Y. Innovative utilization of refractory iron ore via suspension magnetization roasting: A pilot-scale study. Powder Technol. 2019, 352, 16–24. [Google Scholar] [CrossRef]
- Shen, Z. Application examples of flotation machines. In Principles and Technologies of Flotation Machines; Shen, Z., Ed.; Springer: Singapore, 2021; pp. 435–487. [Google Scholar]
- Li, H.; Zhang, Z.; Li, L.; Zhang, Z.; Chen, J.; Yao, T. Types and general characteristics of the BIF-related iron deposits in China. Ore Geol. Rev. 2014, 57, 264–287. [Google Scholar] [CrossRef]
- Liu, L.; Wu, F.; Tan, W. Effect of cetyl trimethyl ammonium bromide on shrinkage cracks in filter cakes during pressure filtration of iron ore concentrates. Powder Technol. 2016, 297, 239–246. [Google Scholar] [CrossRef]
- Luo, L.; Huang, H.; Yu, Y. Characterization and technology of fast reducing roasting for fine iron materials. J. Cent. South Univ. 2012, 19, 2272–2278. [Google Scholar] [CrossRef]
- Roy, S.K.; Nayak, D.; Rath, S.S. A review on the enrichment of iron values of low-grade iron ore resources using reduction roasting-magnetic separation. Powder Technol. 2020, 367, 796–808. [Google Scholar] [CrossRef]
- Sun, Y.S.; Zhu, X.R.; Han, Y.X.; Li, Y.J.; Gao, P. Iron recovery from refractory limonite ore using suspension magnetization roasting: A pilot-scale study. J. Clean. Prod. 2020, 261, 121221. [Google Scholar] [CrossRef]
- Tang, Z.; Zhang, Q.; Sun, Y.; Gao, P.; Han, Y. Pilot-scale extraction of iron from flotation tailings via suspension magnetization roasting in a mixture of CO and H2 followed by magnetic separation. Resour. Conserv. Recy. 2021, 172, 105680. [Google Scholar] [CrossRef]
- Zhang, Q.; Sun, Y.; Wang, S.; Han, Y.; Li, Y.; Gao, P. Growth behavior and kinetics of magnetite during magnetization roasting. J. Ind. Eng. Chem. 2022. [Google Scholar] [CrossRef]
- Yuan, S.; Wang, R.; Gao, P.; Han, Y.; Li, Y. Suspension magnetization roasting on waste ferromanganese ore: A semi-industrial test for efficient recycling of value minerals. Powder Technol. 2022, 396, 80–91. [Google Scholar] [CrossRef]
- Fernández-Martínez, M.; Sardans, J.; Chevallier, F.; Ciais, P.; Obersteiner, M.; Vicca, S.; Canadell, J.G.; Bastos, A.; Friedlingstein, P.; Sitch, S.; et al. Global trends in carbon sinks and their relationships with CO2 and temperature. Nat. Clim. Chang. 2019, 9, 73–79. [Google Scholar] [CrossRef] [Green Version]
- Fung, I.Y.; Doney, S.C.; Lindsay, K.; John, J. Evolution of carbon sinks in a changing climate. Proc. Natl. Acad. Sci. USA 2005, 102, 11201. [Google Scholar] [CrossRef] [Green Version]
- Ritchie, H.; Roser, M. CO₂ and Greenhouse Gas Emissions.2020. Available online: OurWorldInData.org (accessed on 20 December 2021).
- Nayak, D.; Dash, N.; Ray, N.; Rath, S.S. Utilization of waste coconut shells in the reduction roasting of overburden from iron ore mines. Powder Technol. 2019, 353, 450–458. [Google Scholar] [CrossRef]
- Altiner, M. Roasting of a low-grade goethite ore using horse residue and its beneficiation by magnetic separation. Mining. Metall. Explor. 2020, 37, 1357–1365. [Google Scholar] [CrossRef]
- Rath, S.S.; Rao, D.S.; Tripathy, A.; Biswal, S.K. Biomass briquette as an alternative reductant for low grade iron ore resources. Biomass Bioenergy 2018, 108, 447–454. [Google Scholar] [CrossRef]
- Ponomar, V.P.; Dudchenko, N.O.; Brik, A.B. Reduction roasting of hematite to magnetite using carbohydrates. Int. J. Miner. Process. 2017, 164, 21–25. [Google Scholar] [CrossRef]
- Rath, S.S.; Rao, D.S.; Mishra, B.K. A novel approach for reduction roasting of iron ore slime using cow dung. Int. J. Miner. Process. 2016, 157, 216–226. [Google Scholar] [CrossRef]
- Petrus, H.T.B.M.; Putera, A.D.P.; Sugiarto, E.; Perdana, I.; Warmada, I.W.; Nurjaman, F.; Astuti, W.; Mursito, A.T. Kinetics on roasting reduction of limonitic laterite ore using coconut-charcoal and anthracite reductants. Miner. Eng. 2019, 132, 126–133. [Google Scholar] [CrossRef]
- Zhu, X.; Han, Y.; Sun, Y.; Li, Y.J.; Wang, H. Siderite as a novel reductant for clean utilization of refractory iron ore. J. Clean. Prod. 2020, 245, 118704. [Google Scholar] [CrossRef]
- Ponomar, V.P.; Dudchenko, N.O.; Brik, A.B. Synthesis of magnetite powder from the mixture consisting of siderite and hematite iron ores. Miner. Eng. 2018, 122, 277–284. [Google Scholar] [CrossRef]
- Zhang, Q.; Sun, Y.; Wang, S.; Han, Y.; Li, W.; Li, Y. Whether magnetization roasting requires complete phase reconstruction of iron minerals: A study of phase transition and microstructure evolution. Powder Technol. 2022, 411, 117934. [Google Scholar] [CrossRef]
- Nunna, V.; Hapugoda, S.; Pownceby, M.I.; Sparrow, G.J. Beneficiation of low-grade, goethite-rich iron ore using microwave-assisted magnetizing roasting. Miner. Eng. 2021, 166, 106826. [Google Scholar] [CrossRef]
- Zhu, X.; Han, Y.; Sun, Y.; Gao, P.; Li, Y. Thermal Decomposition of Siderite Ore in Different Flowing Atmospheres: Phase Transformation and Magnetism. Miner. Process. Extr. Metall. Rev. 2022, 1–8. [Google Scholar] [CrossRef]
- Zhang, Q.; Sun, Y.; Han, Y.; Li, Y.; Gao, P. Producing magnetite concentrate via self-magnetization roasting in N2 atmosphere: Phase and structure transformation, and extraction kinetics. J. Ind. Eng. Chem. 2021, 104, 571–581. [Google Scholar] [CrossRef]
- Zhang, Q.; Sun, Y.; Han, Y.; Li, Y.; Gao, P. Reaction behavior and non-isothermal kinetics of suspension magnetization roasting of limonite and siderite. Int. J. Miner. Metall. Mater. 2022. [Google Scholar] [CrossRef]
- Sun, Y.S.; Zhu, X.R.; Han, Y.X.; Li, Y.J. Green magnetization roasting technology for refractory iron ore using siderite as a reductant. J. Clean. Prod. 2019, 206, 40–50. [Google Scholar] [CrossRef]
- Lu, Z.; Cai, M. Disposal Methods on Solid Wastes from Mines in Transition from Open-Pit to Underground Mining. Procedia Environ. Sci. 2012, 16, 715–721. [Google Scholar] [CrossRef]
- Li, C.; Sun, H.; Yi, Z.; Li, L. Innovative methodology for comprehensive utilization of iron ore tailings: Part 2: The residues after iron recovery from iron ore tailings to prepare cementitious material. J. Hazard. Mater. 2010, 174, 78–83. [Google Scholar] [CrossRef]
- Sun, H.; Wu, J.; Yu, P.; Li, J. Geology, geochemistry and sulfur isotope composition of the Late Proterozoic Jingtieshan (Superior-type) hematite-jasper-barite iron ore deposits associated with stratabound Cu mineralization in the Gansu Province, China. Miner. Depos. 1998, 34, 102–112. [Google Scholar] [CrossRef]








| Composition | TFe | FeO | SiO2 | Al2O3 | CaO | MgO | Ba | K | P | S | LOI * |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Content | 34.15 | 16.90 | 23.62 | 2.30 | 1.34 | 2.75 | 2.61 | 0.81 | 0.016 | 0.947 | 15.90 |
| Iron Phase | Fe in Siderite | Fe in Magnetite | Fe in Hematite/Limonite | Fe in Pyrite | Fe in Silicate | Total |
|---|---|---|---|---|---|---|
| Content | 12.87 | 0.32 | 20.45 | 0.21 | 0.31 | 34.15 |
| Percentage | 37.69 | 0.94 | 59.88 | 0.61 | 0.91 | 100.00 |
| Samples | Yield | Fe Grade | Fe Recovery |
|---|---|---|---|
| Raw ore | 100.00 | 34.15 | 100.00 |
| Roasted ore | 85.70 | 39.85 | 100.00 |
| Loss | 14.30 | 0.00 | 0.00 |
| Iron concentrate | 54.24 | 57.40 | 91.17 |
| Iron tailing | 31.46 | 9.59 | 8.83 |
| Roasting Time/min | Minerals Content/Mass% | |||||||
|---|---|---|---|---|---|---|---|---|
| Hematite | Quartz | Siderite | Goethite | Dolomite | Muscovite | Barite | Magnetite | |
| raw ore | 16.49 | 16.79 | 35.84 | 0.57 | 13.46 | 12.10 | 4.74 | 0 |
| 2.0 | 25.18 | 17.40 | 17.63 | 0 | 8.13 | 9.23 | 7.21 | 15.21 |
| 4.0 | 8.92 | 16.16 | 0.54 | 0 | 14.77 | 26.70 | 5.43 | 27.47 |
| 6.0 | 5.83 | 17.84 | 0.22 | 0 | 10.20 | 17.43 | 5.46 | 43.02 |
| 8.0 | 5.08 | 15.66 | 0.17 | 0 | 2.97 | 29.77 | 5.68 | 40.66 |
| 10.0 | 4.47 | 16.16 | 0 | 0 | 0 | 32.96 | 5.25 | 41.15 |
| Samples | Points | Minerals | Elements/At% | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| O | Si | Fe | Mn | Mg | Al | S | Ba | |||
| (a) raw ore | 1 | Barite | 58.69 | - | - | - | - | - | 15.36 | 15.21 |
| 2 | Hematite | 54.16 | - | 36.00 | - | - | - | - | - | |
| 3 | Quartz | 59.40 | 29.15 | - | - | - | - | - | - | |
| 4 | Hematite | 54.51 | - | 34.70 | - | - | - | - | - | |
| 5 | Siderite | 52.24 | - | 15.24 | 0.55 | 3.80 | - | - | - | |
| (b) roasted ore under the optimum conditions | 1 | Magnetite | 51.04 | - | 38.57 | - | - | - | - | - |
| 2 | Jasper * | 62.19 | 16.53 | 0.82 | - | 0.52 | 8.05 | - | - | |
| 3 | Magnetite | 50.53 | - | 38.61 | - | - | - | - | - | |
| 4 | Magnetite | 52.04 | - | 31.83 | 0.73 | 4.05 | - | - | - | |
| (c) iron concentrate | 1 | Magnetite | 50.24 | 0.64 | 39.01 | - | - | - | - | - |
| 2 | Magnetite | 50.01 | - | 31.24 | 3.55 | 4.90 | - | - | - | |
| 3 | Hematite | 54.54 | 0.23 | 34.90 | - | - | 0.23 | - | - | |
| 4 | Magnetite | 48.34 | - | 38.73 | - | - | - | - | - | |
| (d) iron tailing | 1 | Hematite | 53.49 | - | 35.85 | - | - | - | - | - |
| 2 | Jasper * | 55.60 | 9.30 | 7.46 | - | 8.51 | 8.94 | - | - | |
| 3 | Barite | 58.34 | - | - | - | - | - | 16.63 | 15.65 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhu, X.; Zhang, Q.; Sun, Y.; Li, Y.; Han, Y. Fluidization Roasting Technology of Jingtieshan Iron Ore in the Absence of Carbon Additives. Sustainability 2022, 14, 13629. https://doi.org/10.3390/su142013629
Zhu X, Zhang Q, Sun Y, Li Y, Han Y. Fluidization Roasting Technology of Jingtieshan Iron Ore in the Absence of Carbon Additives. Sustainability. 2022; 14(20):13629. https://doi.org/10.3390/su142013629
Chicago/Turabian StyleZhu, Xinran, Qiang Zhang, Yongsheng Sun, Yanjun Li, and Yuexin Han. 2022. "Fluidization Roasting Technology of Jingtieshan Iron Ore in the Absence of Carbon Additives" Sustainability 14, no. 20: 13629. https://doi.org/10.3390/su142013629
APA StyleZhu, X., Zhang, Q., Sun, Y., Li, Y., & Han, Y. (2022). Fluidization Roasting Technology of Jingtieshan Iron Ore in the Absence of Carbon Additives. Sustainability, 14(20), 13629. https://doi.org/10.3390/su142013629