Nickel-Cadmium-Sulfide Anchored on rGO Nanocomposite for Removal of Textile Industry Dyes
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of Ni–Cd–S/rGO
2.3. Material Characterization
2.4. Photocatalytic Degradation of Textile Dye
3. Results and Discussion
3.1. XRD Analysis
3.2. FTIR Analysis
3.3. Morphological Analysis
3.4. XPS Analysis
3.5. Optical Property
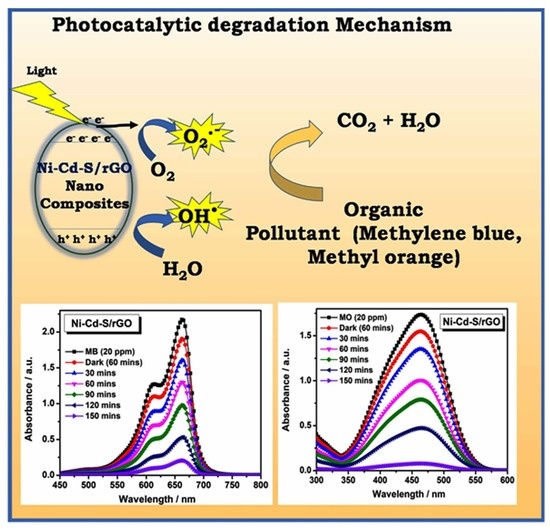
3.6. Photocatalytic Activity
- (i)
- Formation of exciton (electron hole pair)Ni–Cd–S/rGO + hν → h+ (VB) + e− (CB)
- (ii)
- Formation of hydroxyl radicalsOH− + h+ → OH●H2O + h+ → OH● + H+
- (iii)
- Formation of superoxide radicalsO2 + e− → O2●−
- (iv)
- Photo degradation of FG and RB dye
3.7. Possible Mechanism of Photocatalysis for Selective dye Adsorption Degradation
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Mathon, B.; Coquery, M.; Miege, C.; Vandycke, A.; Choubert, J.-M. Influence of water depth and season on the photodegradation of micropollutants in a free-water surface constructed wetland receiving treated wastewater. Chemosphere 2019, 235, 260–270. [Google Scholar]
- Salari, H.; Sadeghinia, M. MOF-templated synthesis of nano Ag2O/ZnO/CuO heterostructure for photocatalysis. J. Photochem. Photobiol. A Chem. 2019, 376, 279–287. [Google Scholar] [CrossRef]
- Liu, Y.; Zhang, Q.; Xu, M.; Yuan, H.; Chen, Y.; Zhang, J.; Luo, K.; Zhang, J.; You, B. Novel and efficient synthesis of Ag-ZnO nanoparticles for the sunlight induced photocatalytic degradation. Appl. Surf. Sci. 2019, 476, 632–640. [Google Scholar] [CrossRef]
- Peera, S.G.; Koutavarapu, R.; Chao, L.; Singh, L.; Murugadoss, G.; Rajeshkhanna, G. 2D MXene Nanomaterials as Electrocatalysts for Hydrogen Evolution Reaction (HER): A Review. Micromachines 2022, 13, 1499. [Google Scholar] [CrossRef] [PubMed]
- Lai, C.; Zhang, M.M.; Lia, B.S.; Huang, D.L.; Zeng, G.M.; Qin, L.; Liu, X.G.; Yi, H.; Cheng, M.; Li, L.; et al. Fabrication of CuS/BiVO4 (040) binary heterojunction photocatalysts with enhanced photocatalytic activity for Ciprofloxacin degradation and mechanism insight. Chem. Eng. J. 2019, 358, 891–902. [Google Scholar] [CrossRef]
- Liu, X.W.; Xua, J.J.; Ni, Z.Y.; Wang, R.C.; You, J.H.; Guo, R. Adsorption and visible light-driven photocatalytic properties of Ag3PO4/WO3composites: A discussion of the mechanism. Chem. Eng. J. 2019, 356, 22–33. [Google Scholar] [CrossRef]
- Zhang, B.; Wang, Q.; Zhuang, J.; Guan, S.; Li, B. Molten salt assisted in-situ synthesis of TiO2/g-C3N4 composites with enhanced visible-light-driven photocatalytic activity and adsorption ability. J. Photochem. Photobiol. A Chem. 2018, 362, 1–13. [Google Scholar] [CrossRef]
- Wang, Q.; Li, B. Flux Growth of Highly Crystalline Photocatalytic BaTiO3 Particle Layers on Porous Titanium Sponge Substrate and Insights into the Formation Mechanism. IOP Conf. Ser. Mater. Sci. Eng. 2017, 239, 012013. [Google Scholar] [CrossRef] [Green Version]
- Murugadoss, G.; Thiruppathi, K.; Venkatesh, N.; Hazra, S.; Mohankumar, A.; Thiruppathi, G.; Kumar, M.R.; Sundararaj, P.; Rajabathar, J.R.; Sakthivel, P. Construction of novel quaternary nanocomposite and its synergistic effect towards superior photocatalytic and antibacterial application. J. Environ. Chem. Eng. 2022, 10, 106961. [Google Scholar]
- Alomar, M.; Liu, Y.L.; Chen, W.; Fida, H. Controlling the growth of ultrathin MoS2 nanosheets/CdS nanoparticles by two-step solvothermal synthesis for enhancing photocatalytic activities under visible light. Appl. Surf. Sci. 2019, 480, 1078–1088. [Google Scholar] [CrossRef]
- Lv, J.X.; Zhang, Z.M.; Wang, J.; Lu, X.L.; Zhang, W.; Lu, T.B. In situ synthesis of CdS/Graphdiyne heterojunction for enhanced photocatalytic activity of hydrogen production. ACS Appl. Mater. Interfaces 2019, 11, 2655–2661. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Liu, S.Q.; Yang, M.Q.; Xu, Y.J. Synthesis of uniform CdS nanospheres/graphene hybrid nanocomposites and their application as visible light photocatalyst for selective reduction of nitro organics in water. ACS Appl. Mater. Interfaces 2013, 5, 4309–4319. [Google Scholar] [CrossRef] [PubMed]
- Park, H.; Kim, Y.K.; Choi, W.Y. Reversing CdS preparation order and its effects on photocatalytic hydrogen production of CdS/Pt-TiO2 hybrids under visible light. J. Phys. Chem. C 2011, 115, 6141–6148. [Google Scholar]
- Shi, W.L.; Guo, F.; Li, M.Y.; Shi, Y.; Tang, Y.B. N-doped carbon dots/CdS hybrid photocatalyst that responds to visible/ near-infrared light irradiation for enhanced photocatalytic hydrogen production. Sep. Purif. Technol. 2019, 212, 142–149. [Google Scholar]
- Wang, B.; He, S.; Zhang, L.L.; Huang, X.Y.; Gao, F.; Feng, W.H.; Liu, P. CdS nanorods decorated with inexpensive NiCd bimetallic nanoparticles as efficient photocatalysts for visible-light-driven photocatalytic hydrogen evolution. Appl. Catal. B 2019, 243, 229–235. [Google Scholar] [CrossRef]
- Irfan, R.M.; Tahir, M.H.; Khan, S.A.; Shaheen, M.A.; Ahmed, G.; Iqbal, S. Enhanced photocatalytic H2production under visible light on composite photocatalyst (CdS/NiSe nanorods) synthesized in aqueous solution. J. Colloid Interface Sci. 2019, 557, 1–9. [Google Scholar] [CrossRef]
- El-Katori, E.E.; Ahmed, M.A.; El-Bindary, A.A.; Oraby, A.M. Impact of CdS/SnO2 heterostructured nanoparticle as visible light active photocatalyst for the removal methylene blue dye. J. Photochem. Photobiol. A Chem. 2020, 392, 12403. [Google Scholar]
- Hou, J.; Huang, B.; Kong, L.; Xie, Y.; Liu, Y.; Chen, M.; Wang, Q. One-pot hydrothermal synthesis of CdS–CuS decorated TiO2 NTs for improved photocatalytic dye degradation and hydrogen production. Ceram. Int. 2021, 47, 30860–30868. [Google Scholar] [CrossRef]
- Singh, A.; Ahmed, A.; Sharma, A.; Sharma, C.; Paul, S.; Khosla, A.; Gupta, V.; Arya, S. Promising photocatalytic degradation of methyl orange dye via sol-gel synthesized Ag–CdS@Pr-TiO2 core/shell nanoparticles. Phys. B Condens. Matter. 2021, 616, 413121. [Google Scholar]
- Kokilavani, S.; Al-Kheraif, A.A.; Thomas, A.M.; Syed, A.; Elgorban, A.M.; Raju, L.L.; Das, A.; Khan, S.S. Novel NiS/Ag2MoO4 heterostructure nanocomposite: Synthesis, characterization and superior antibacterial and enhanced photocatalytic activity. Phys. E Low Dimens. Syst. Nanostruct. 2021, 133, 114767. [Google Scholar] [CrossRef]
- Cheng, L.; Xiang, Q.J.; Liao, Y.L.; Zhang, H.W. CdS-Based photocatalysts. Energy Environ. Sci. 2018, 11, 1362–1391. [Google Scholar] [CrossRef]
- Lia, L.; Wu, J.; Liu, B.B.; Liu, X.J.; Li, C.; Gong, Y.Y.; Huang, Y.L.; Pan, L.K. NiS sheets modified CdS/reduced graphene oxide composite for efficient visible light photocatalytic hydrogen evolution. Catal. Today 2018, 315, 110–116. [Google Scholar]
- Xu, J.L.; Zhang, L.; Xu, G.C.; Sun, Z.P.; Zhang, C.; Ma, X.; Qi, C.L.; Zhang, L.; Jia, D.Z. Facile synthesis of NiS anchored carbon nanofibers for high-performance supercapacitors. Appl. Surf. Sci. 2018, 434, 112. [Google Scholar] [CrossRef]
- Wang, Q.; Zhang, L.; Guo, Y.; Shen, M.; Wang, M.; Li, B.; Shi, J. Multifunctional 2D porous g-C3N4 nanosheets hybridized with 3D hierarchical TiO2 microflowers for selective dye adsorption, antibiotic degradation and CO2 reduction. Chem. Eng. J. 2020, 396, 125347. [Google Scholar] [CrossRef]
- Murugadoss, G.; Kumar, M.R.; Kathalingam, A.; Rajabathar, J.R.; Al-Lohedan, H.; Al-Dhayan, D.M. Facile synthesis of heterostructure CeO2/CdS and CdS/CeO2 nanocomposites for photocatalytic application of methylene blue textile dye. J. Ovonic Res. 2021, 17, 595–603. [Google Scholar]
- Ramalingam, G.; Magdalane, C.M.; Kumar, B.A.; Yuvakkumar, R.; Ravi, G.; Jothi, A.I.; Rotte, N.K.; Murugadoss, G.; Ananth, A. Enhanced visible light-driven photocatalytic performance of CdSe nanorods. Environ. Res. 2022, 203, 111855. [Google Scholar]
- Muruganandam, S.; Parivathini, K.; Murugadoss, G. Effect of co-doped (Ni2+: Co2+) in CdS nanoparticles: Investigation on structural and magnetic properties. Appl. Phys. A 2021, 127, 1–9. [Google Scholar]
- Narthana, K.; Durai, G.; Kuppusami, P.; Theerthagiri, J.; Sujatha, S.; Lee, S.J.; Choi, M.Y. One-step synthesis of hierarchical structured nickel copper sulfide nanorods with improved electrochemical supercapacitor properties. Int. J. Energy Res. 2021, 45, 9983–9998. [Google Scholar]
- Murugadoss, G.; Prakash, J.; Kumar, M.R.; Alothman, A.A.; Habila, M.A.; Peera, S.G. Controlled Synthesis of Europium-Doped SnS Quantum Dots for Ultra-Fast Degradation of Selective Industrial Dyes. Catalyst 2022, 12, 1128. [Google Scholar] [CrossRef]
- Fazli, Y.; Pourmortazavi, S.M.; Kohsari, I.; Sadeghpur, M. Electrochemical synthesis and structure characterization of nickel sulfide nanoparticles. Mater. Sci. Semicond. Process. 2014, 27, 362–367. [Google Scholar] [CrossRef]
- Jansi Rani, B.; Dhivya, N.; Ravi, G.; Zance, S.S.; Yuvakkumar, R.; Hong, S.I. Electrochemical performance of β-Nis@ Ni(OH)2 nanocomposite for water splitting applications. ACS Omega 2018, 4, 10302–10310. [Google Scholar] [CrossRef] [PubMed]
- Liang, D.; Gao, J.; Sun, H.; Chen, P.; Hou, Z.; Zheng, X. Selective oxidation of glycerol with oxygen in a base-free aqueous solution over MWNTs supported Pt catalysts. Appl. Catal. B Environ. 2011, 106, 423–432. [Google Scholar]
- Manikandan, A.; Hema, E.; Durka, M.; Selvi, M.A.; Alagesan, T.; Antony, S.A. Mn2+ doped NiS (MnxNi1−xS: X = 0.0, 0.3 and 0.5) nanocrystals: Structural, morphological, opto-magnetic and photocatalytic properties. J. Inorg. Organomet. Polym. Mater. 2015, 25, 804–815. [Google Scholar]
- Ponnaiah, S.K.; Prakash, P.; Vellaichamy, B. A new analytical device incorporating a nitrogen doped lanthanum metal oxide with reduced graphene oxide sheets for paracetamol sensing. Ultrason. Sonochem. 2018, 44, 196–203. [Google Scholar] [CrossRef] [PubMed]
- Pal, D.B.; Rathoure, A.K.; Singh, A. Investigation of surface interaction in rGO-CdS photocatalyst for hydrogen production: An insight from XPS studies. Int. J. Hydrog. Energy 2021, 46, 26757–26769. [Google Scholar] [CrossRef]
- Murugadoss, G.; Kandhasamy, N.; Kumar, M.R.; Alanazi, A.K.; Khan, F.; Salhi, B.; Yadav, H.M. Role of the dopant (silver) inclusion on before and after core metal-oxide reaction: Application on textile dyes removal. Inorg. Chem. Commun. 2022, 137, 109186. [Google Scholar] [CrossRef]
- Liu, X.; Bie, C.; He, B.; Zhu, B.; Zhang, L.; Cheng, B. 0D/2D NiS/CdS nanocomposite heterojunction photocatalyst with enhanced photocatalytic H2 evolution activity. Appl. Surf. Sci. 2021, 554, 149622. [Google Scholar] [CrossRef]
- Jayaraman, V.; Mani, A. Ag, Ni bimetallic supported g-C3N4 2D/Cd2Sb2O6.8 pyrochlore interface photocatalyst for efficient removal of organic pollutants. J. Mater. Sci. Mater. Electron. 2020, 31, 11247–11267. [Google Scholar]
- Zhang, H.; Yu, Z.; Jiang, R.; Hou, Y.; Huang, J.; Zhu, H.; Yang, F.; Li, M.; Li, F.; Ran, Q. Metal organic frameworks constructed heterojunction with α-NiS-β-NiS/CdS: The effect of organic-ligand in UiO-66 for charge transfer of photocatalytic hydrogen evolution. Renew. Energy 2021, 168, 1112–1121. [Google Scholar] [CrossRef]
- Muninathan, S.; Arumugam, S. Enhanced photocatalytic activities of NiS decorated reduced graphene oxide for hydrogen production and toxic dye degradation under visible light irradiation. Int. J. Hydrogen Energy 2021, 46, 6532–6546. [Google Scholar]
- Some, S.; Kim, Y.; Yoon, Y.; Yoo, H.; Lee, S.; Park, Y.; Lee, H. High-quality reduced graphene oxide by a dual-function chemical reduction and healing process. Sci Rep. 2013, 3, 1–5. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hamid, A.A.A.; Yang, X.; Yang, J.; Chen, X.; Ying, J.Y. Graphene-wrapped nickel sulfide nanoprisms with improved performance for Li-ion battery anodes and supercapacitors. Nano Energy 2016, 26, 425–437. [Google Scholar]
- Liu, G.; Thummavichai, K.; Lv, X.; Chen, W.; Lin, T.; Tan, S.; Zeng, M.; Chen, Y.; Wang, N.; Zhu, Y. Defect-Rich Heterogeneous MoS2/rGO/NiS Nanocomposite for Efficient pH-Universal Hydrogen Evolution. J. Nanomater. 2021, 11, 662. [Google Scholar] [CrossRef] [PubMed]
- Zhao, G.; Zhang, Y.; Yang, L.; Jiang, Y.; Zhang, Y.; Hong, W.; Tian, Y.; Zhao, H.; Hu, J.; Zhou, L.; et al. Nickel Chelate Derived NiS2 Decorated with Bifunctional Carbon: An Efficient Strategy to Promote Sodium Storage Performance. Adv. Funct. Mater. 2018, 28, 1803690. [Google Scholar]
- Alhammadi, M.R.; Minnam Reddy, V.R.; Gedi, S.; Park, H.; Sayed, M.S.; Shim, J.J.; Kim, W.K. Performance of Graphene–CdS Hybrid Nanocomposite Thin Film for Applications in Cu(In,Ga)Se2 Solar Cell and H2 Production. J. Nanomater. 2020, 10, 245. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, Z.; Wang, X.; Wang, X.; Lin, Y.; Meng, A.; Yang, L.; Li, Q. Mn-Cd-S@ amorphous-Ni3S2 hybrid catalyst with enhanced photocatalytic property for hydrogen production and electrocatalytic OER. Appl. Surf. Sci. 2019, 491, 799–806. [Google Scholar] [CrossRef]
- Wang, Q.; Zhang, B.; Lu, X.; Zhang, X.; Zhu, H.; Li, B. Multifunctional 3D K2Ti6O13 nanobelt-built architectures towards wastewater remediation: Selective adsorption, photodegradation, mechanism insight and photoelectrochemical investigation. Catal. Sci. Technol. 2018, 8, 6180–6195. [Google Scholar] [CrossRef]
- Zhu, J.; Shen, Y.; Yu, X.; Guo, J.; Zhu, Y.; Zhang, Y. A facile two-step method to synthesize immobilized CdS/BiOCl film photocatalysts with enhanced photocatalytic activities. J. Alloys Compd. 2019, 771, 309–316. [Google Scholar] [CrossRef]
- Zhang, J.; Fang, J.; Ye, X.; Guo, Z.; Liu, Y.; Song, Q.; Zheng, S.; Chen, X.; Wang, S.; Yang, S. Visible photoactivity and anti-photocorrosion performance of CdS photocatalysts by the hybridization of N-substituted carboxyl group polyaniline. Appl. Surf. Sci. 2019, 480, 557–564. [Google Scholar] [CrossRef]
- Zhao, W.; Li, J.; She, T.; Ma, S.; Cheng, Z.; Wang, G.; Zhao, P.; Wei, W.; Xia, D.; Leung, D.Y. Study on the photocatalysis mechanism of the Z-Scheme cobalt oxide nanocubes/carbon nitride nanosheets heterojunction photocatalyst with high photocatalytic performances. J. Hazard. Mater. 2021, 402, 123839. [Google Scholar]
- Shen, X.; Shao, H.; Liu, Y.; Zhai, Y. Synthesis and photocatalytic performance of ZnO with flower-like structure from zinc oxide ore. J. Mater. Sci. Technol. 2020, 51, 1–7. [Google Scholar] [CrossRef]
- Zhuang, J.; Zhang, B.; Wang, Q.; Guan, S.; Li, B. Construction of novel ZnTiO3/g-C3N4 heterostructures with enhanced visible light photocatalytic activity for dye wastewater treatment. J. Mater. Sci.: Mater. Electron. 2019, 30, 6322–6334. [Google Scholar] [CrossRef]
- Venkatesh, N.; Sabarish, K.; Murugadoss, G.; Thangamuthu, R.; Sakthivel, P. Visible light–driven photocatalytic dye degradation under natural sunlight using Sn-doped CdS nanoparticles. Environ. Sci. Pollut. Res. 2020, 27, 43212–43222. [Google Scholar]
- Abdelwahab, M.A.; El Rayes, S.M.; Kamel, M.M.; Abdelrahman, E.A. Encapsulation of NiS and ZnS in analcime nanoparticles as novel nanocomposites for the effective photocatalytic degradation of orange G and methylene blue dyes. Int. J. Environ. Anal. Chem. 2022, 1–18. [Google Scholar] [CrossRef]
- Qayoom, A.; Ashraf, I.; Rashid, A.; Ayoub, M.; Kumar, D. Synthesis and Characterization of NiS and Zn doped NiS Quantum Dots and their application in methylene blue degradation in aqueous solution. Res Sq. 2022. [Google Scholar] [CrossRef]
- Jing, C.; Zhang, Y.; Zheng, J.; Ge, S.; Lin, J.; Pan, D.; Naik, N.; Guo, Z. In-situ constructing visible light CdS/Cd-MOF photocatalyst with enhanced photodegradation of methylene blue. Particuology 2022, 69, 111–122. [Google Scholar] [CrossRef]
- Ramasubbu, V.; Kumar, P.R.; Chellapandi, T.; Madhumitha, G.; Mothi, E.M.; Shajan, X.S. Zn (II) porphyrin sensitized (TiO2@ Cd-MOF) nanocomposite aerogel as novel photocatalyst for the effective degradation of methyl orange (MO) dye. Opt. Mater. 2022, 132, 112558. [Google Scholar] [CrossRef]
- Alijani, H.; Abdouss, M.; Khataei, H. Efficient photocatalytic degradation of toxic dyes over BiFeO3/CdS/rGO nanocomposite under visible light irradiation. Diam. Relat. Mater. 2022, 122, 108817. [Google Scholar]
- Shakil, M.; Inayat, U.; Khalid, N.R.; Tanveer, M.; Gillani, S.S.A.; Tariq, N.H.; Shah, A.; Mahmood, A.; Dahshan, A. Enhanced structural, optical, and photocatalytic activities of Cd–Co doped Zn ferrites for degrading methyl orange dye under irradiation by visible light. J. Phys. Chem. Solids 2022, 161, 110419. [Google Scholar]
- Wang, Q.; Guo, Q.; Wang, L.; Li, B. The flux growth of single-crystalline CoTiO3 polyhedral particles and improved visible-light photocatalytic activity of heterostructured CoTiO3/gC3N4 composites. Dalton Trans. 2016, 45, 17748–17758. [Google Scholar] [CrossRef] [PubMed]
- Ma, C.M.; Yang, B.Y.; Hong, G.B. Husk of agarwood fruit-based hydrogel beads for adsorption of cationic and anionic dyes in aqueous solutions. Molecules 2021, 26, 1437. [Google Scholar] [CrossRef] [PubMed]











| Catalyst | Light Source | Dye | Dye Concentration (mg L−1) | Irradiation Time | Efficiency (%) | Ref. |
|---|---|---|---|---|---|---|
| TiO2 NTs/CdS–CuS (8%) | Solar | MB, MO | 1 mol/L | 6 h | 89.28, 63.36 | [18] |
| Ag–CdS@Pr-TiO2 core/shell Nanoparticles | Visible light | MO | 0.0049 g | 30 min | 98 | [19] |
| NiS/Ag2MoO4 | Visible light | MB | 10 | 140 min | 90.8 | [20] |
| 3D/2D TiO2/p-g-C3N4 | Visible light | MO | 10 | 4 h | 99 | [24] |
| K2Ti6O13 Nanobelt | UV | MB | 10 | 120 min | 82.1 | [47] |
| ZnTiO3/g-C3N4 | Visible light | MO | 10 | 180 min | 76 | [52] |
| Sn-doped CdS NPs | Sunlight | MB | 20 | 180 min | 97.5 | [53] |
| NiS/analcime | Visible light | MB | 10 | 180 min | 88.76 | [54] |
| Zn doped NiS QDs | Visible light | MB | 10 | 2 h | 78 | [55] |
| Ni–Cd–S/rGO | Sunlight | MO | 25 | 150 min | 97 | This work |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kandhasamy, N.; Murugadoss, G.; Kannappan, T.; Kirubaharan, K.; Kumar Manavalan, R. Nickel-Cadmium-Sulfide Anchored on rGO Nanocomposite for Removal of Textile Industry Dyes. Sustainability 2022, 14, 16184. https://doi.org/10.3390/su142316184
Kandhasamy N, Murugadoss G, Kannappan T, Kirubaharan K, Kumar Manavalan R. Nickel-Cadmium-Sulfide Anchored on rGO Nanocomposite for Removal of Textile Industry Dyes. Sustainability. 2022; 14(23):16184. https://doi.org/10.3390/su142316184
Chicago/Turabian StyleKandhasamy, Narthana, Govindasamy Murugadoss, Thiruppathi Kannappan, Kamalan Kirubaharan, and Rajesh Kumar Manavalan. 2022. "Nickel-Cadmium-Sulfide Anchored on rGO Nanocomposite for Removal of Textile Industry Dyes" Sustainability 14, no. 23: 16184. https://doi.org/10.3390/su142316184
APA StyleKandhasamy, N., Murugadoss, G., Kannappan, T., Kirubaharan, K., & Kumar Manavalan, R. (2022). Nickel-Cadmium-Sulfide Anchored on rGO Nanocomposite for Removal of Textile Industry Dyes. Sustainability, 14(23), 16184. https://doi.org/10.3390/su142316184